Re-Equilibration Processes in Fluid Inclusion Assemblages
Abstract
:1. Introduction
2. Hydrothermal Laboratory
3. Fluid Inclusion Synthesis
4. Re-Equilibration in the D2O Environment
5. Fluid Inclusion Re-Equilibration Experiments
5.1. “Blank” Re-Equilibration Experiments
5.2. Synthesis in H2O and Re-Equilibration in D2O
5.3. Synthesis in H2O-NaCl and Re-Equilibration in H2O
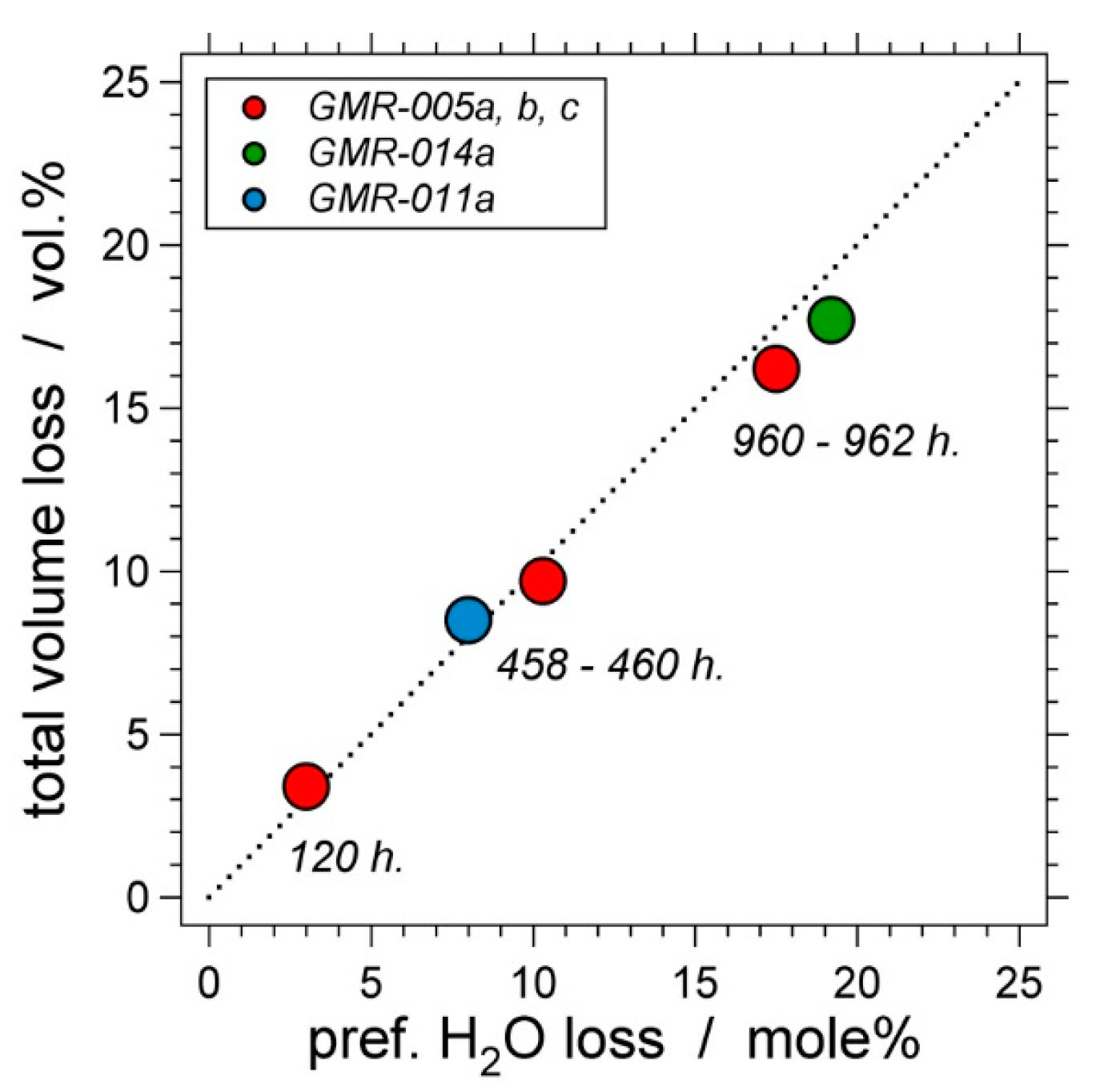
6. Preferential H2O Loss
7. Natural Fluid Inclusions
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Spear, F.S. Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths; Monograph Series; Mineralogical Society of America: Washington, DC, USA, 1995; p. 799. [Google Scholar]
- Faure, G. Principles of Isotope Geology; John Wiley & Sons: New York, NY, USA, 1986; p. 589. [Google Scholar]
- Geyh, M.A. Handbuch. der Physicalischen und Chemischen Altersbestimmung; Wissenschaftliche Buchgesellschaft: Darmstadt, Germany, 2005; p. 211. [Google Scholar]
- Roedder, E. Fluid Inclusions, Reviews in Mineralogy; Mineralogical Society of America: Chelsea, MA, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Bakker, R.J.; Jansen, J.B.H. Preferential water leakage from fluid inclusions by means of mobile dislocations. Nature 1990, 345, 58–60. [Google Scholar] [CrossRef]
- Sterner, S.M.; Bodnar, R.J. Synthetic fluid inclusions-VII. Re-equilibration of fluid inclusions in quartz during laboratory-simulated metamorphic burial and uplift. J. Metam. Geol. 1989, 7, 243–260. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford Science Publications: Oxford, UK, 1975; p. 414. [Google Scholar]
- Bakker, R.J. Re-equilibration of fluid inclusions: Bulk diffusion. Lithos 2009, 112, 277–288. [Google Scholar] [CrossRef]
