Concentration at the Minimum Bubble Velocity (CMV) for Various Types of Flotation Frothers
Abstract
:1. Introduction
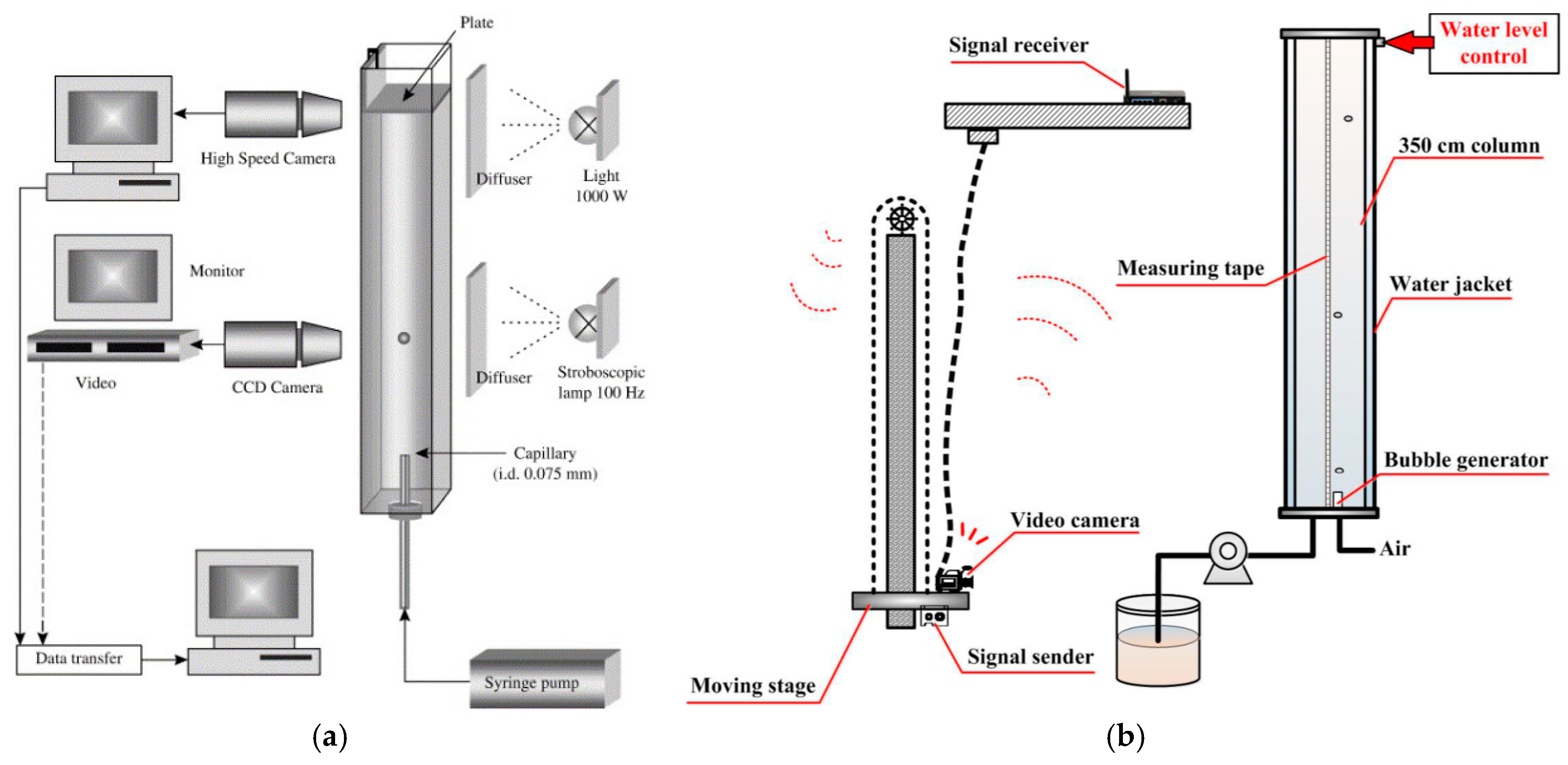
2. Columns Used to Measure Bubble Velocity
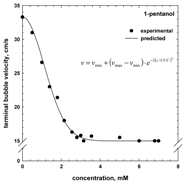
3. Terminal Bubble Velocity
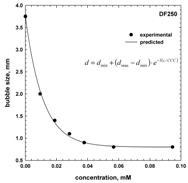
