Ammonium-Carbamate-Rich Organogels for the Preparation of Amorphous Calcium Carbonates
Abstract
:1. Introduction
2. Materials and Methods
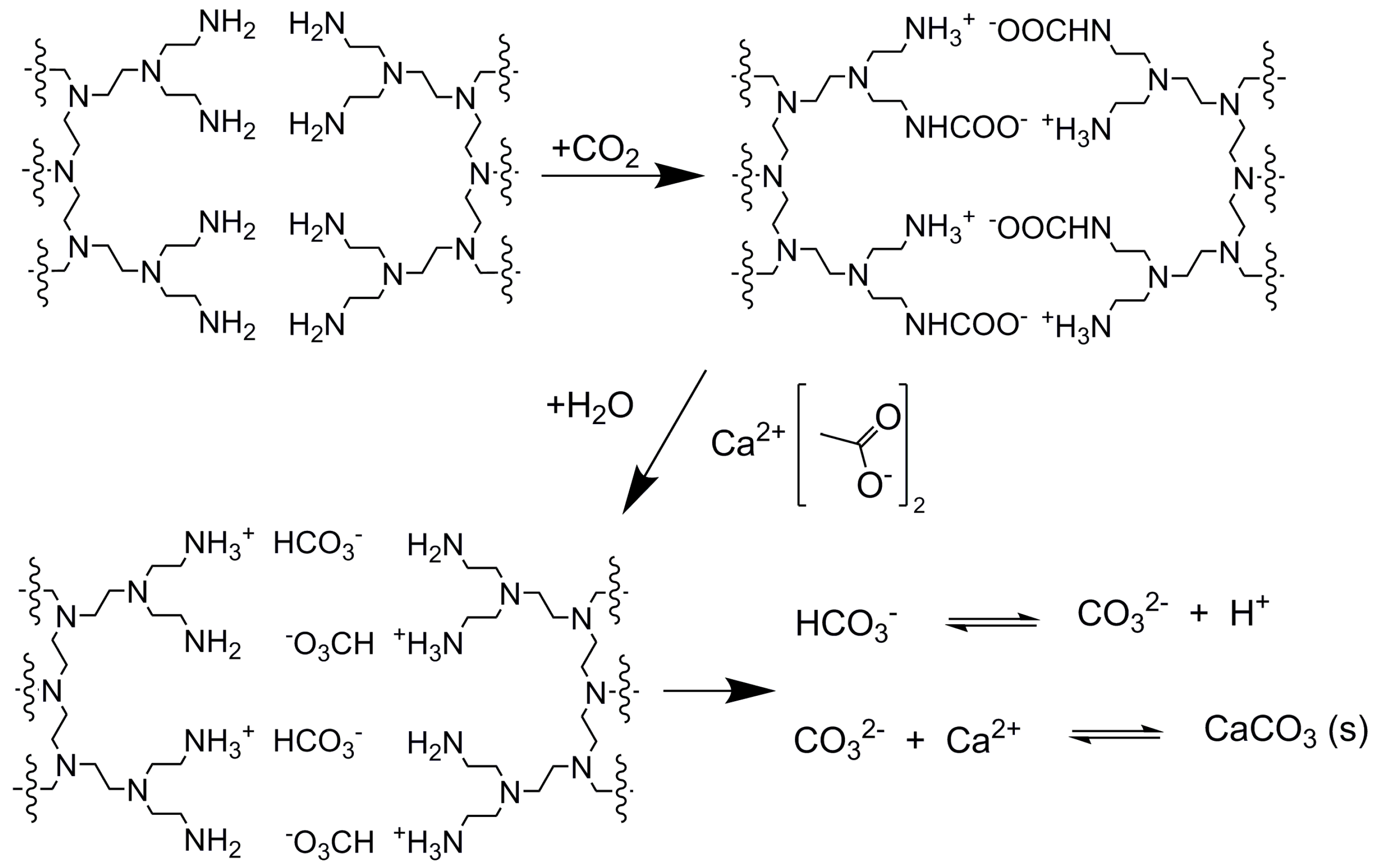
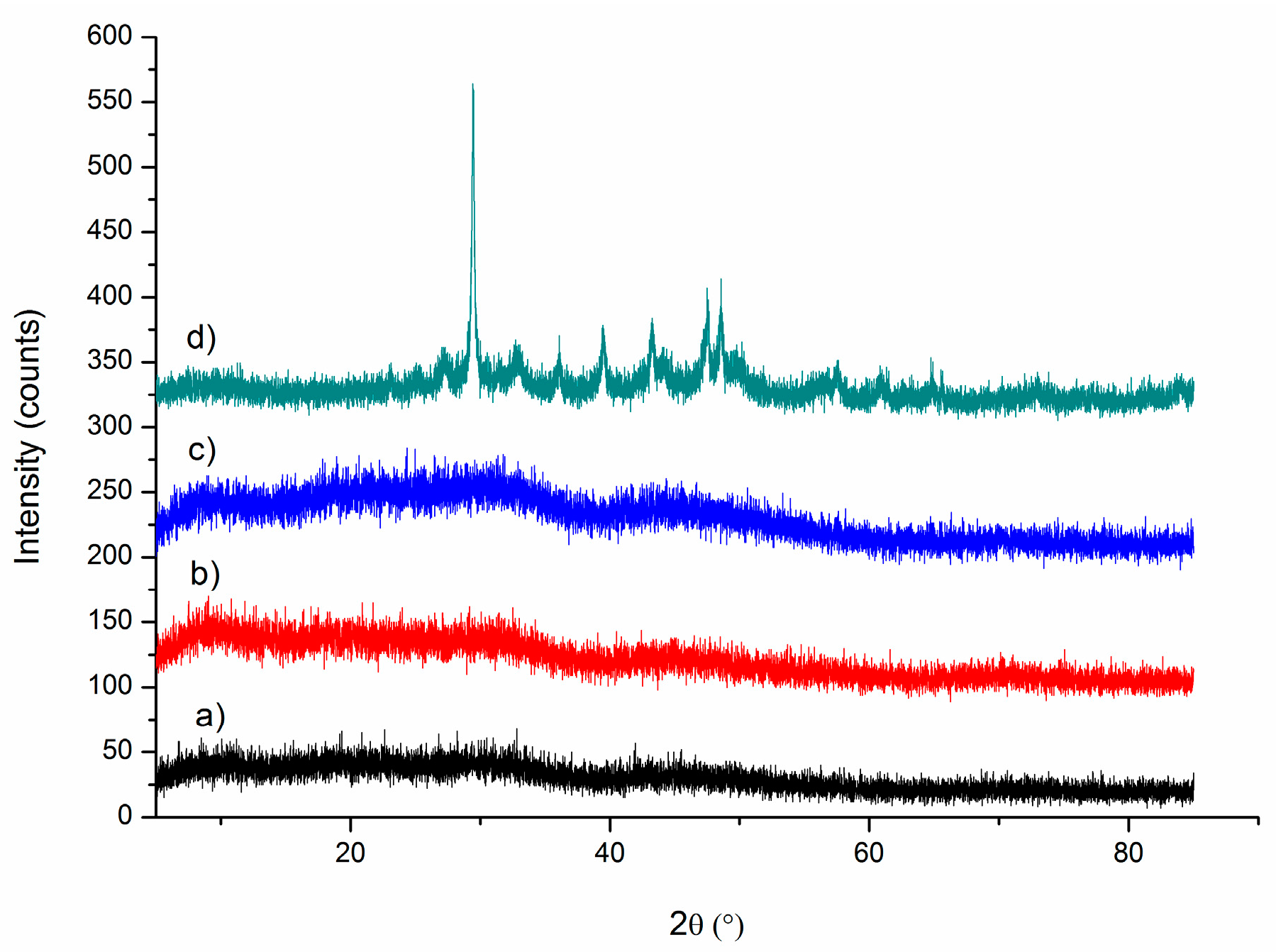
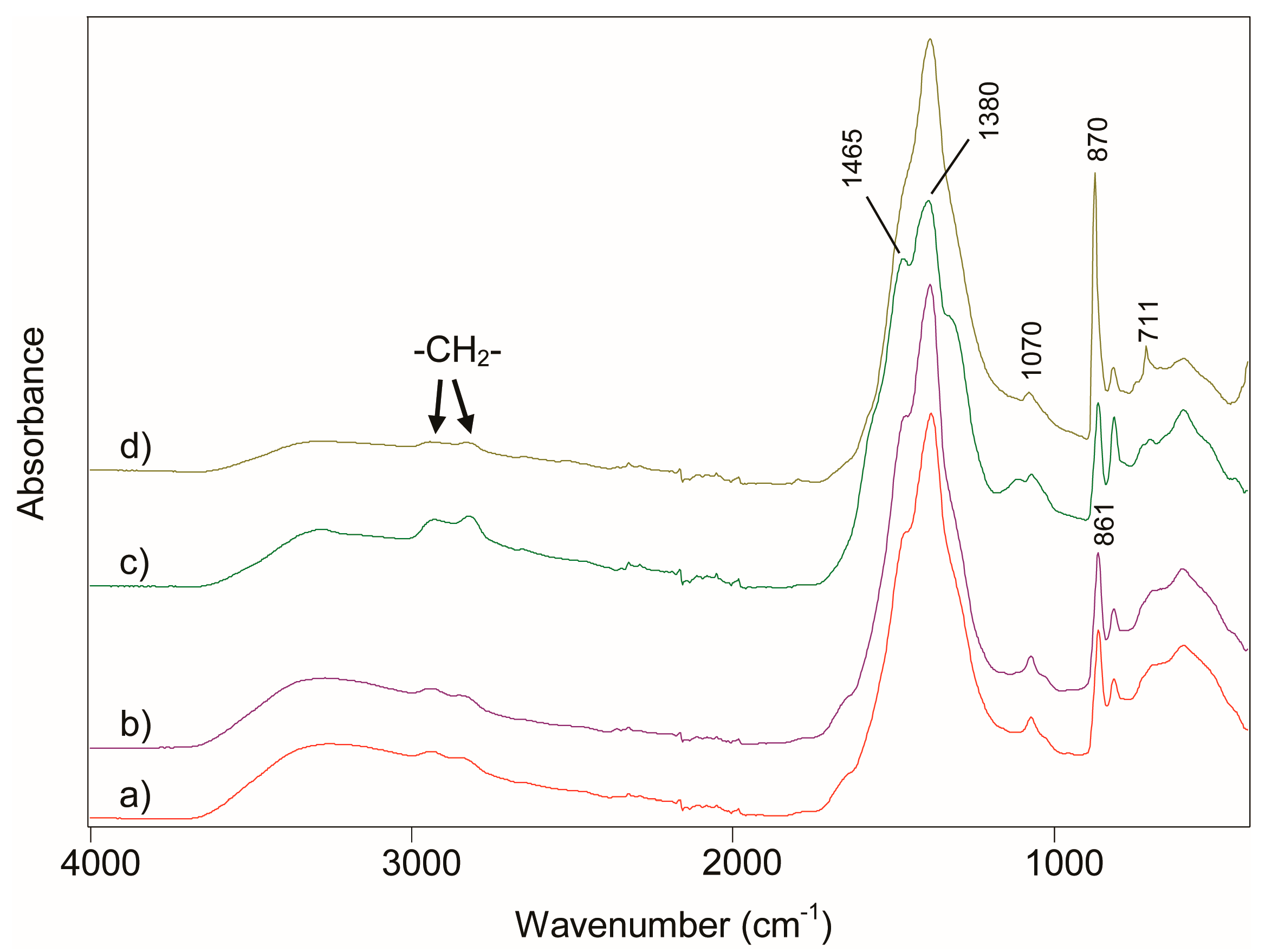
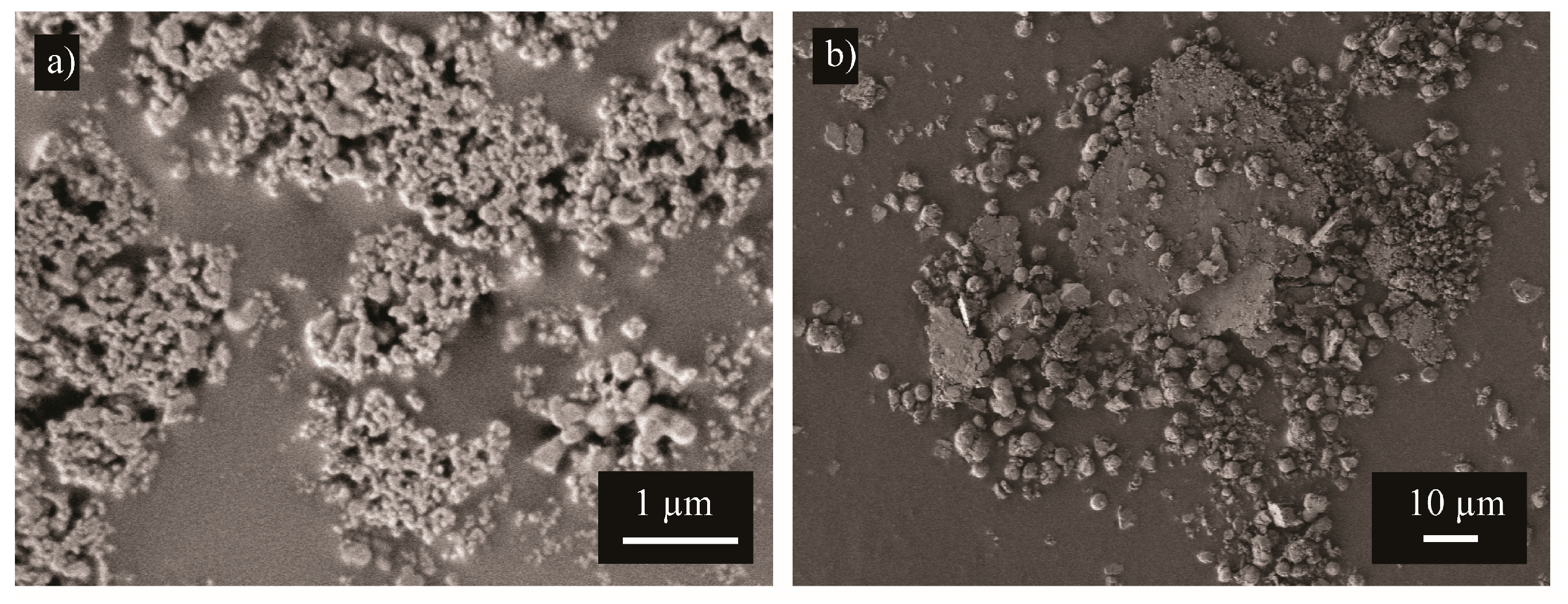
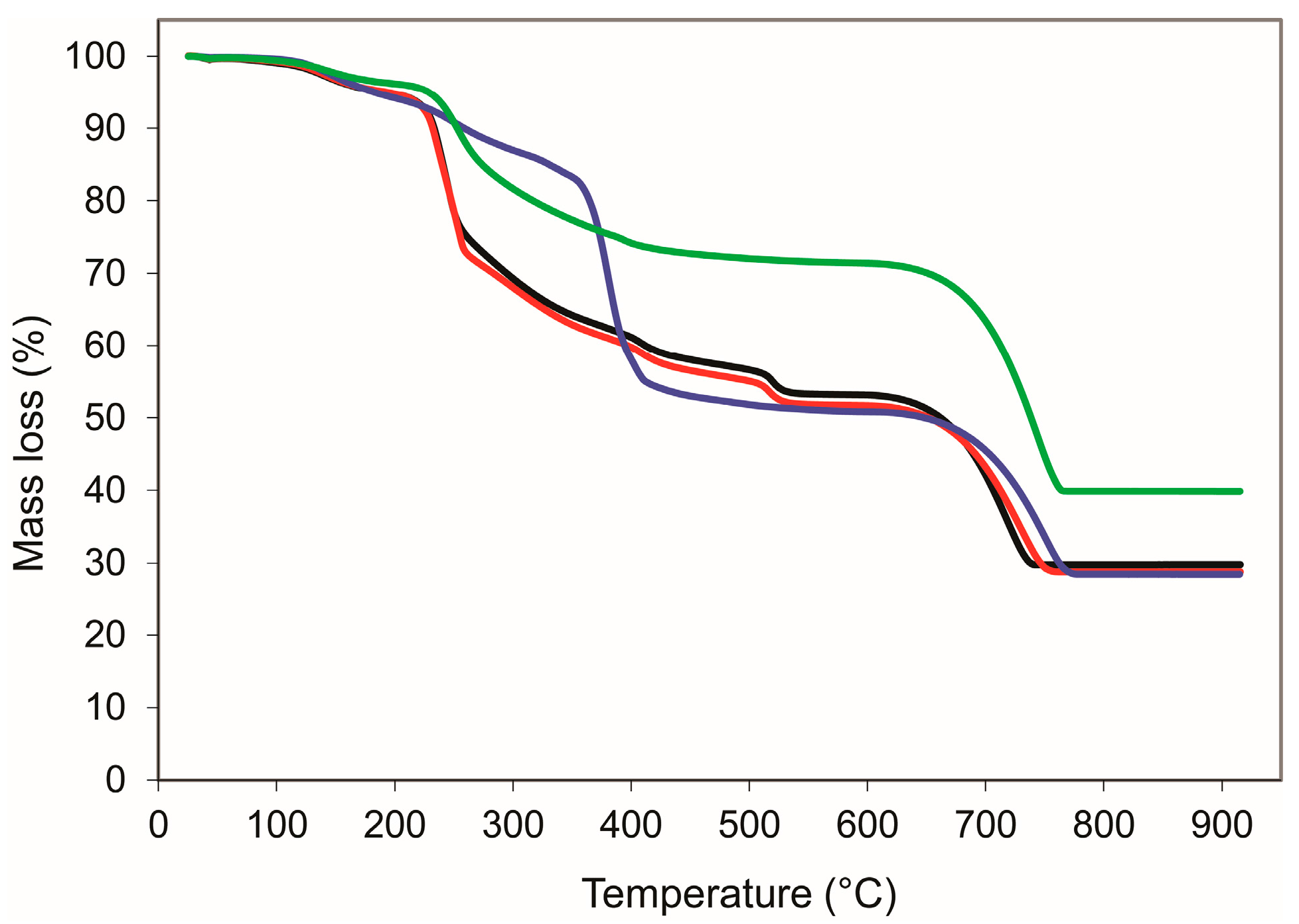
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.; Caldeira, L.; Hosfelt, J.; Kwiatkowski, L.; Maclaren, J.K.; Mason, B.M.; Nebuchina, Y.; Ninokawa, A.; Pongratz, J.; Ricke, K.L.; et al. Reversal of ocean acidification enhances net coral reef calcification. Nature 2016, 531, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Tegethoff, F.W. Calcium Carbonate—From the Cretaceous Period into the 21st Century, 1st ed.; Springer: Basel, Switzerland, 2001. [Google Scholar]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea Urchin Spine Calcite Forms via a Transient Amorphous Calcium Carbonate Phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Burgos Cara, A.; Elert, K.; Putnis, C.V.; Ruiz-Agudo, E. Direct Nanoscale Imaging Reveals the Growth of Calcite Crystals via Amorphous Nanoparticles. Cryst. Growth Des. 2016, 16, 1850–1860. [Google Scholar] [CrossRef]
