PbSO4 Leaching in Citric Acid/Sodium Citrate Solution and Subsequent Yielding Lead Citrate via Controlled Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Procedures
3. Results
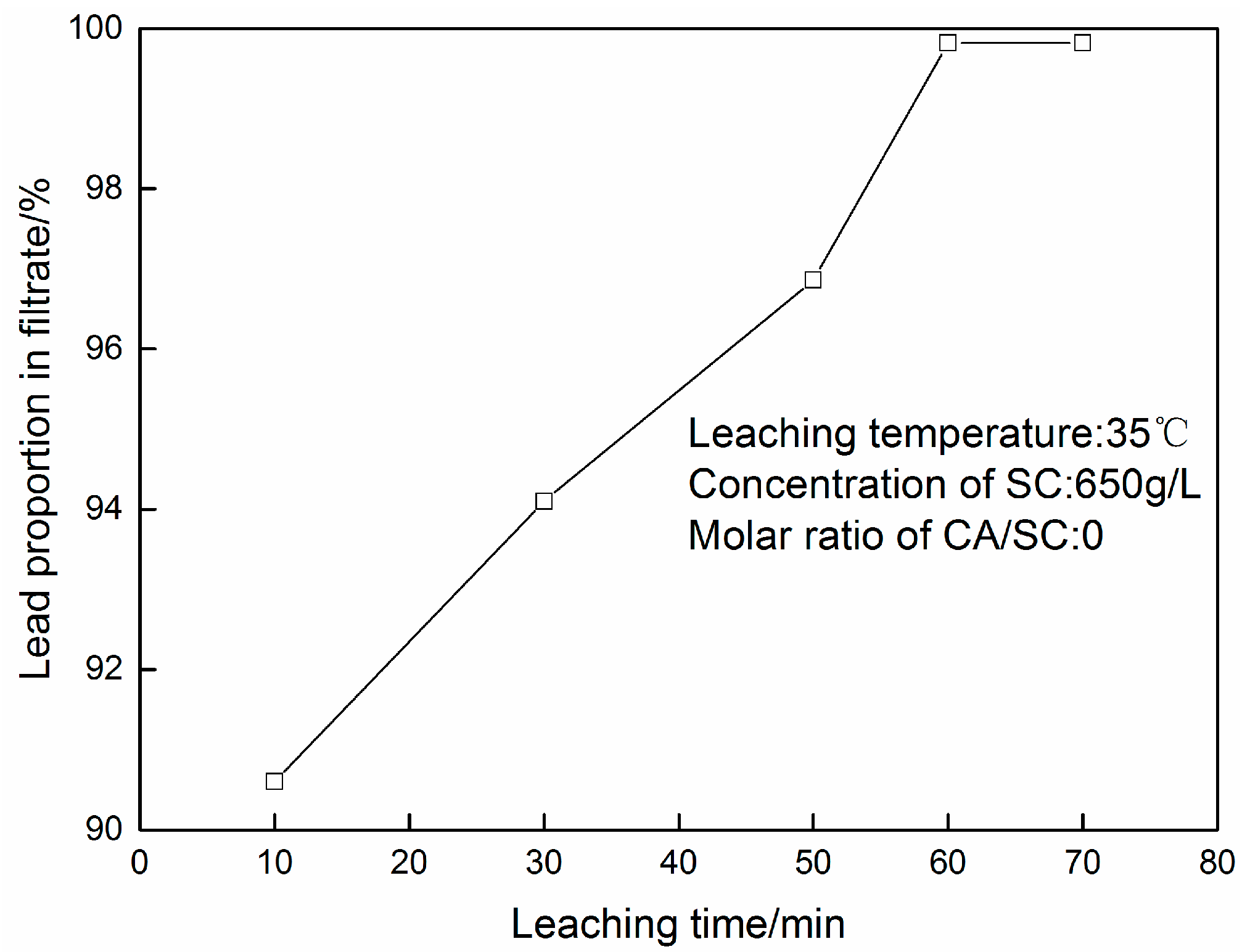
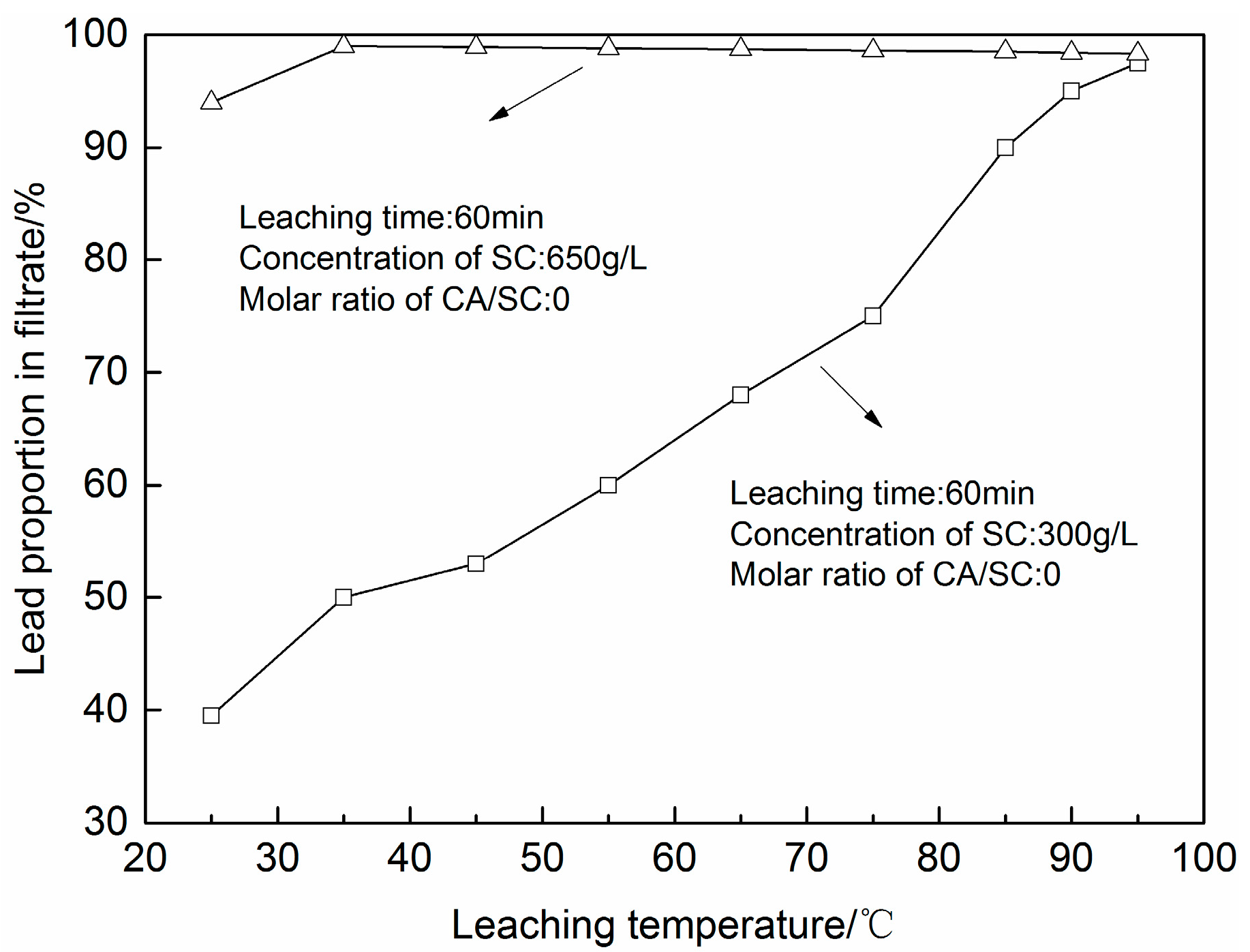
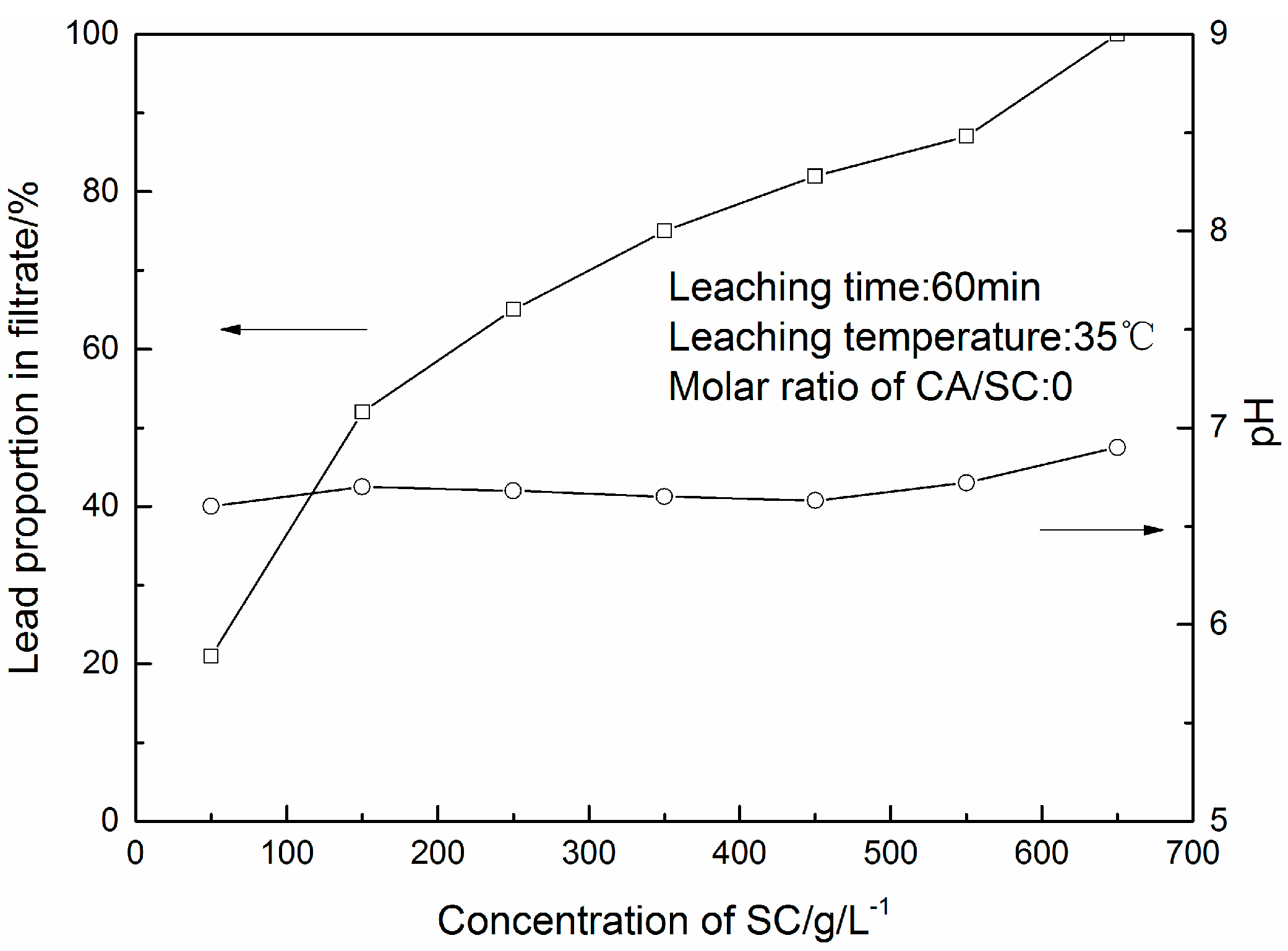
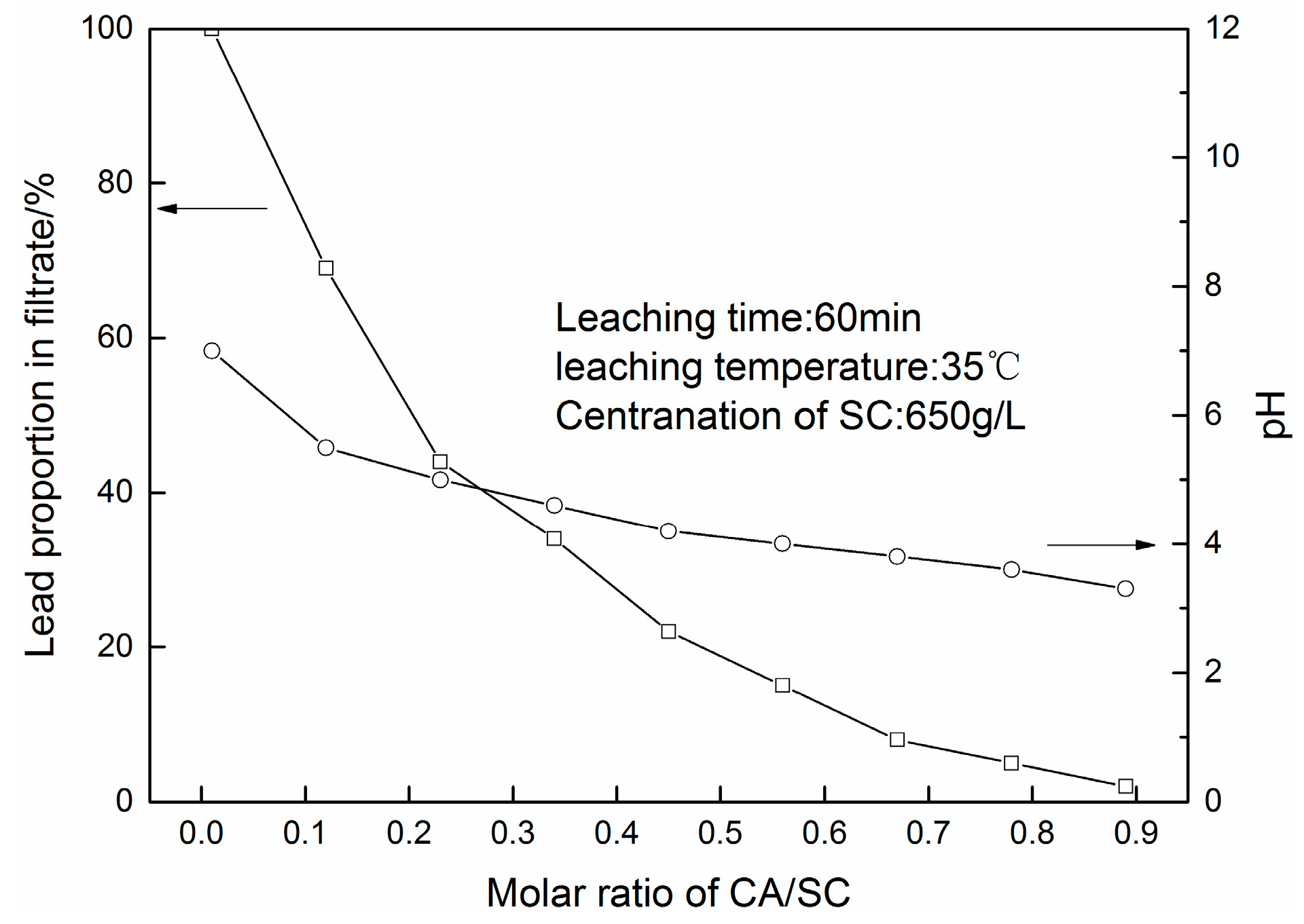
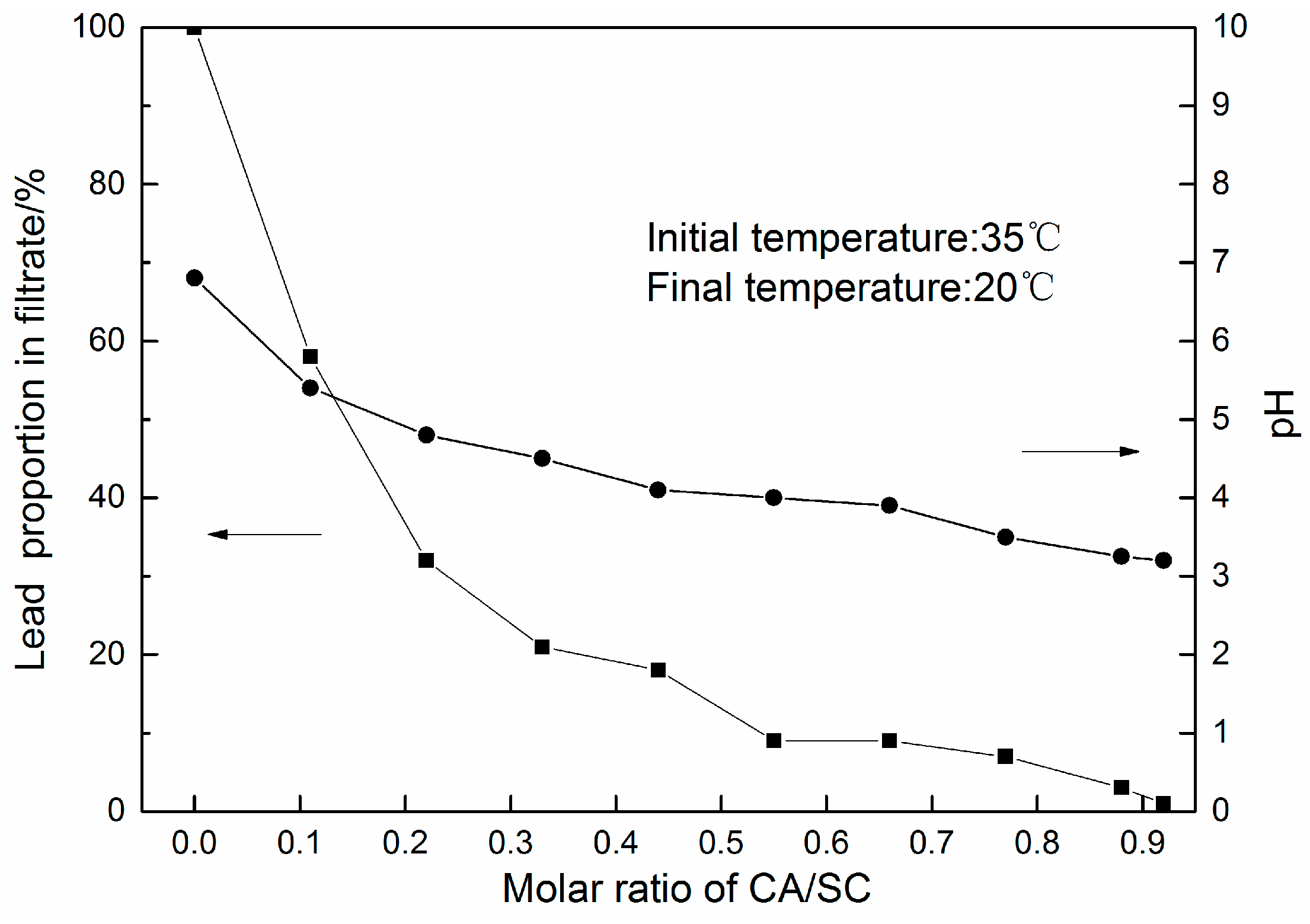
3.1. PbSO4 Leaching in Citric Acid/Sodium Citrate Solution
3.2. Lead Citrate Yielded from CA/SC Solution via Controlled Crystallization
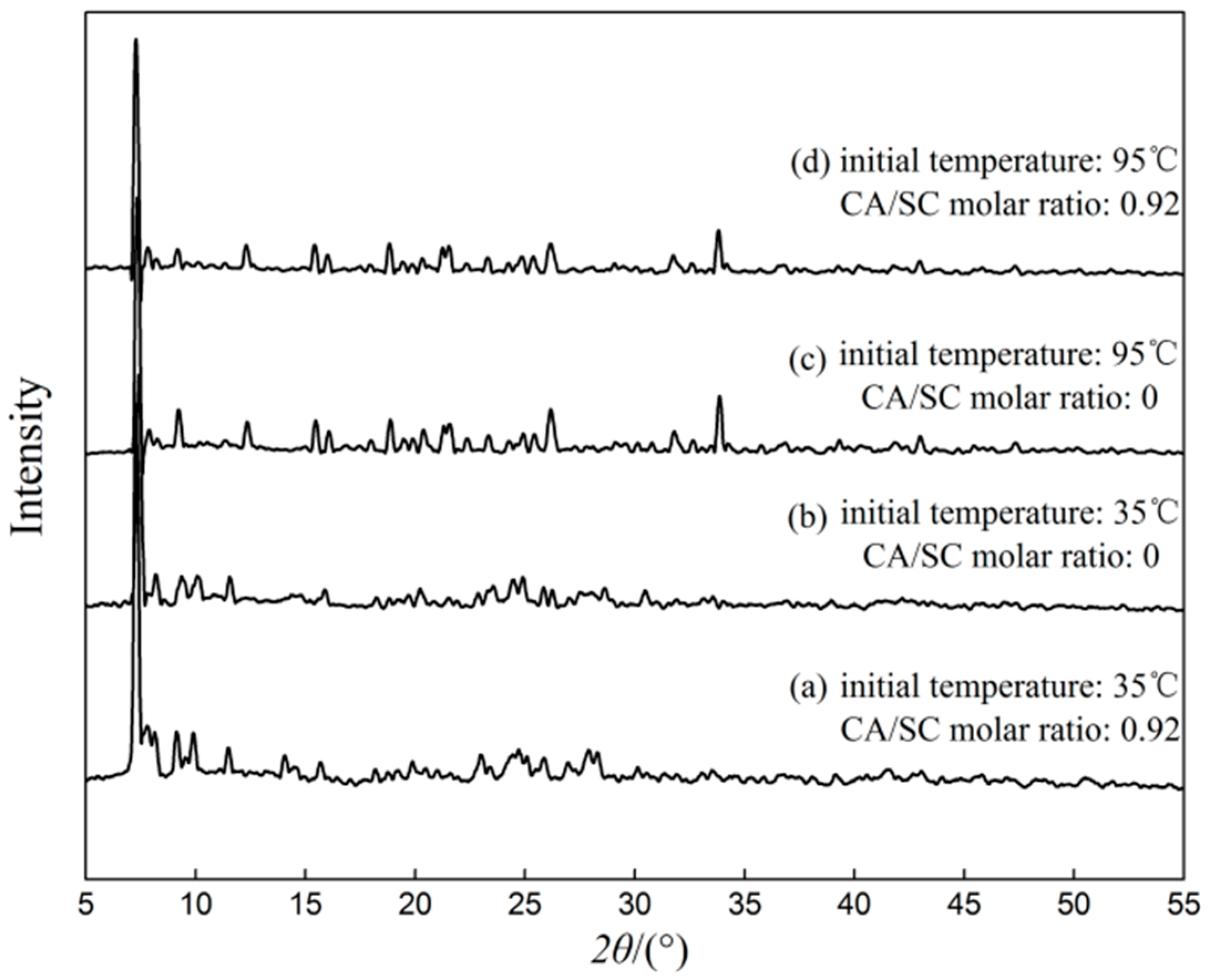
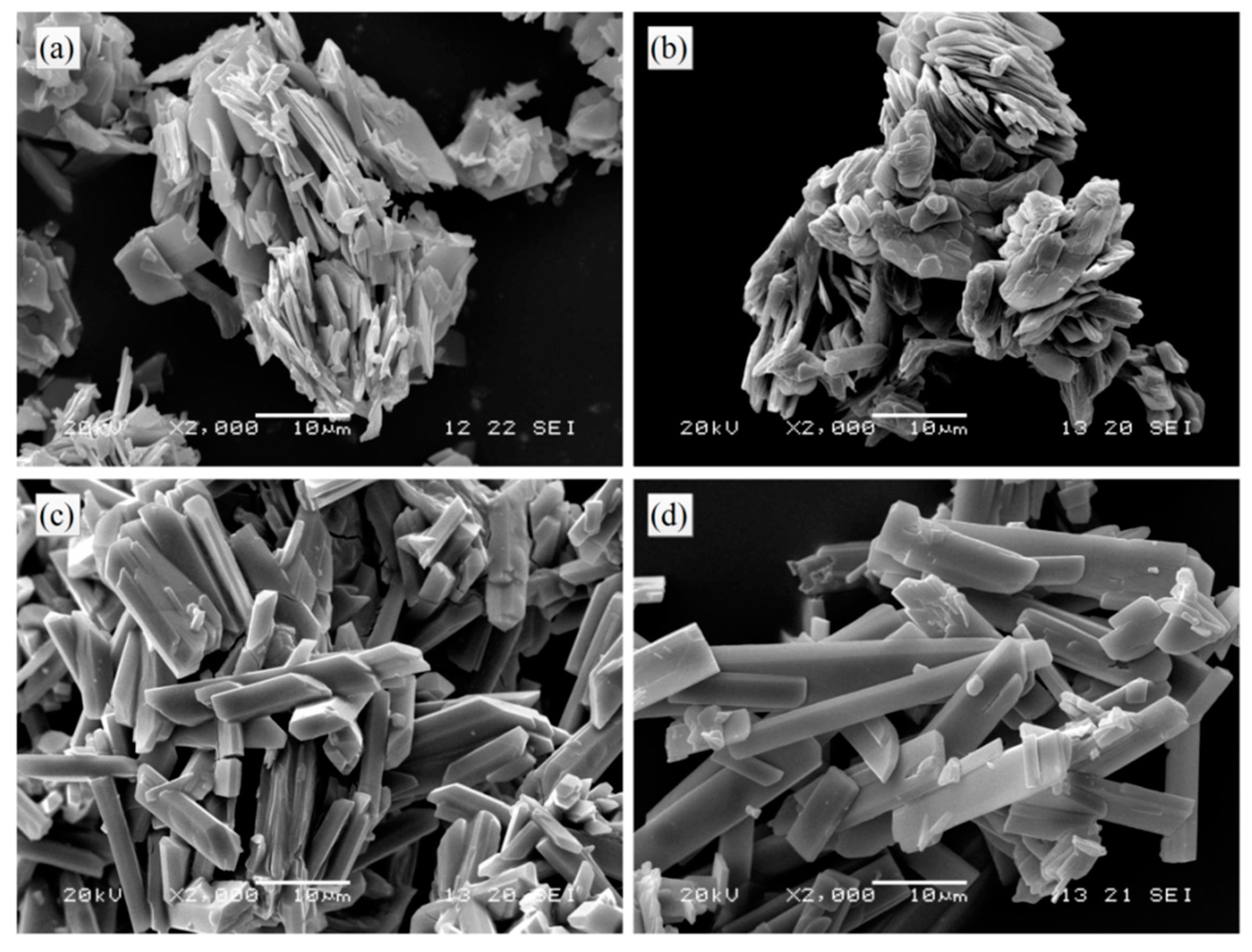