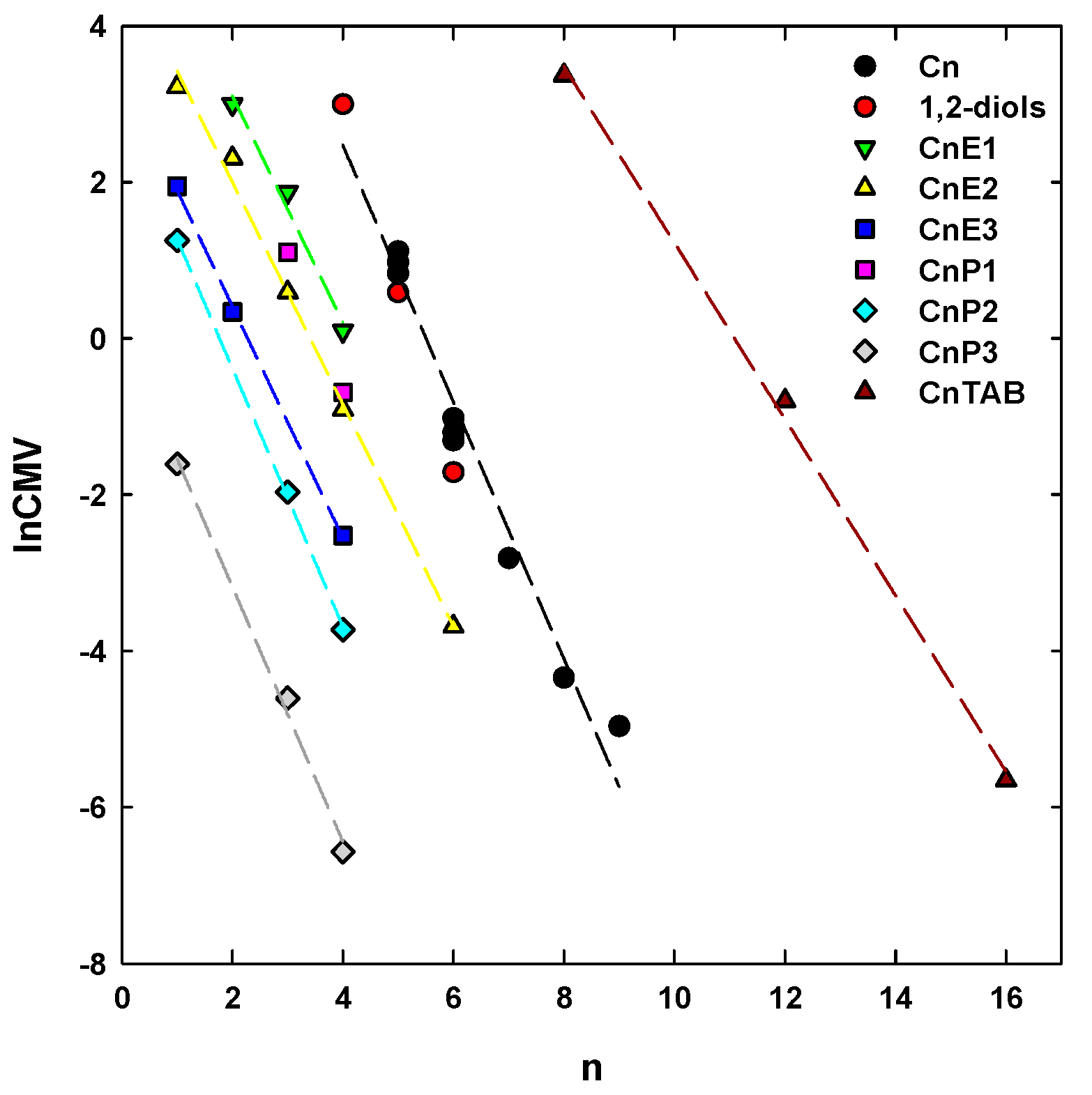
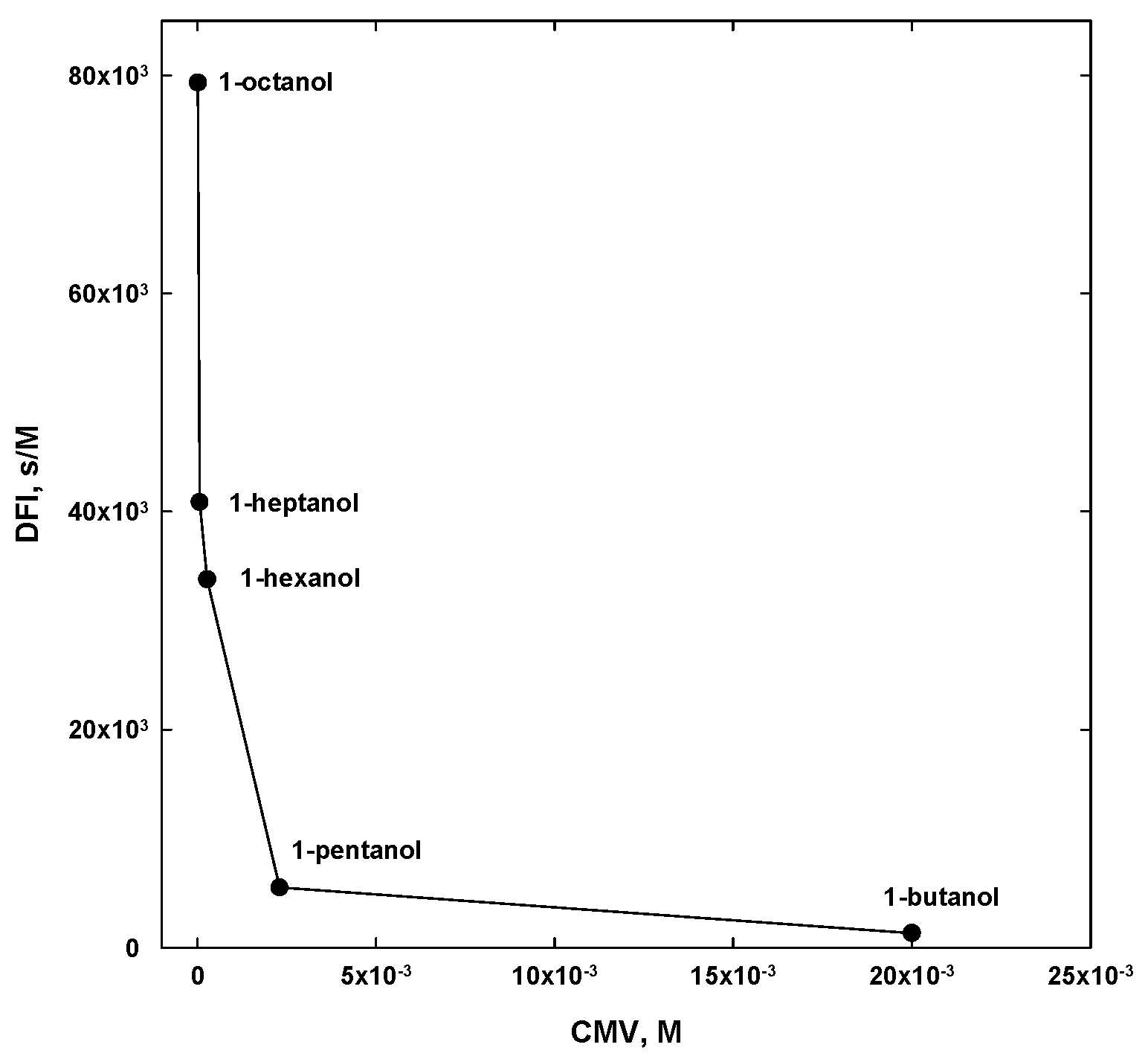
4. Concentration at the Minimum Bubble Velocity (CMV)
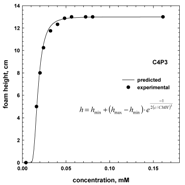
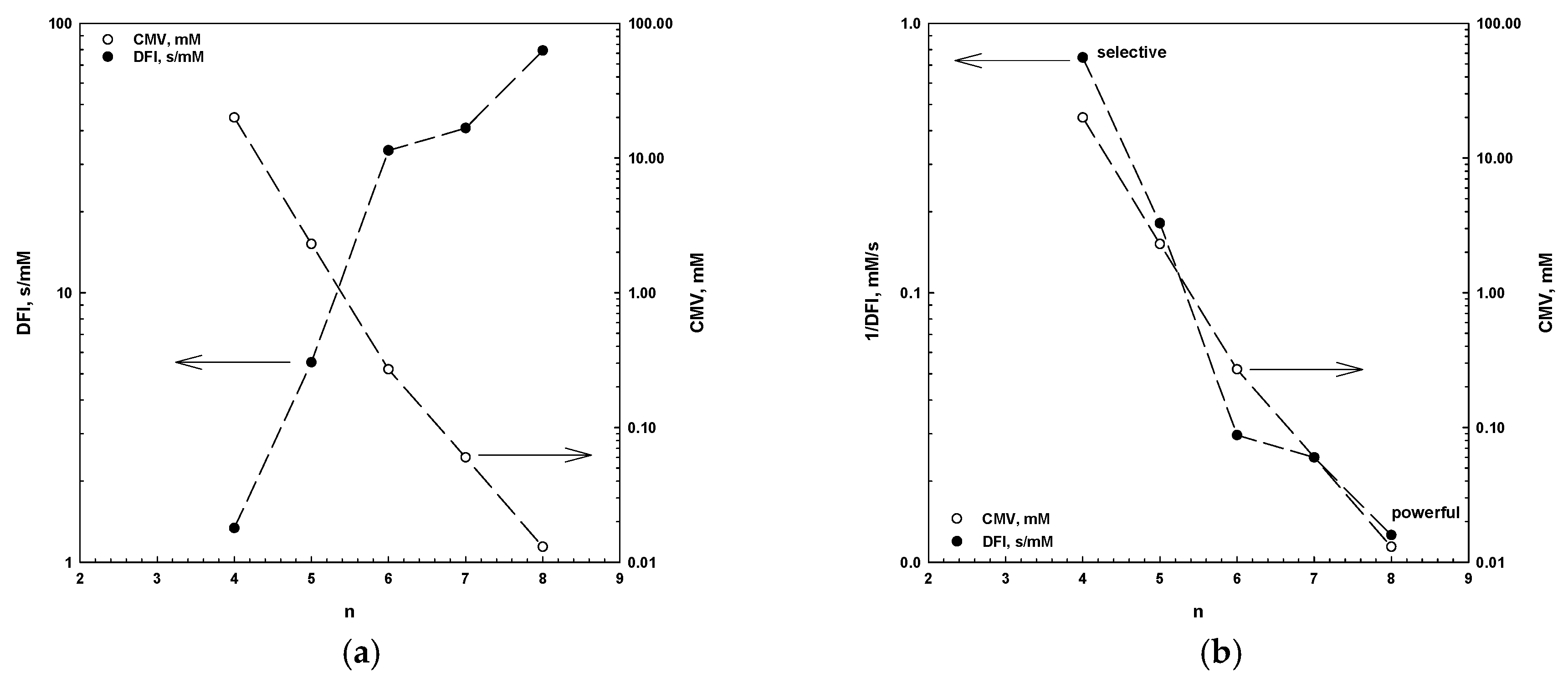
5. CMV in Relation to Other Frother Characterization Parameters
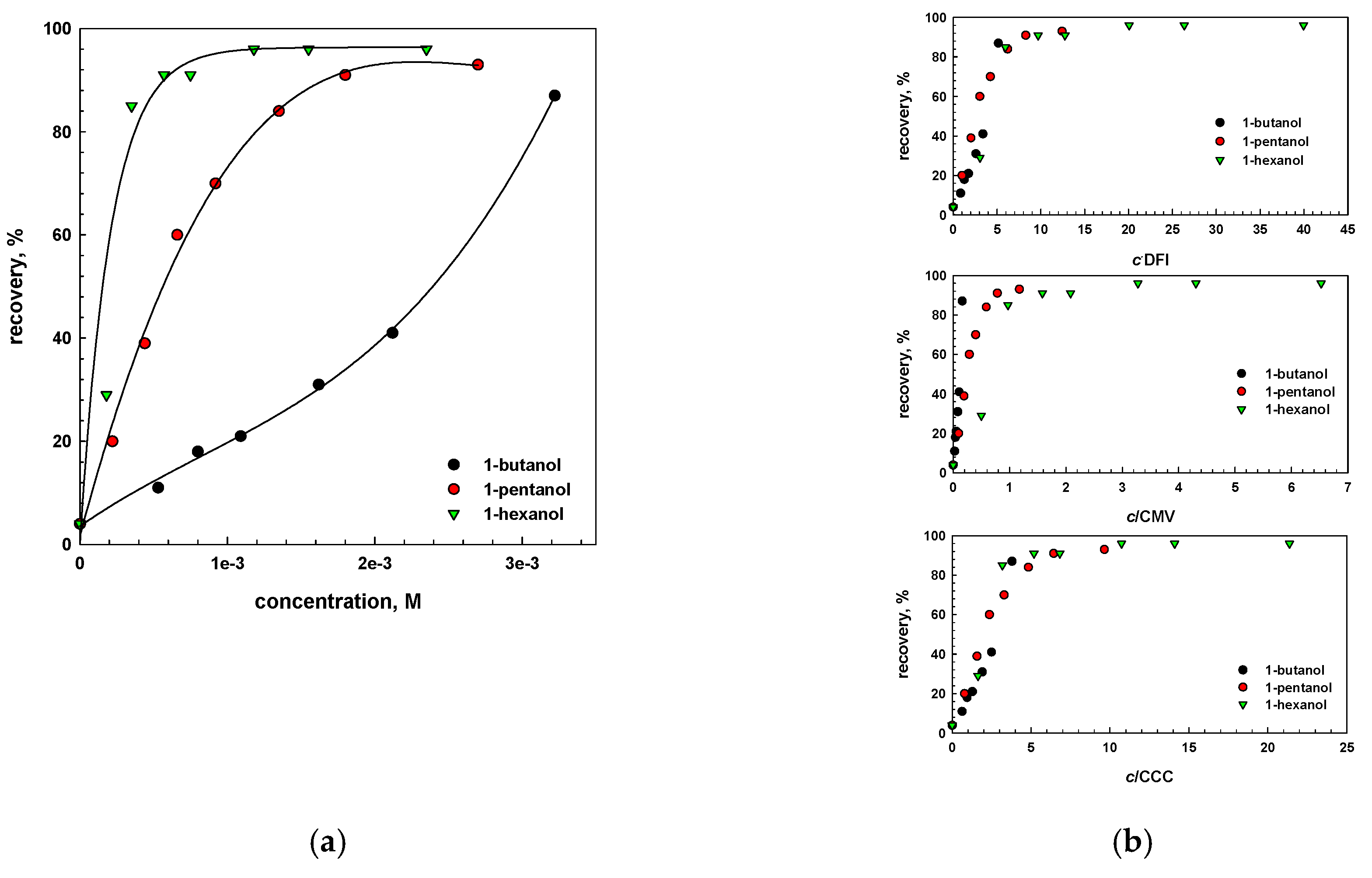