- Gayathri, S.; Lakshminarayanan, R.; Weaver, J.C.; Morse, D.E.; Kini, R.M.; Valiyaveettil, S. In Vitro Study of Magnesium-Calcite Biomineralization in the Skeletal Materials of the Seastar Pisaster giganteus. Chem. Eur. J. 2007, 13, 3262–3268. [Google Scholar] [CrossRef] [PubMed]
- Wilt, F.H. Developmental biology meets materials science: Morphogenesis of biomineralized structures. Dev. Biol. 2005, 280, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Brečević, L.; Nielsen, A.E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Reddy, M.M.; Nancollas, G.H. The crystallization of calcium carbonate. J. Cryst. Growth 1976, 35, 33–38. [Google Scholar] [CrossRef]
- Nakahara, Y.; Tazawa, T.; Miyata, K. Properties of Calcium Carbonate Prepared by Interfacial Reaction Method. Nippon Kagaku Kaishi 1976, 5, 732–736. [Google Scholar] [CrossRef]
- Sun, S.; Chevrier, D.M.; Zhang, P.; Gebauer, D.; Cölfen, H. Distinct Short-Range Order Is Inherent to Small Amorphous Calcium Carbonate Clusters (<2 nm). Angew. Chem. Int. Ed. 2016, 55, 12206–12209. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.-W.; Sham, T.-K.; et al. Proto-Calcite and Proto-Vaterite in Amorphous Calcium Carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef] [PubMed]
- Farhadi-Khouzani, M.; Chevrier, D.M.; Zhang, P.; Hedin, N.; Gebauer, D. Water as the Key to Proto-Aragonite Amorphous CaCO3. Angew. Chem. Int. Ed. 2016, 55, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Xu, A.-W.; Antonietti, M.; Yu, S.-H.; Cölfen, H. Polymer-Mediated Mineralization and Self-Similar Mesoscale-Organized Calcium Carbonate with Unusual Superstructures. Adv. Mater. 2008, 20, 1333–1338. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.; Hedin, N.; Bacsik, Z. Spherical and Porous Particles of Calcium Carbonate Synthesized with Food Friendly Polymer Additives. Cryst. Growth Des. 2015, 15, 3609–3616. [Google Scholar] [CrossRef]
- Schenk, A.S.; Cantaert, B.; Kim, Y.-Y.; Li, Y.; Read, E.S.; Semsarilar, M.; Armes, S.P.; Meldrum, F.C. Systematic Study of the Effects of Polyamines on Calcium Carbonate Precipitation. Chem. Mater. 2014, 26, 2703–2711. [Google Scholar] [CrossRef]