3.3. Comparison Test on the Characterization of Lead Citrate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, H.Y.; Li, A.J.; Finlow, D.E. The lead and lead-acid battery industries during 2002 and 2007 in China. J. Power Sources 2009, 191, 22–27. [Google Scholar] [CrossRef]
- Tian, X.; Gong, Y.; Wu, Y.F.; Agyeiwaa, A.; Zuo, T.Y. Management of used lead acid battery in China: Secondary lead industry progress, policies and problems. Resour. Conserv. Recycl. 2014, 93, 75–84. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH Press: Weinheim, Germany, 1997. [Google Scholar]
- Prengman, R.D.; Morgan, C.; Hine, E.; Homer, P.; Griffin, G.M. Process for Recycling Lead-Acid Batteries. U.S. Patent 6,177,056, 23 January 2001. [Google Scholar]
- Liang, J.; Mao, J.S. A dynamic analysis of environmental losses from anthropogenic lead flow and their accumulation in China. Trans. Nonferr. Met. Soc. 2014, 24, 1125–1133. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. The mineralogical characterization of lead-acid battery paste. Hydrometallurgy 1996, 40, 223–245. [Google Scholar] [CrossRef]
- Gong, Y.; Dutrizac, J.E.; Chen, T.T. The conversion of lead sulphate to lead carbonate in sodium carbonate media. Hydrometallurgy 1992, 28, 399–421. [Google Scholar] [CrossRef]
- Lyakov, N.K.; Atanasova, D.A.; Vassilev, V.S.; Haralampiev, G.A. Desulphurization of damped battery paste by sodium carbonate and sodium hydroxide. J. Power Sources 2007, 171, 960–965. [Google Scholar] [CrossRef]
- Zhu, X.F.; Yang, J.K.; Gao, L.X.; Liu, J.W.; Yang, D.N.; Sun, X.J.; Zhang, W.; Wang, Q.; Li, L.; He, D.S.; et al. Preparation of lead carbonate from lead paste by hydrometallurgical processes. Hydrometallurgy 2013, 134–135, 47–53. [Google Scholar] [CrossRef]