6. Influence of Frother Characterization Parameters on Flotation
7. Conclusions
- (a)
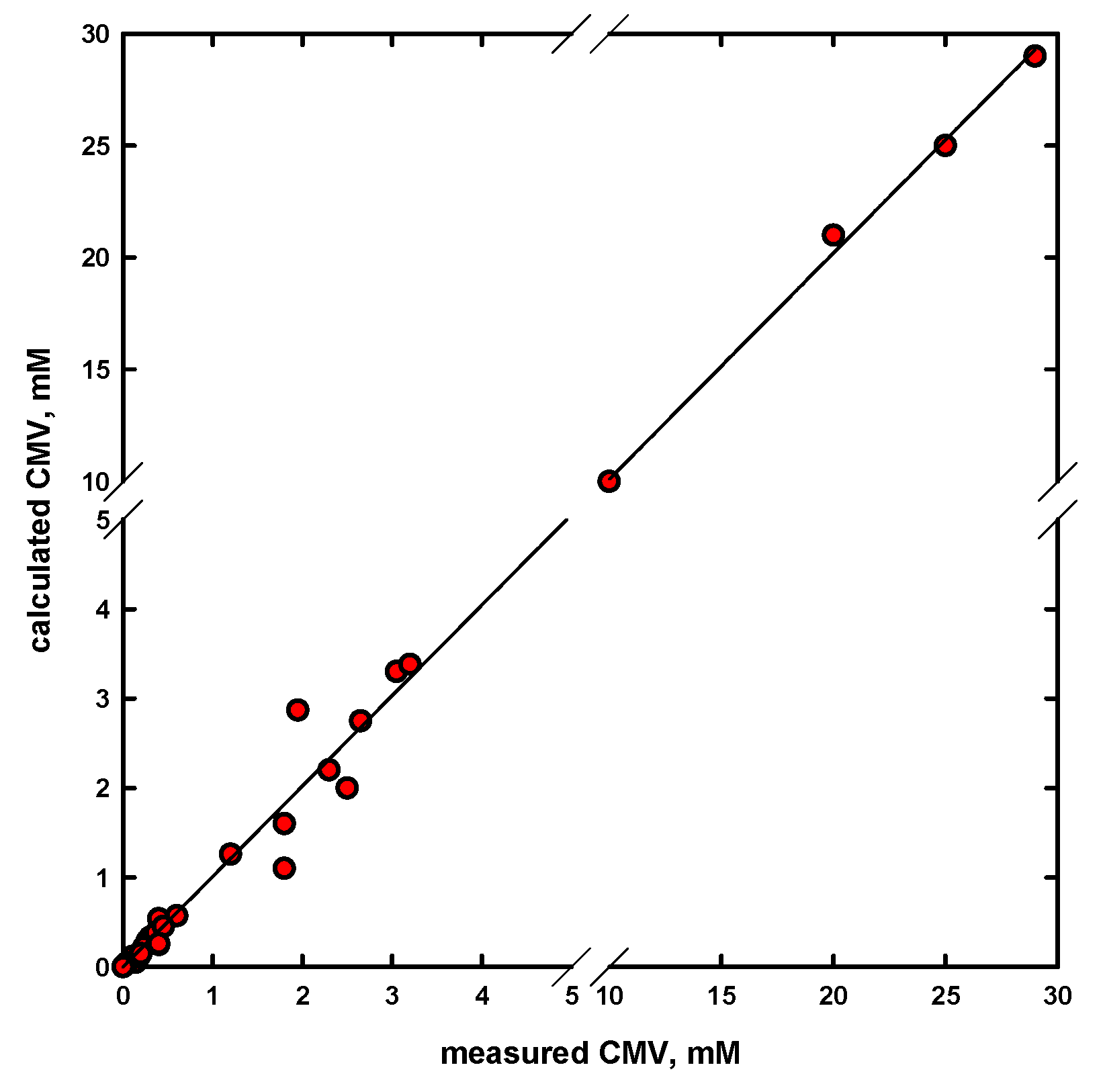
- The newly obtained empirical equation allows one to rapidly and accurately determine a concentration at the minimum bubble velocity (CMV), as a characterization parameter for different types of frothers, such as straight and branched alkyl chain aliphatic alcohols, 1,ω-diols, poly(propylene glycol) and poly(ethylene glycol) alkyl ethers, n-alkyltrimethylammonium bromides, commercial frothers and others. An excellent agreement was observed between the determined and experimental values.