- Yasumoto, K.; Yasumoto-Hirose, M.; Yasumoto, J.; Murata, R.; Sato, S.; Baba, M.; Mori-Yasumoto, K.; Jimbo, M.; Oshima, Y.; Kusumi, T.; et al. Biogenic Polyamines Capture CO2 and Accelerate Extracellular Bacterial CaCO3 Formation. Mar. Biotechnol. 2014, 16, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Vinoba, M.; Bhagiyalakshmi, M.; Grace, A.N.; Chu, D.H.; Nam, S.C.; Yoon, Y.; Yoon, S.H.; Jeong, S.K. CO2 Absorption and Sequestration as Various Polymorphs of CaCO3 Using Sterically Hindered Amine. Langmuir 2013, 29, 15655–15663. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Song, C.S.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Schwartz, V.; Clark, J.C.; Overbury, S.H.; Zhao, S.; Xu, X.; Song, C. A solid molecular basket sorbent for CO2 capture from gas streams with low CO2 concentration under ambient conditions. Phys. Chem. Chem. Phys. 2012, 14, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Bacsik, Z.; Atluri, R.; Garcia-Bennett, A.E.; Hedin, N. Temperature-Induced Uptake of CO2 and Formation of Carbamates in Mesocaged Silica Modified with n-Propylamines. Langmuir 2010, 26, 10013–10024. [Google Scholar] [CrossRef] [PubMed]