- Schmidt, C.; Bruhn, D.; Wirth, R. Experimental evidence of transformation plasticity in silicates: Minimum of creep strength in quartz. Earth Plant. Sci. Lett. 2003, 205, 273–280. [Google Scholar] [CrossRef]
- Doppler, G.; Bakker, R.J.; Baumgartner, M. Fluid inclusion modification by H2O and D2O diffusion: The influence of inclusion depth, size, and shape in re-equilibration experiments. Contrib. Mineral. Petrol. 2013, 165, 1259–1274. [Google Scholar] [CrossRef]
- Doppler, G.; Bakker, R.J. The influence of the α-β phase transition of quartz on fluid inclusions during re-equilibration experiments. Lithos 2014, 198, 14–23. [Google Scholar] [CrossRef]
- Bakker, R.J.; Doppler, G. Salinity and density modifications of synthetic H2O and H2O-NaCl fluid inclusions in re-equilibration experiments at constant temperature and confining pressure. Chem. Geol. 2016, 424, 43–85. [Google Scholar] [CrossRef]
- Kerrick, D.M. Cold-seal systems. In Hydrothermal Experimental Techniques; Ulmer, G.C., Barnes, H.L., Eds.; Wiley: New York, NY, USA, 1987; pp. 293–323. [Google Scholar]
- Shelton, K.L.; Orville, P.M. Formation of synthetic fluid inclusions in natural quartz. Am. Mineral. 1980, 65, 1233–1236. [Google Scholar]
- Smith, D.L.; Evans, B. Diffusional crack healing in quartz. J. Geoph. Res. 1984, 89, 4125–4135. [Google Scholar] [CrossRef]
- Brantley, S.L.; Evans, B.; Hickman, S.H.; Crerar, D.A. Healing of microcracks in quartz: Implications for fluid flow. Geology 1990, 18, 136–139. [Google Scholar] [CrossRef]
- Haar, L.; Gallagher, J.S.; Kell, G.S. NBS/NRC Steam Tables; Hemisphere Publishing Corporation: Washington, DC, USA, 1984. [Google Scholar]