- (b)
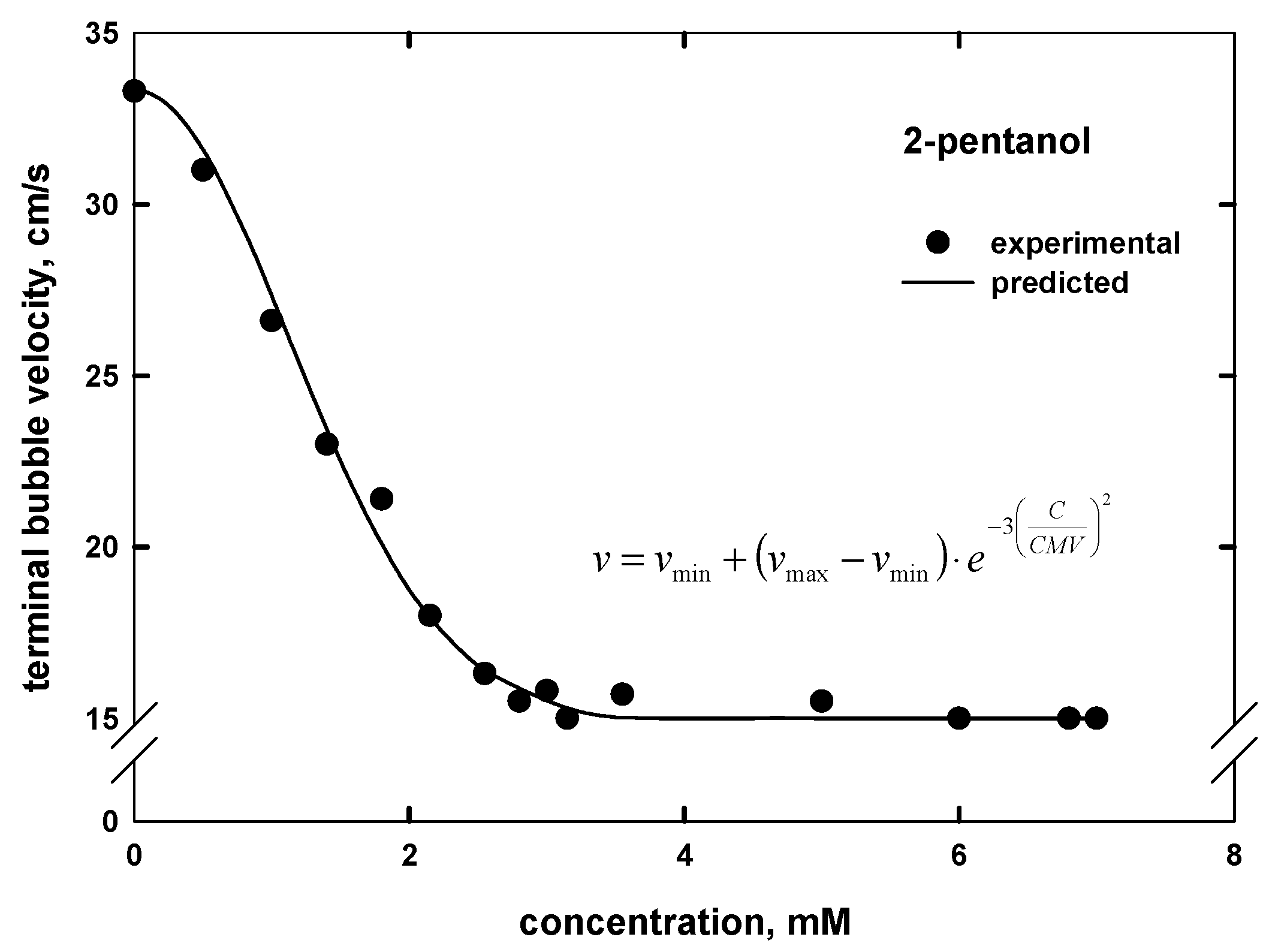
- The proposed empirical model can also be used to predict the terminal bubble velocity–frother concentration curve by knowing the maximum and minimum terminal velocities, as well as the values of the CMV. This approach is universal under similar experimental conditions and can be also extended for the prediction of bubble size when the minimum and maximum bubble sizes, as well as CCC value, are known.
- (c)
- There is a strong correlation between the CMV, the DFI and the number of carbons in the alkyl chain (n). This correlation allows one to determine these parameters, but also to classify frothers as selective and powerful.
- (d)
- The assessment and usefulness of frother characterization parameters, e.g., CMV, DFI and CCC, were shown in the flotation of coal.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, Y.H.; Rafiei, A.A.; Elmahdy, A.; Finch, J.A. Bubble size, gas holdup and bubble velocity profile of some alcohols and commercial frothers. Int. J. Miner. Process. 2013, 119, 1–5. [Google Scholar] [CrossRef]
- Tan, Y.H.; Finch, J.A. Frother structure–property relationship: Effect of hydroxyl position in alcohols on bubble rise velocity. Miner. Eng. 2016, 92, 1–8. [Google Scholar] [CrossRef]
- Tan, Y.H.; Finch, J.A. Frother structure–property relationship: Effect of alkyl chain length in alcohols and polyglycols ethers on bubble rise velocity. Miner. Eng. 2016, 95, 14–20. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Rao, R.; Finch, J.A. Characterizing frothers through critical coalescence concentration (CCC) 95-hydrophile-lipophile balance (HLB) relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef]
- Kowalczuk, P.B. Determination of critical coalescence concentration and bubble size for surfactants used as flotation frothers. Ind. Eng. Chem. Res. 2013, 52, 11752–11757. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Siedlarz, M.; Szczerkowska, S.; Wojcik, M. Facile determination of foamability index of non-ionic and cationic frothers and its effect on flotation of quartz. Sep. Sci. Technol. 2017, 1–9. [Google Scholar] [CrossRef]
- Czarnecki, J.; Malysa, K.; Pomianowski, A. Dynamic frothability index. J. Colloid Interface Sci. 1982, 86, 570–572. [Google Scholar] [CrossRef]