- Bacsik, Z.; Ahlsten, N.; Ziadi, A.; Zhao, G.; Garcia-Bennett, A.E.; Martín-Matute, B.; Hedin, N. Mechanisms and kinetics for sorption of CO2 on bicontinuous mesoporous silica modified with n-propylamine. Langmuir 2011, 27, 11118–11128. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. J. Phys. Chem. C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Mafra, L.; Čendak, T.; Schneider, S.; Wiper, P.V.; Pires, J.; Gomes, J.R.B.; Pinto, M.L. Structure of Chemisorbed CO2 Species in Amine-Functionalized Mesoporous Silicas Studied by Solid-State NMR and Computer Modeling. J. Am. Chem. Soc. 2017, 139, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Ito, Y.; Horiguchi, M.; Fujita, H. Studies on the solvent dependence of the carbamic acid formation from ω-(1-naphthyl)alkylamines and carbon dioxide. Tetrahedron 2005, 61, 213–229. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Carretti, E.; Dei, L.; Baglioni, P.; Weiss, R.G. Synthesis and Characterization of Gels from Polyallylamine and Carbon Dioxide as Gellant. J. Am. Chem. Soc. 2003, 125, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Carretti, E.; Dei, L.; Weiss, R.G.; Baglioni, P. A new class of gels for the conservation of painted surfaces. J. Cult. Herit. 