- Driesner, T. The system H2O-NaCl. Part II: Correlation for molar volume, enthalpy, and isobaric heat capacity from 0 to 1000 °C, 1 to 5000 bar, and 0 to 1 XNaCl. Geochim. Cosmochim. Acta 2007, 71, 4902–4919. [Google Scholar] [CrossRef]
- Anderko, A.; Pitzer, K.S. Phase equilibria and volumetric properties of the system KCl-H2O and NaCl-KCl-H2O above 573 K: Equation of state representation. Geochim. Cosmochim. Acta 1993, 57, 4885–4897. [Google Scholar] [CrossRef]
- Hosieni, K.R.; Howald, R.A.; Scanlon, M.W. Thermodynamics of the lambda transition and the equation of state of quartz. Am. Mineral. 1985, 70, 782–793. [Google Scholar]
- Hill, P.G.; MacMillan, C.R.D.; Lee, V. A fundamental equation of state for heavy water. J. Phys. Chem. Ref. Data 1982, 11, 1–14. [Google Scholar] [CrossRef]
- Baumgartner, M.; Doppler, G.; Bakker, R.J. Preliminary results of experimental re-equilibration studies of natural H2O–CO2–NaCl bearing fluid inclusions in quartz. ECROFI-XXI. In Berichte der Geologischen Bundesanstalt; Geological Survey of Austria: Vienna, Austria, 2011; Volume 87, pp. 44–45. [Google Scholar]
- Rull, F. Structural investigation of water and aqueous solutions by Raman spectroscopy. Pure Appl. Chem. 2002, 74, 1859–1870. [Google Scholar] [CrossRef]
- Schrotter, H.W.; Klockner, H.W. Raman scattering cross-sections in gases and liquids. In Raman Spectroscopy of Gases and Liquids; Weber, A., Ed.; Springer-Verlag: Berlin, Germany, 1979; pp. 123–166. [Google Scholar]
- Dubessy, J.; Poty, B.; Ramboz, C. Advances in C–O–H–N–S fluid geochemistry based on micro-Raman spectrometric analysis of fluid inclusions. Eur. J. Mineral. 1989, 1, 517–534. [Google Scholar] [CrossRef]