- Malysa, E.; Malysa, K.; Czarnecki, J. A method of comparison of the frothing and collecting properties of frothers. Colloids Surf. 1987, 23, 29–39. [Google Scholar] [CrossRef]
- Khoshdast, H.; Mirshekari, S.; Zahab-Nazouri, A. A model for predicting dynamic frothability index for dual-frother blends. J. Min. Environ. 2015, 6, 119–124. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114 115, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Zawala, J.; Malysa, E.; Krzan, M.; Malysa, K. Monitoring of contamination of coal processing plants and environmental waters using bubble velocity measurements—Advantages and limitations. Physicochem. Probl. Miner. Process. 2014, 50, 143–157. [Google Scholar] [CrossRef]
- Frumkin, A.; Levich, V. On surfactants and interfacial motion. Zhur. Fiz. Khim. 1947, 21, 1183–1204. [Google Scholar]
- Fuerstenau, D.W.; Wayman, C.H. Effect of chemical reagents on the motion of single air bubbles in water. Trans. AIME 1958, 211, 694–699. [Google Scholar]
- Clift, R.; Grace, J.R.; Weber, M.E. Bubbles, Drops and Particles; Academic Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Zhou, Z.A.; Egiebor, N.O.; Plitt, L.R. Frother effect on single bubble motion in a water column. Can. Metall. Q. 1992, 31, 11–16. [Google Scholar] [CrossRef]
- Sam, A.; Gomez, C.O.; Finch, J.A. Axial velocity profiles of single bubbles in water/frother solutions. Int. J. Miner. Process. 1996, 47, 177–196. [Google Scholar] [CrossRef]
- Zhang, W.; Sam, A.; Finch, J.A. Temperature effect on single bubble velocity profile in water and surfactant solution. Colloids Surf. A Physicochem. Eng. Aspects 2003, 223, 45–54. [Google Scholar] [CrossRef]
- Krzan, M.; Lunkenheimer, K.; Malysa, K. On the influence of the surfactant’s polar group on the local and terminal velocities of bubbles. Colloids Surf. A Physicochem. Eng. Aspects 2004, 250, 431–441. [Google Scholar] [CrossRef]
- Alves, S.S.; Orvalho, S.P.; Vasconcelos, J.M.T. Effect of bubble contamination on rise velocity and mass transfer. Chem. Eng. Sci. 2005, 60, 1–9. [Google Scholar] [CrossRef]
- Krzan, M.; Malysa, K. Influence of solution pH and electrolyte presence on bubble velocity in anionic surfactant solutions. Physicochem. Probl. Miner. Process. 2009, 43, 43–58. [Google Scholar]