- Kumar, R.V.; Sonmez, M.S.; Kotezva, V.P. Lead Recycling. UK Patent 2010/0040938, 16 March 2006. [Google Scholar]
- Kumar, R.V.; Sonmez, M.S.; Kotzeva, V.P. Lead Recycling. International Patent Application No. PCT/GB2007/004222, 15 May 2008. [Google Scholar]
- Sonmez, M.S.; Kumar, R.V. Leaching of waste battery paste components. Part 1: Lead citrate synthesis from PbO and PbO2. Hydrometallurgy 2009, 95, 53–60. [Google Scholar] [CrossRef]
- Sonmez, M.S.; Kumar, R.V. Leaching of waste battery paste components. Part 2: Leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution. Hydrometallurgy 2009, 95, 82–86. [Google Scholar] [CrossRef]
- Hu, Y.C.; Yang, J.K.; Zhang, W.; Xie, Y.L.; Wang, J.X.; Yuan, X.Q.; Liang, S.; Hu, J.P.; Wu, X. A novel leady oxide combined with porous carbon skeleton synthesized from lead citrate precursor recovered from spent lead-acid battery paste. J. Power Sources 2016, 304, 128–135. [Google Scholar] [CrossRef]
- Yang, D.N.; Liu, J.W.; Wang, Q.; Yuan, X.Q.; Zhu, X.F.; Li, L.; Zhang, W.; Hu, Y.C.; Sun, X.J.; Kumar, R.V.; et al. A novel ultrafine leady oxide prepared from spent lead pastes for application as cathode of lead acid battery. J. Power Sources 2014, 257, 27–36. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.K.; Wu, X.; Hu, Y.C.; Yu, W.H.; Wang, J.X.; Dong, J.X.; Li, M.Y.; Liang, S.; Hu, J.P.; et al. A critical review on secondary lead recycling technology and its prospect. Renew. Sustain. Energery Rev. 2016, 61, 108–122. [Google Scholar] [CrossRef]
- China Standards Publication. Methods for Chemical Analysis of Crude Gold. Part 4: Determination of Lead Content. EDTA Titrimetric Method; GB/T 15249.4-2009; China Standards Press: Beijing, China, 2009.