- Bakker, R.J.; Jansen, J.B.H. A mechanism for preferential H2O leakage from fluid inclusions in quartz, based on TEM observations. Contrib. Mineral. Petrol. 1994, 116, 7–20. [Google Scholar] [CrossRef]
- Audétat, A.; Günther, D. Mobility and H2O loss from fluid inclusions in natural quartz crystals. Contrib. Mineral. Petrol. 1999, 137, 1–14. [Google Scholar]
- Diamond, L.W.; Tarantola, A.; Stünitz, H. Modification of fluid inclusions in quartz by deviatoric stress II: Experimentally induced changes in inclusion volume and composition. Contrib. Mineral. Petrol. 2010, 160, 845–864. [Google Scholar] [CrossRef]
- Baumgartner, M.; Bakker, R.J.; Doppler, G. Re-equilibration of natural H2O-CO2-salt-rich fluid inclusions in quartz—Part 1: Experiments in pure water at constant pressures and differential pressures at 600 °C. Contrib. Mineral. Petrol. 2014, 168, 1017. [Google Scholar] [CrossRef]

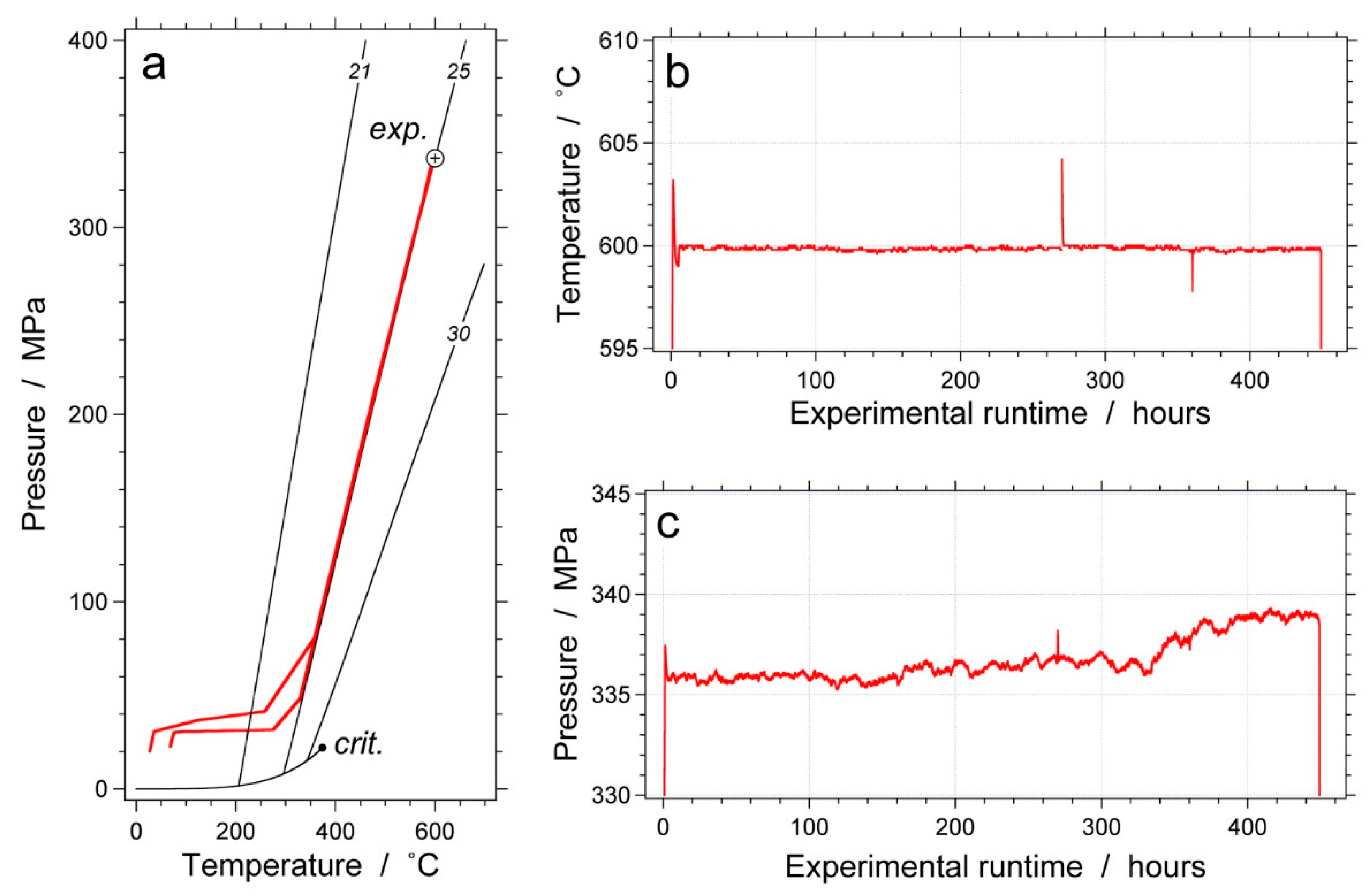
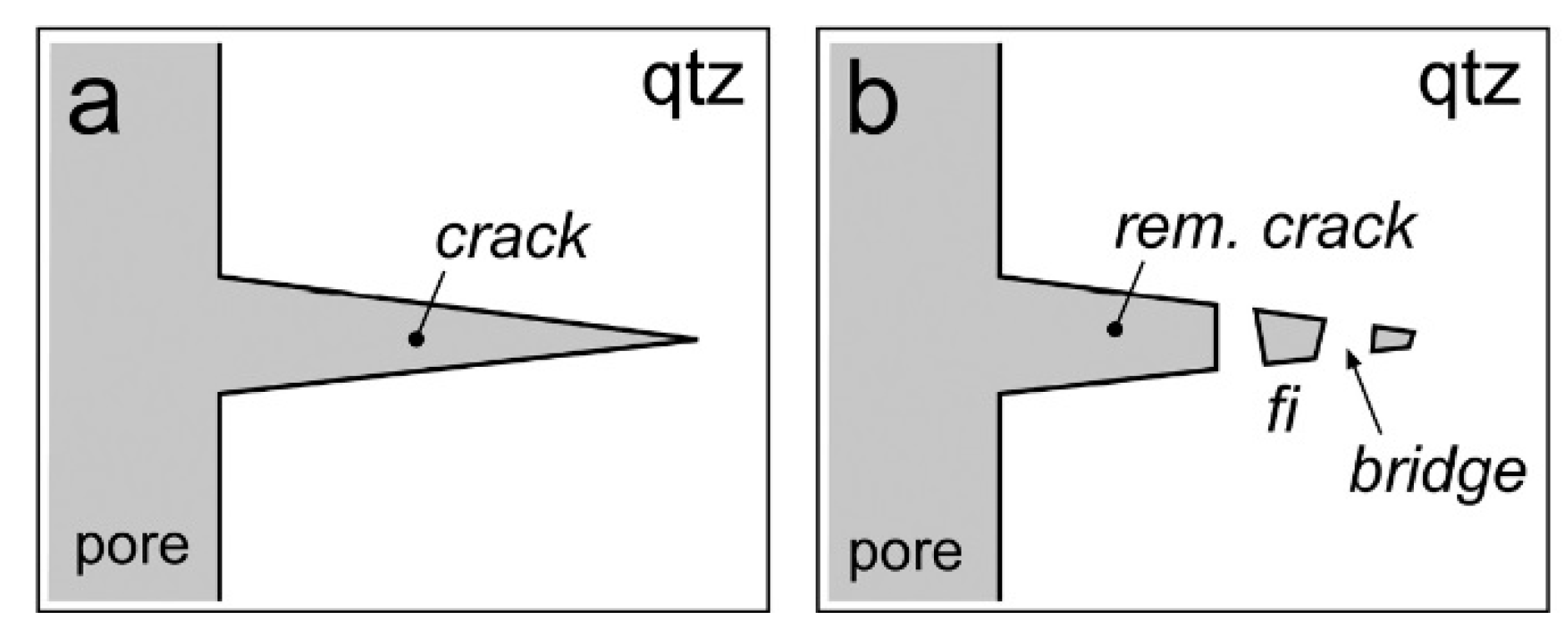


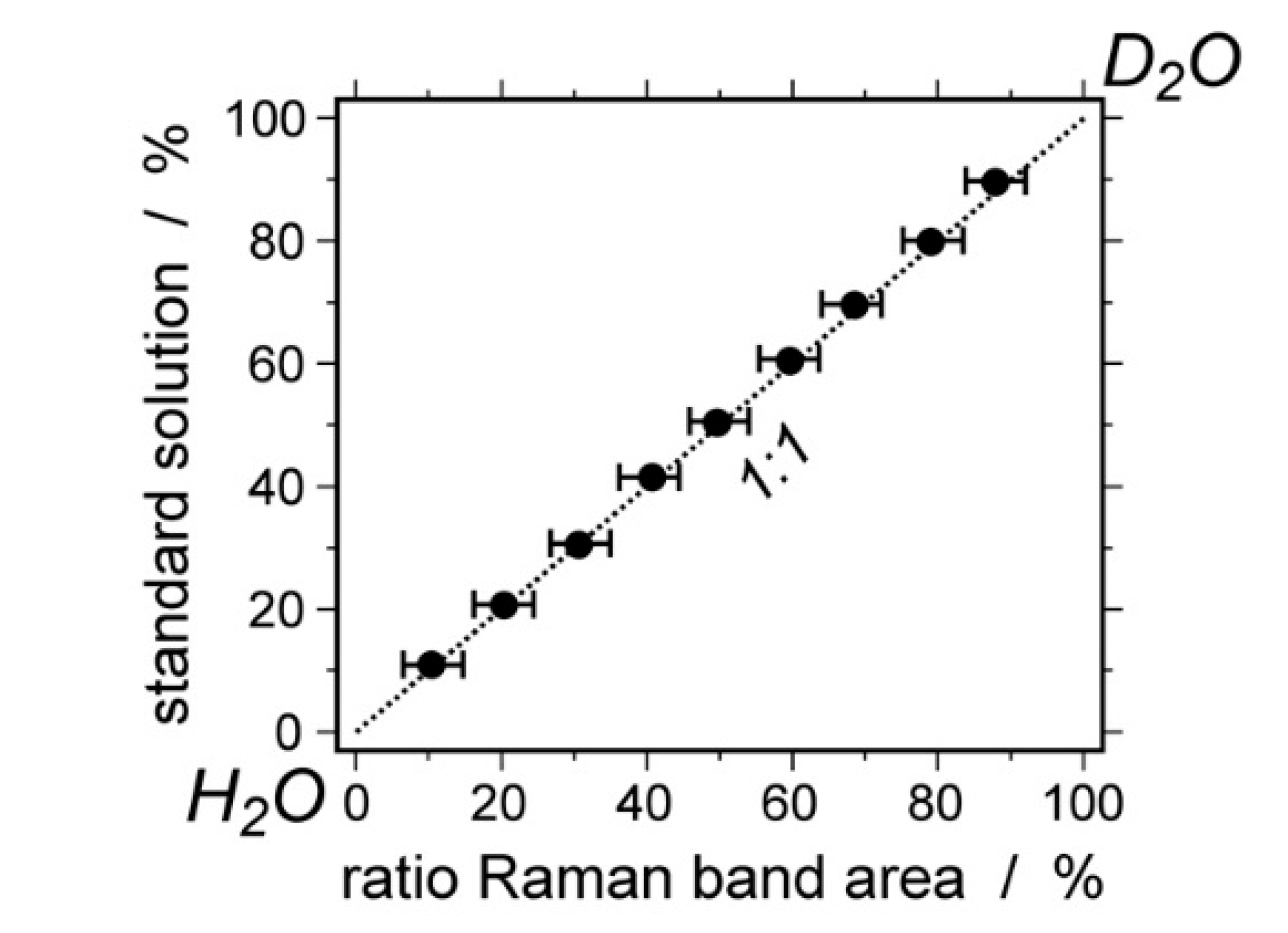
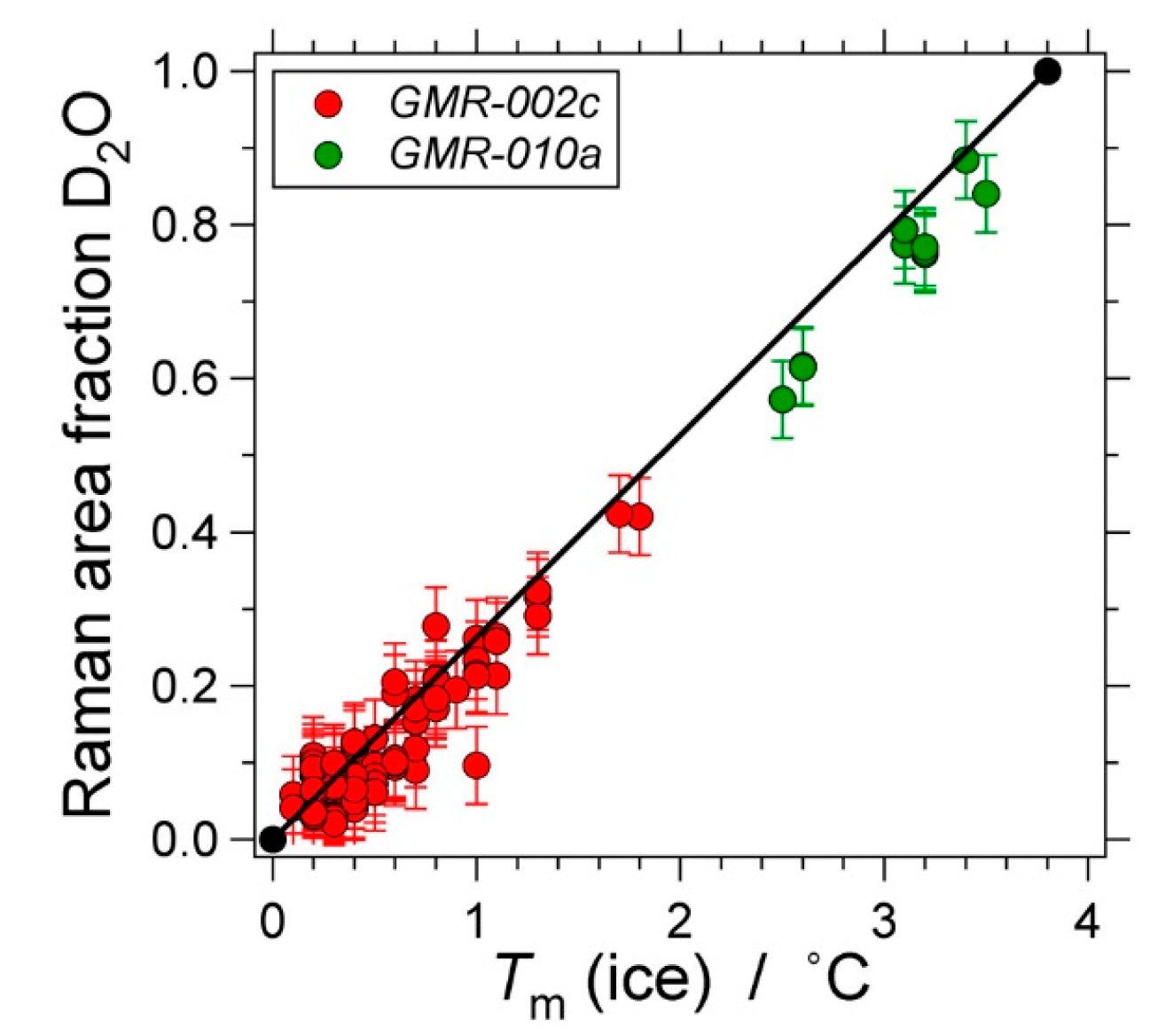
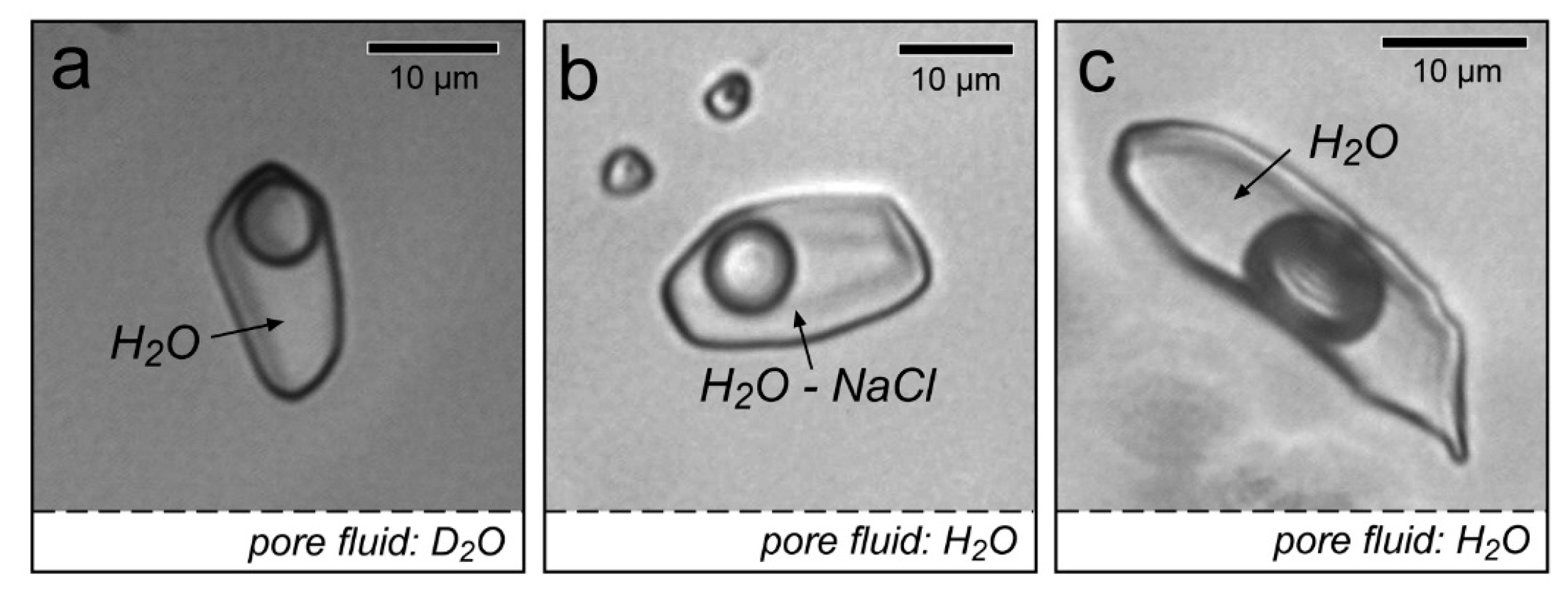
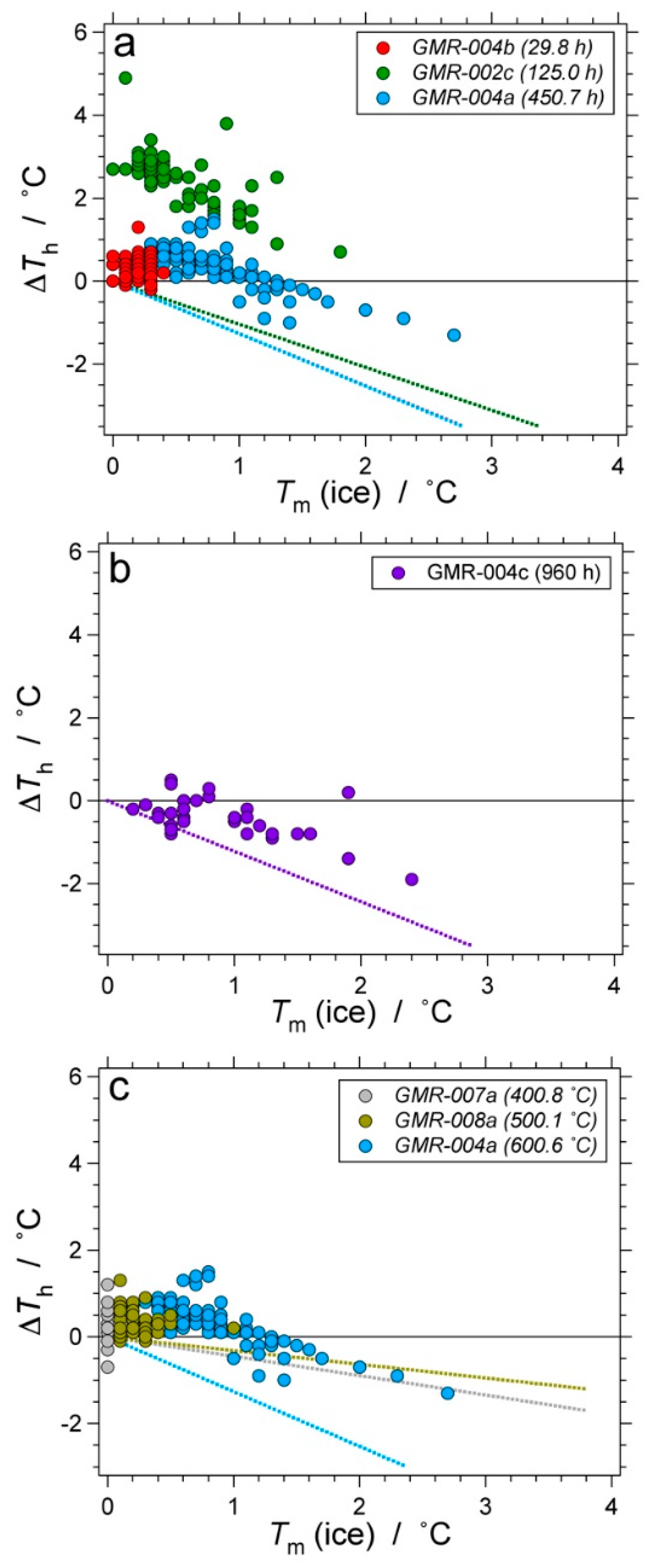
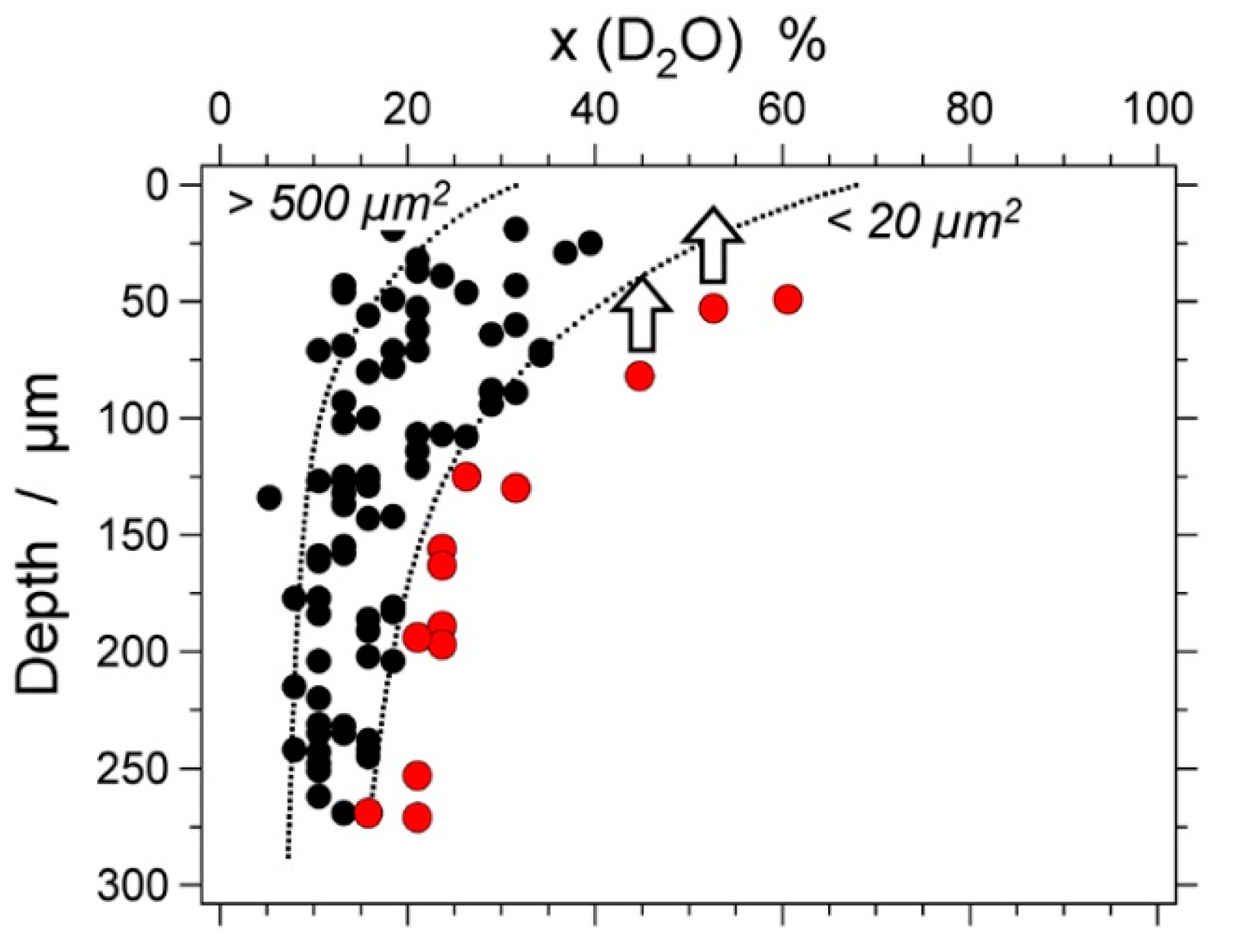
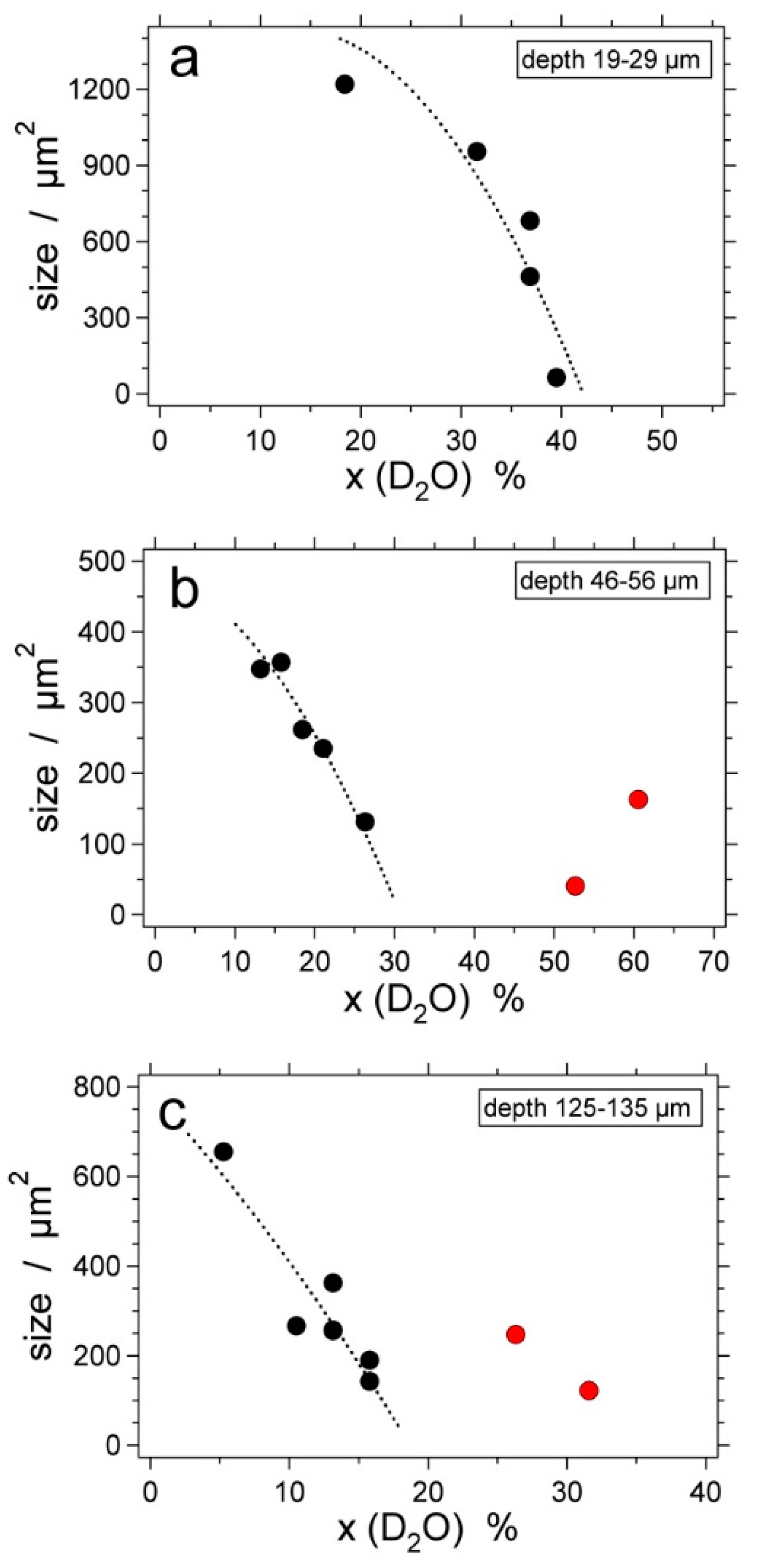
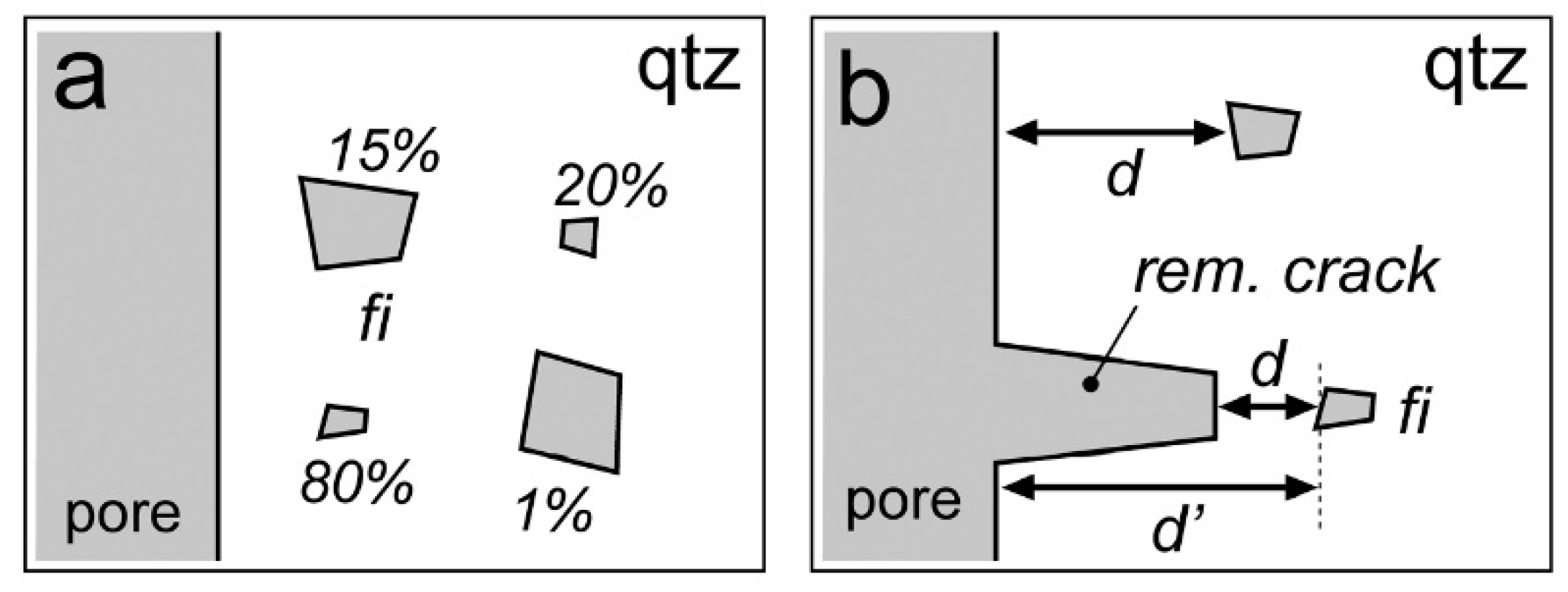
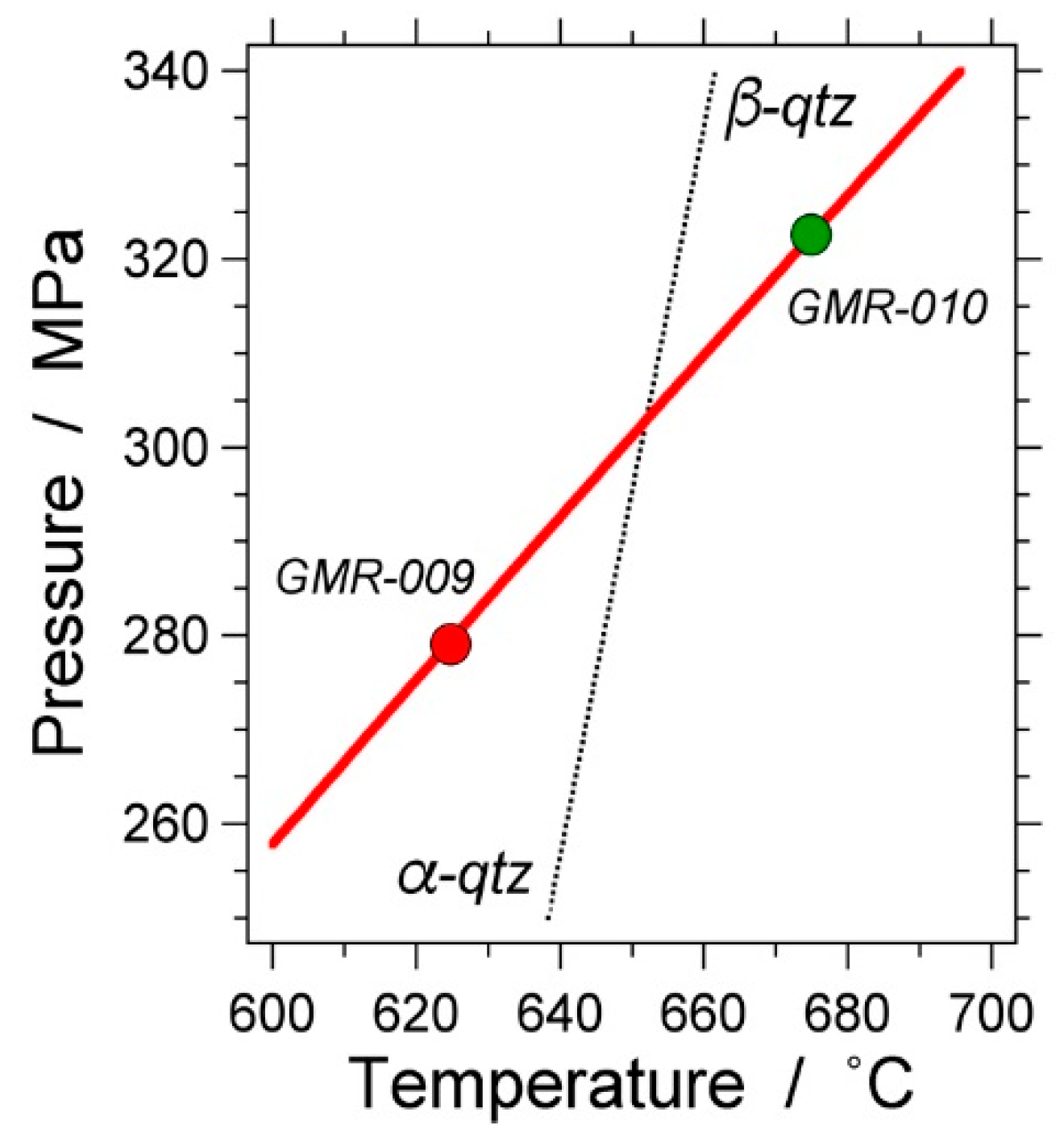

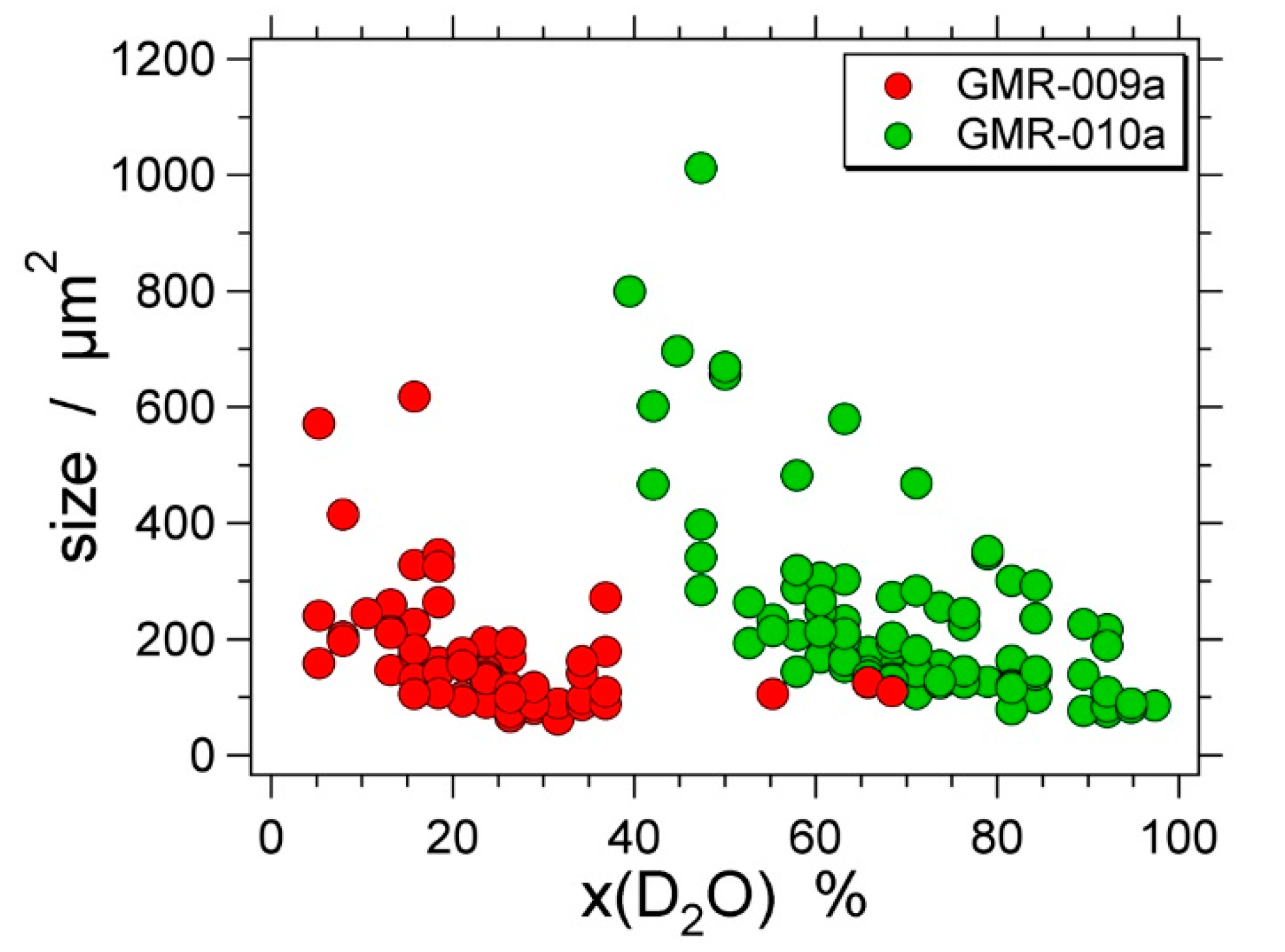
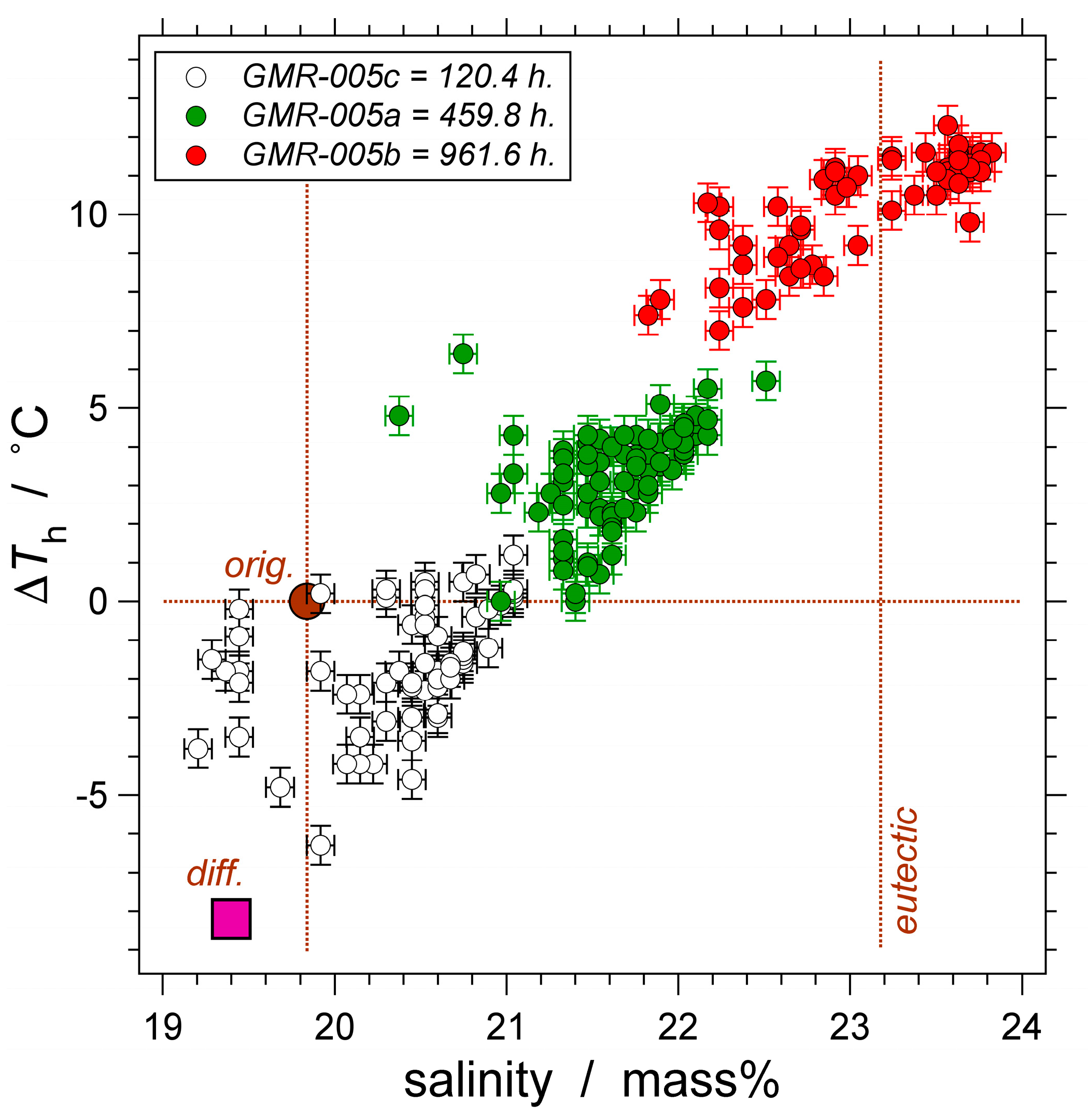
| Experiment | Loaded Fluid | Pressure (MPa) | Temperature (°C) | Duration (Hours) | |||
|---|---|---|---|---|---|---|---|
| H2O 1 | NaCl 1 | Average 2 | Range 3 | Average 2 | Range 3 | ||
| GMR-002 | 100 | - | 338.5 (1.3) | 335.7–341.3 | 600.2 (0.10) | 600.0–600.4 | 460.4 |
| GMR-004 | 100 | - | 336.3 (0.46) | 334.4–337.6 | 600.6 (0.41) | 598.4–603.3 | 457.4 |