- Javor, Z.; Schreithofer, N.; Heiskanen, K. The effect of bubble release techniques on their behavior at the initial stages of rise. Miner. Eng. 2012, 36, 254–261. [Google Scholar] [CrossRef]
- Varadaraj, R.; Bock, J.; Valint, P. Jr.; Zushma, S.; Thomas, R. Fundamental interfacial properties of alkyl-branched sulfate and ethoxy sulfate surfactants derived from Guerbet alcohols. 1. Surface and instantaneous interfacial tensions. J. Phys. Chem. 1991, 95, 1671–1676. [Google Scholar] [CrossRef]
- Krzan, M.; Zawala, J.; Malysa, K. Development of steady state adsorption distribution over interface of a bubble rising in solutions of n-alkanols (C5, C8) and n-alkyltrimethylammonium bromides (C8, C12, C16). Colloids Surf. A Physicochem. Eng. Aspects. 2007, 298, 42–51. [Google Scholar] [CrossRef]
- Krzan, M.; Malysa, K. Profiles of local velocities of bubbles in n-butanol, n-hexanol and n-nonanol solutions. Colloids Surf. A Physicochem. Eng. Aspects. 2004, 207, 279–291. [Google Scholar] [CrossRef]
- Kosior, D.; Zawala, J.; Krasowska, M.; Malysa, K. Influence of n-octanol and a-terpineol on thin film stability and bubble attachment to hydrophobic surface. Phys. Chem. Chem. Phys. 2013, 15, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Klevens, H. Structure and aggregation in dilate solution of surface active agents. J. Am. Oil Chem. Soc. 1953, 30, 74–80. [Google Scholar] [CrossRef]
- Finch, J.A.; Zhang, W. Frother function-structure relationship: Dependence of CCC95 on HLB and the H-ratio. Miner. Eng. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Laskowski, J.S. Testing flotation frothers. Physicochem. Probl. Miner. Process. 2004, 38, 13–22. [Google Scholar]
- Sweet, C.; van Hoogstraten, J.; Harris, M.; Laskowski, J.S. The effect of frothers on bubble size and frothability of aqueous solutions. In Processing of Complex Ores: Proceedings of the Second UBC-McGill Bi-Annual International Symposium on Fundamentals of Mineral Processing and the Environment, Sudbury, Ontario, 17–19 August 1997; Finch, J.A., Rao, S.R., Holubec, I., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 1997; pp. 235–245. [Google Scholar]
- Mukai, S.; Wakamatsu, T.; Takahashi, K. Mutual interaction between collectors and frothers in flotation. Mem. Fac. Eng. Kyoto Univ. 1972, 34, 279–288. [Google Scholar]