- Bottari, E.; Vicedomini, M. On the complex formation between lead(II) and citrate ions in alkaline solution. J. Inorg. Nucl. Chem. 1973, 35, 2447–2453. [Google Scholar] [CrossRef]
- Zárate-gutiérrez, R.; Lapidus, G.T. A novel process for silver recovery from a refractory Au–Ag ore in cyanidation by pretreatment with sulfating leaching using pyrite as reductant. Hydrometallurgy 2014, 144–145, 124–128. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Motekaitis, R.J. NIST Standard Reference Database 46; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2004.
- Zhu, X.; He, X.; Yang, J.; Gao, L.; Liu, J.; Yang, D.; Sun, X.; Zhang, W.; Wang, Q.; Kumar, R.V. Leaching of spent lead acid battery paste components by sodium citrate and acetic acid. J. Hazard. Mater. 2013, 250–251, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Guiomar, M.J.; Lito, H.M.; Filomena, M.; Camões, G.F.C.; Covington, A.K. Effect of citrate impurities on the reference pH value of potassium dihydrogen buffer solution. Anal. Chim. Acta 2003, 482, 137–146. [Google Scholar]
- Bottari, E.; Vicedomini, M. On the complex formation between lead(II) and citrate ions in acid solution. J. Inorg. Nucl. Chem. 1973, 35, 1269–1278. [Google Scholar] [CrossRef]
- Sun, X.X.; Sun, Y.Z.; Yu, J.G. Cooling crystallization of aluminum sulfate in pure water modulated by sodium dodecylbenzenesulfonate. J. Cryst. Growth 2015, 419, 94–101. [Google Scholar] [CrossRef]
- Ma, R.J. Principle on Hydrometallurgy; Metallurgical Industrial Press: Beijing, China, 2007. [Google Scholar]

| No. | Time (min) | Temperature (°C) | Concentration of SC (g/L) | Molar Ratio of CA/SC |
|---|---|---|---|---|
| 1 | 60 | 25 | 700 | 0 |
| 2 | 60 | 35 | 650 | 0 |
| 3 | 60 | 45 | 600 | 0 |
| 4 | 60 | 55 | 550 | 0 |
| 5 | 60 | 65 | 500 | 0 |
| 6 | 60 | 75 | 450 | 0 |
| 7 | 60 | 85 | 400 | 0 |
| 8 | 60 | 95 | 300 | 0 |
| No. | Initial Temperature (°C) | Molar Ratio of CA/SC |
|---|---|---|
| a | 35 | 0.92 |
| b | 35 | 0 |
| c | 95 | 0 |
| d | 95 | 0.92 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Yang, C.; Wu, Y.; Liu, X.; Xie, W.; Yang, J. PbSO4 Leaching in Citric Acid/Sodium Citrate Solution and Subsequent Yielding Lead Citrate via Controlled Crystallization. Minerals 2017, 7, 93. https://doi.org/10.3390/min7060093
He D, Yang C, Wu Y, Liu X, Xie W, Yang J. PbSO4 Leaching in Citric Acid/Sodium Citrate Solution and Subsequent Yielding Lead Citrate via Controlled Crystallization. Minerals. 2017; 7(6):93. https://doi.org/10.3390/min7060093
Chicago/Turabian StyleHe, Dongsheng, Cong Yang, Yuyuan Wu, Xing Liu, Weimin Xie, and Jiakuan Yang. 2017. "PbSO4 Leaching in Citric Acid/Sodium Citrate Solution and Subsequent Yielding Lead Citrate via Controlled Crystallization" Minerals 7, no. 6: 93. https://doi.org/10.3390/min7060093