| GMR-005 | 80.2 | 19.8 | 336.9 (0.55) | 335.3–338.5 | 600.7 (0.31) | 598.8–601.1 | 458.6 |
| GMR-006 | 100 | - | 337.6 (0.77) | 335.9–338.9 | 300.1 (0.52) | 299.7–300.6 | 457.3 |
| GMR-007 | 100 | - | 337.7 (0.45) | 336.7–338.6 | 400.8 (0.60) | 400.1–401.2 | 457.2 |
| GMR-008 | 100 | - | 334.9 (0.79) | 333.0–337.0 | 500.1 (0.34) | 499.7–500.3 | 459.2 |
| GMR-009 | 100 | - | 279.1 (0.48) | 278.3–280.0 | 624.8 (0.33) | 623.9–625.2 | 458.1 |
| GMR-010 | 100 | - | 322.6 (0.30) | 321.7–323.3 | 674.9 (0.31) | 674.1–675.3 | 459.2 |
| GMR-011 | 90 | 10 | 337.4 (0.19) | 336.6–338.0 | 599.7 (0.13) | 598.2–600.3 | 462.4 |
| GMR-013 | 100 | - | 280.9 (0.17) | 280.4–281.3 | 675.0 (0.22) | 674.1–675.2 | 456.2 |
| GMR-014 | 83.7 | 16.3 | 337.4 (0.54) | 332.5–338.2 | 600.4 (0.35) | 597.2–601.1 | 454.3 |
| Experiment | Experimental Vm (cm3/mol) 12 | Corrected Values 3 | Th Measured (°C) | ||||
|---|---|---|---|---|---|---|---|
| Th (°C) 2 | Vm (cm3/mol) 2 | Disk 4 | Mean | Std. Dev. 5 | Range | ||
| GMR-002 | 24.97 (0.03) | 291.9 (0.4) | 24.73 (0.03) | c | 293.2 | 1.1 | 291.4–297.8 |
| GMR-004 | 25.03 (0.02) | 292.8 (0.3) | 24.78 (0.02) | a | 293.1 | 0.8 | 288.5–295.6 |
| b | 292.7 | 0.9 | 290.5–295.8 | ||||
| c | 293.8 | 1.1 | 290.4–296.8 | ||||
| g | 292.8 | 1.4 | 286.5–295.2 | ||||
| GMR-005 | 23.73 (0.01) | 324.1 (0.3) | 23.54 (0.01) | a | 329.7 | 1.5 | 324.4–332.8 |
| b | 327.0 | 3.0 | 316.6–330.8 | ||||
| c | 328.3 | 2.5 | 321.5–332.1 | ||||
| GMR-006 | 19.06 (0.01) | 118.9 (0.7) | 19.08 (0.01) | a | 130.9 | 2.9 | 126.0–136.8 |
| GMR-007 | 20.66 (0.02) | 193.7 (0.7) | 20.66 (0.02) | a | 199.8 | 2.8 | 191.4–205.0 |