2008, 9, 386–393. [Google Scholar] [CrossRef]
- Carretti, E.; Bonini, M.; Dei, L.; Berrie, B.H.; Angelova, L.V.; Baglioni, P.; Weiss, R.G. New Frontiers in Materials Science for Art Conservation: Responsive Gels and Beyond. Acc. Chem. Res. 2010, 43, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Prah, J.; Maček, J.; Dražič, G. Precipitation of calcium carbonate from a calcium acetate and ammonium carbamate batch system. J. Cryst. Growth 2011, 324, 229–234. [Google Scholar] [CrossRef]
- Bacsik, Z.; Hedin, N. Effects of carbon dioxide captured from ambient air on the infrared spectra of supported amines. Vib. Spectrosc. 2016, 87, 215–221. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brečević, L. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Zou, Z.; Bertinetti, L.; Politi, Y.; Jensen, A.C.S.; Weiner, S.; Addadi, L.; Fratzl, P.; Habraken, W.J.E.M. Opposite Particle Size Effect on Amorphous Calcium Carbonate Crystallization in Water and during Heating in Air. Chem. Mater. 2015, 27, 4237–4246. [Google Scholar] [CrossRef]
- Gorna, K.; Hund, M.; Vučak, M.; Gröhn, F.; Wegner, G. Amorphous calcium carbonate in form of spherical nanosized particles and its application as fillers for polymers. Mater. Sci. Eng. A 2008, 477, 217–225. [Google Scholar] [CrossRef]
- Foran, E.; Weiner, S.; Fine, M. Biogenic Fish-gut Calcium Carbonate is a Stable Amorphous Phase in the Gilt-head Seabream, Sparus aurata. Sci. Rep. 2013, 3, 1700. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacsik, Z.; Zhang, P.; Hedin, N. Ammonium-Carbamate-Rich Organogels for the Preparation of Amorphous Calcium Carbonates. Minerals 2017, 7, 110. https://doi.org/10.3390/min7070110
Bacsik Z, Zhang P, Hedin N. Ammonium-Carbamate-Rich Organogels for the Preparation of Amorphous Calcium Carbonates. Minerals. 2017; 7(7):110. https://doi.org/10.3390/min7070110
Chicago/Turabian StyleBacsik, Zoltán, Peng Zhang, and Niklas Hedin. 2017. "Ammonium-Carbamate-Rich Organogels for the Preparation of Amorphous Calcium Carbonates" Minerals 7, no. 7: 110. https://doi.org/10.3390/min7070110