| GMR-008 | 22.67 (0.02) | 251.3 (0.5) | 22.60 (0.02) | a | 254.3 | 2.9 | 236.2–259.4 |
| GMR-009 | 27.50 (0.03) | 320.6 (0.3) | 27.05 (0.03) | a | 323.7 | 0.8 | 321.3–326.0 |
| b | 323.7 | 0.8 | 321.9–325.5 | ||||
| GMR-010 | 27.48 (0.02) | 317.5 (0.2) | 26.75 (0.02) | a | 322.9 | 0.9 | 320.9–326.8 |
| b | 322.0 | 0.8 | 319.4–324.6 | ||||
| GMR-011 | 24.17 (0.01) | 309.9 (0.2) | 23.96 (0.01) | a | 313.9 | 0.8 | 311.8–318.8 |
| GMR-013 | 29.15 (0.02) | 332.2 (0.2) | 28.38 (0.02) | a | 337.3 | 0.7 | 335.3–338.9 |
| GMR-014 | 23.86 (0.02) | 319.2 (0.5) | 23.66 (0.02) | a | 328.5 | 1.3 | 324.8–331.9 |
| Synthesis | Re-Equilibration Experiments | ||||||
|---|---|---|---|---|---|---|---|
| Loaded Fluid | Pressure (MPa) | Temperature (°C) | Duration (h) | ||||
| Experiment | Disk | Average 1 | Range 2 | Average 1 | Range 2 | ||
| GMR-002 | c | D2O | 336.84 (0.20) | 336.22–337.49 | 599.87 (0.07) | 599.5–600.1 | 120.8 |
| GMR-004 | a | D2O | 336.74 (1.10) | 335.27–339.32 | 599.86 (0.23) | 597.8–610.3 | 450.4 |
| b | D2O | 337.30 (0.49) | 333.47–338.39 | 600.53 (0.32) | 599.2–600.9 | 29.8 | |
| c | D2O | 336.60 (0.83) | 334.35–337.80 | 600.28 (0.26) | 599.7–609.1 | 960.0 | |
| g | H2O | 338.25 (0.32) | 336.03–340.04 | 600.03 (0.32) | 598.7–611.0 | 458.6 | |
| GMR-005 | a | H2O | 336.1 (0.84) | 334.8–338.0 | 600.70 (0.08) | 598.9–600.8 | 458.9 |
| b | H2O | 337.5 (1.57) | 335.1–340.3 | 600.30 (0.30) | 599.8–613.9 | 961.6 | |
| c | H2O | 332.6 (0.30) | 331.5–333.2 | 600.70 (0.50) | 597.5–610.9 | 120.4 | |
| GMR-006 | a | D2O | 336.74 (0.37) | 334.08–343.61 | 300.11 (0.56) | 298.9–319.8 | 458.4 |
| GMR-007 | a | D2O | 337.17 (0.30) | 335.90–340.99 | 400.58 (0.36) | 399.8–416.4 | 457.2 |
| GMR-008 | a | D2O | 337.27 (0.17) | 336.60–340.23 | 499.67 (0.44) | 498.7–512.4 | 459.5 |
| GMR-009 | a | D2O | 278.42 (1.13) | 276.36–281.35 | 624.73 (0.23) | 623.1–636.3 | 457.8 |
| b | H2O | 281.17 (0.93) | 277.10–283.07 | 624.46 (1.72) | 617.0–636.0 | 458.3 | |
| GMR-010 | a | D2O | 321.15 (0.51) | 320.00–322.28 | 674.63 (0.34) | 673.9–675.2 | 458.3 |
| b | H2O | 319.66 (0.39) | 318.94–320.87 | 675.22 (0.39) | 670.5–683.3 | 453.7 | |
| GMR-011 | a | H2O | 337.89 (0.38) | 336.47–339.42 | 600.08 (0.21) | 597.2–608.2 | 458.1 |
| GMR-013 | a | D2O | 279.38 (0.31) | 278.55–280.36 | 674.60 (0.22) | 672.9–680.6 | 457.1 |
| GMR-014 | a | H2O | 338.69 (0.14) | 337.79–341.04 | 599.57 (0.31) | 598.9–609.9 | 959.7 |
| Experiment | Disk | Pressure (MPa) 1 | H2O Fugacity (MPa) 1 | Pressure Gradient (MPa) 2 | ||
|---|---|---|---|---|---|---|
| Average | Min. | Max. | ||||
| GMR-002 | c | 334.34 (329.68–337.46) | 174.18 (171.38–176.10) | −2.50 | −7.36 | +0.82 |
| GMR-004 | a | 334.62 (330.61–337.03) | 174.34 (171.82–175.98) | −2.12 | −7.23 | +1.39 |
| b | 336.35 (331.98–339.14) | 175.75 (172.94–177.62) | −0.95 | −5.81 | +2.33 | |
| c | 331.52 (328.27–336.39) | 172.71 (170.64–175.78) | −5.80 | −9.16 | +0.62 | |
| g | 335.60 (329.87–339.76) | 175.03 (171.42–177.72) | −2.65 | −8.70 | +1.83 | |
| GMR-005 | a | 320.29 (312.77–333.44) | 148.65 (144.86–155.46) * | −15.81 | −23.33 | −2.66 |
| b | 326.94 (317.60–356.74) | 151.86 (147.09–167.89) * | −10.56 | −19.90 | +19.24 | |
| b | 323.72 (314.46–343.69) | 150.40 (145.70–160.93) * | −8.88 | −18.19 | +11.09 | |
| GMR-006 | a | 310.83 (303.38–318.27) | 25.90 (24.97–26.85) | −25.91 | −33.37 | −18.10 |
| GMR-007 | a | 322.53 (315.25–329.85) | 65.11 (63.21–67.07) | −14.64 | −22.22 | −7.02 |
| GMR-008 | a | 326.49 (319.73–333.28) | 115.93 (113.40–118.51) | −10.78 | −17.71 | −3.82 |
| GMR-009 | a | 270.18 (267.78–272.58) | 148.97 (145.54–150.40) | −8.24) | −11.77 | −4.71 |
| b | 269.99 (266.53–273.46) | 148.75 (146.13–151.41) | −11.18 | −15.57 | −6.78 | |
| GMR-010 | a | 307.00 (304.16–309.85) | 193.47 (191.37–195.59) | −14.15 | −17.50 | −10.79 |
| b | 310.05 (307.45–312.65) | 195.85 (193.87–197.84) | −9.61 | −12.60 | −6.62 | |
| GMR-011 | a | 326.02 (313.97–331.23) | 158.09 (151.64–160.94) * | −11.87 | −23.92 | −6.63 |
| GMR-013 | a | 265.55 (263.32–267.80) | 166.16 (164.67–167.69) | −13.83 | −16.37 | −11.27 |
| GMR-014 | a | 312.00 (303.95–320.89) | 146.34 (142.31–150.90) * | −26.69 | −34.74 | −17.80 |
| Experiment | Disk | Vm (cm3/mol) | Corrected Values | |
|---|---|---|---|---|
| Th (°C) | Vm (cm3/mol) | |||
| GMR-002 | c | 24.91 | 288.0 | 24.67 |
| GMR-004 | a | 24.92 | 288.0 | 24.67 |
| b | 24.92 | 288.0 | 24.67 | |
| b | 24.93 | 288.2 | 24.68 | |
| GMR-006 | a | 19.09 | 118.3 | 19.12 |
| GMR-007 | a | 20.77 | 195.4 | 20.76 |
| GMR-008 | a | 22.68 | 250.1 | 22.66 |
| GMR-009 | a | 27.45 | 316.9 | 27.00 |
| GMR-010 | a | 27.26 | 312.0 | 26.53 |
| GMR-013 | a | 29.04 | 328.1 | 28.26 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, R.J. Re-Equilibration Processes in Fluid Inclusion Assemblages. Minerals 2017, 7, 117. https://doi.org/10.3390/min7070117
Bakker RJ. Re-Equilibration Processes in Fluid Inclusion Assemblages. Minerals. 2017; 7(7):117. https://doi.org/10.3390/min7070117
Chicago/Turabian StyleBakker, Ronald J. 2017. "Re-Equilibration Processes in Fluid Inclusion Assemblages" Minerals 7, no. 7: 117. https://doi.org/10.3390/min7070117