- Kurniawan, A.U.; Ozdemir, O.; Nguyen, A.V.; Ofori, P.; Firth, B. Flotation of coal particles in MgCl2, NaCl, and NaClO3 solutions in the absence and presence of Dowfroth 250. Int. J. Miner. Process. 2011, 98, 137–144. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S.; Ramos, O. Effect of sea water main components on frothability in the flotation of Cu-Mo sulphide ore. Physicochem. Probl. Miner. Process. 2013, 50, 17–29. [Google Scholar] [CrossRef]
- Karlkvist, T.; Patra, A.; Rao, K.H.; Bordes, R.; Holmberg, K. Flotation selectivity of novel alkyl dicarboxylate reagents for apatite–calcite separation. J. Colloid Interface Sci. 2015, 445, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Moimane, T.M.; Corin, K.C.; Wiese, J.G. Investigation of the interactive effects of the reagent suite in froth flotation of a Merensky ore. Miner. Eng. 2016, 96, 39–45. [Google Scholar] [CrossRef]
- Park, H.; Wang, J.; Wang, L. A comparative study of methyl cyclohexanemethanol and methyl isobutyl carbinol as frother for coal flotation. Int. J. Miner. Process. 2016, 155, 32–44. [Google Scholar] [CrossRef]
- Drzymala, J.; Ratajczak, T.; Kowalczuk, P.B. Kinetic separation curves based on process rate considerations. Physicochem. Probl. Miner. Process. 2017, 53, 983–995. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Selectivity and power of frothers in copper ore flotation. Physicochem. Probl. Miner. Process. 2017, 53, 515–523. [Google Scholar] [CrossRef]
- Sousa, R.; Futuro, A.; Setas Pires, C.; Machado Leite, M. Froth flotation of Aljustrel sulphide complex ore. Physicochem. Probl. Miner. Process. 2017, 53, 758–769. [Google Scholar] [CrossRef]
- Sun, K.; Liu, T.; Zhang, Y.; Liu, X.; Wang, B.; Xu, C. Application and mechanism of anionic collector sodium dodecyl sulfate (SDS) in phosphate beneficiation. Minerals 2017, 7, 29. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Mroczko, D.; Drzymala, J. Influence of frother type and dose on collectorless flotation of copper-bearing shale in a flotation column. Physicochem. Probl. Miner. Process. 2015, 51, 547–558. [Google Scholar] [CrossRef]

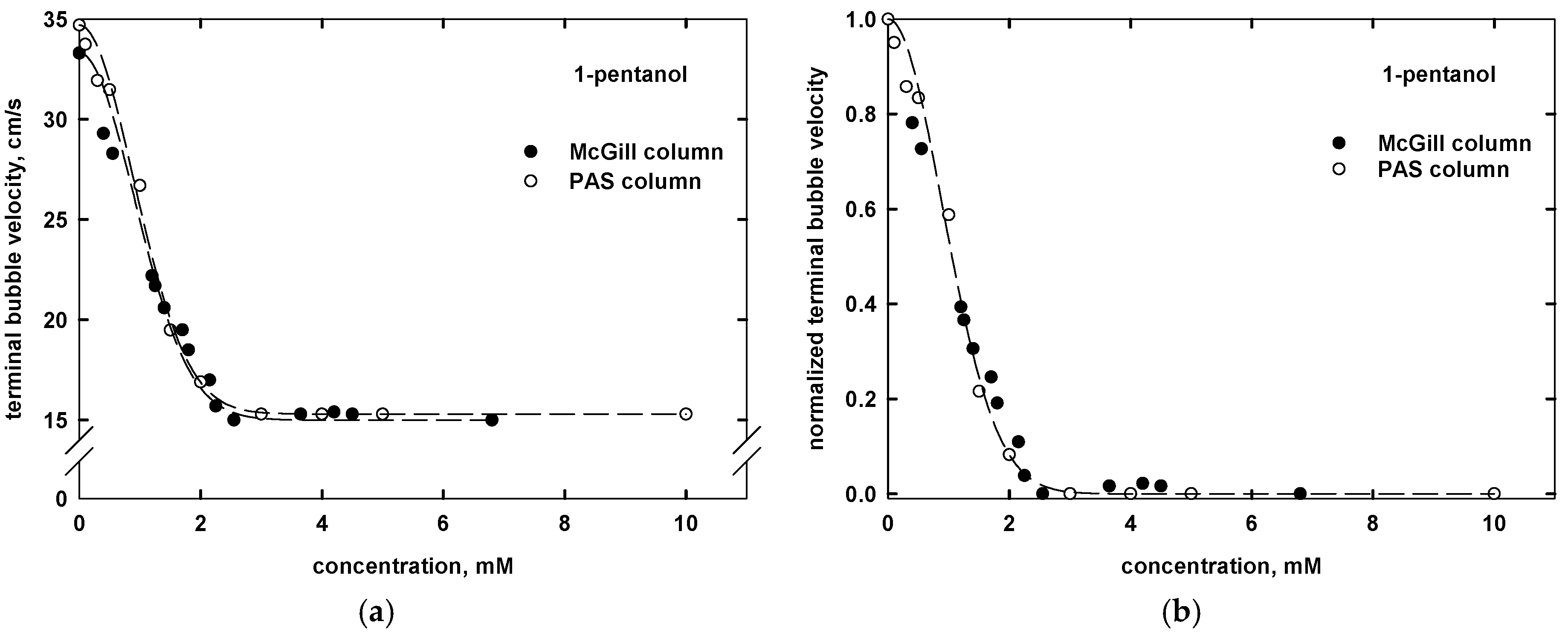

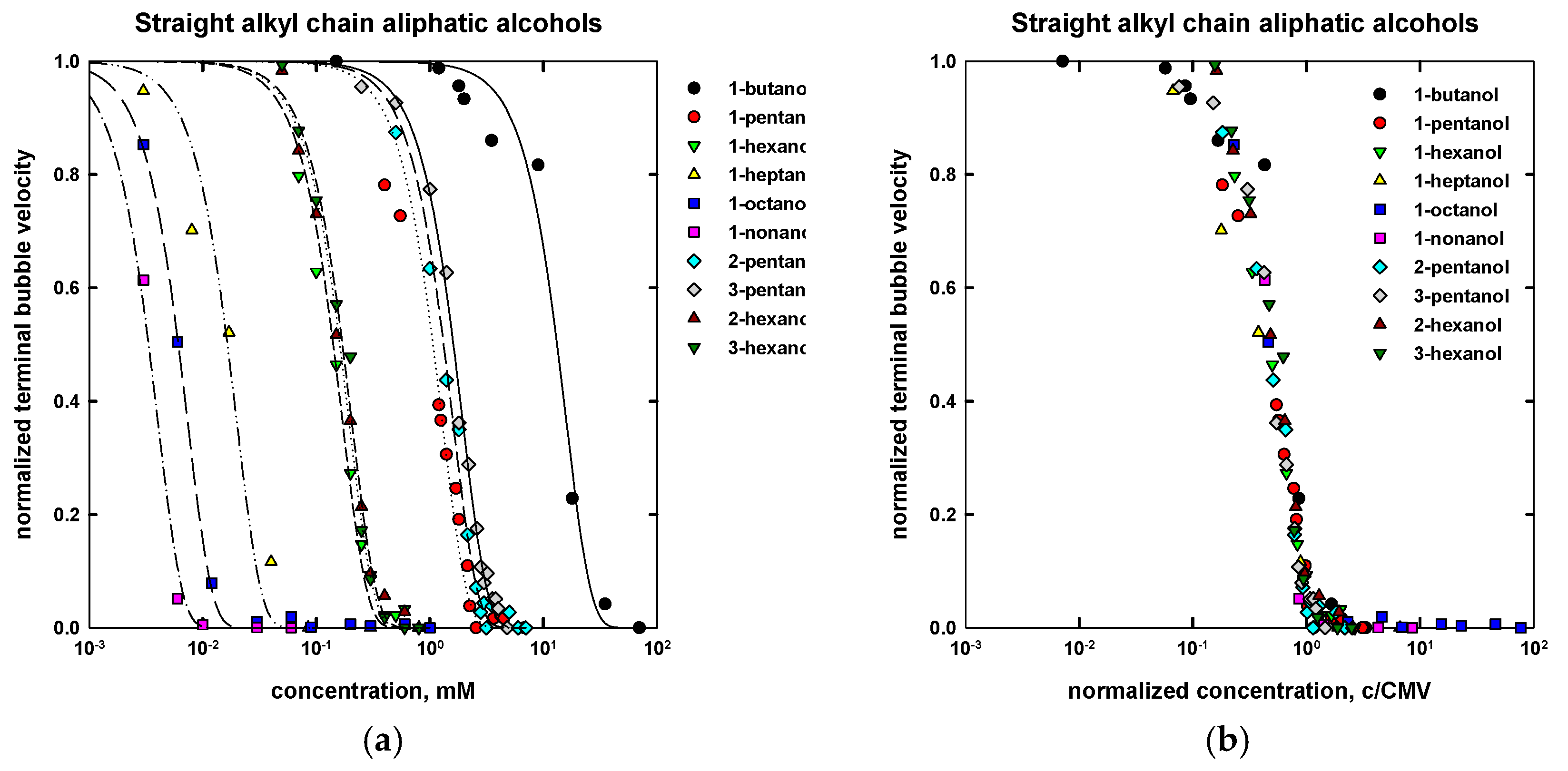
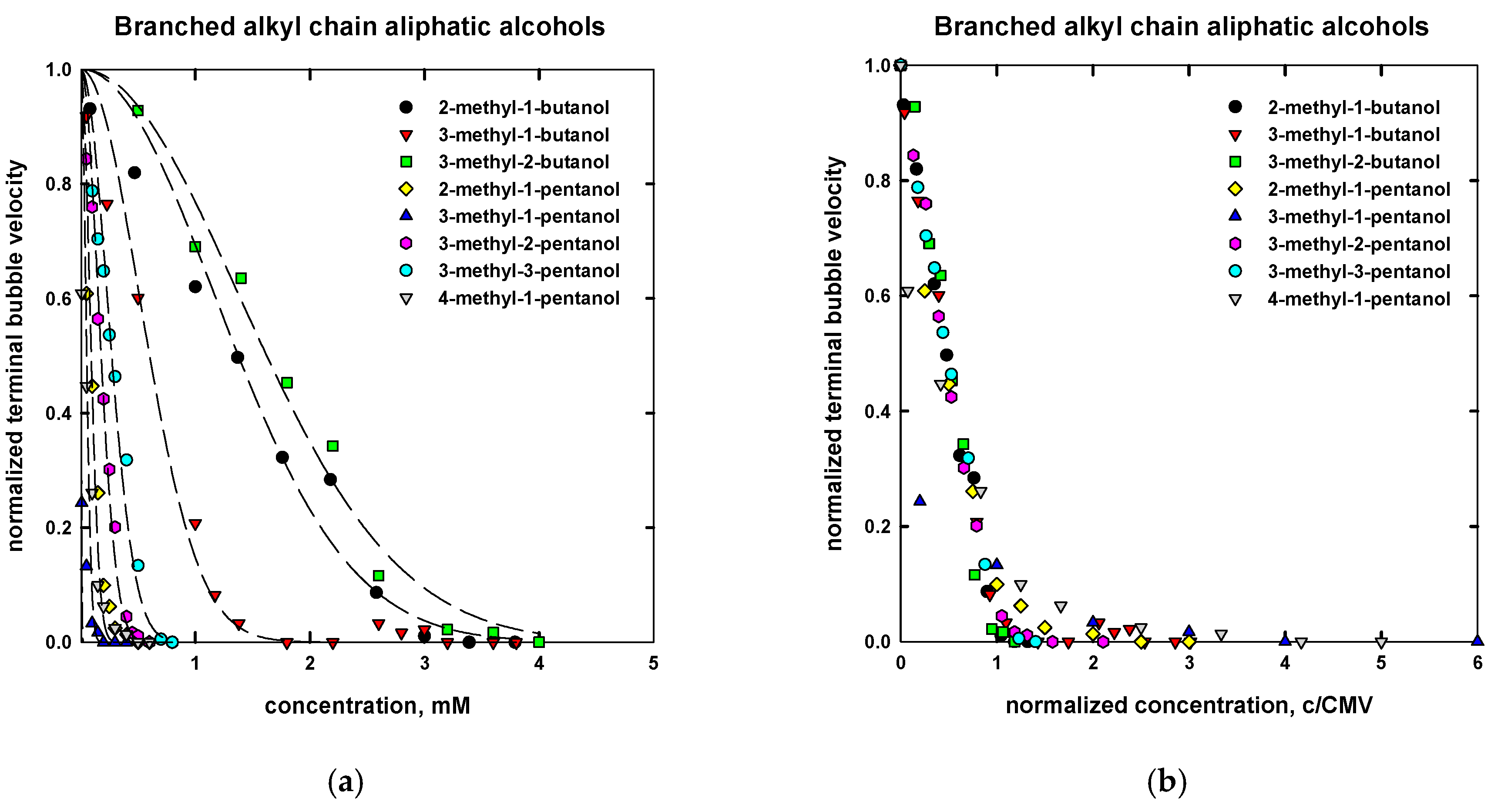
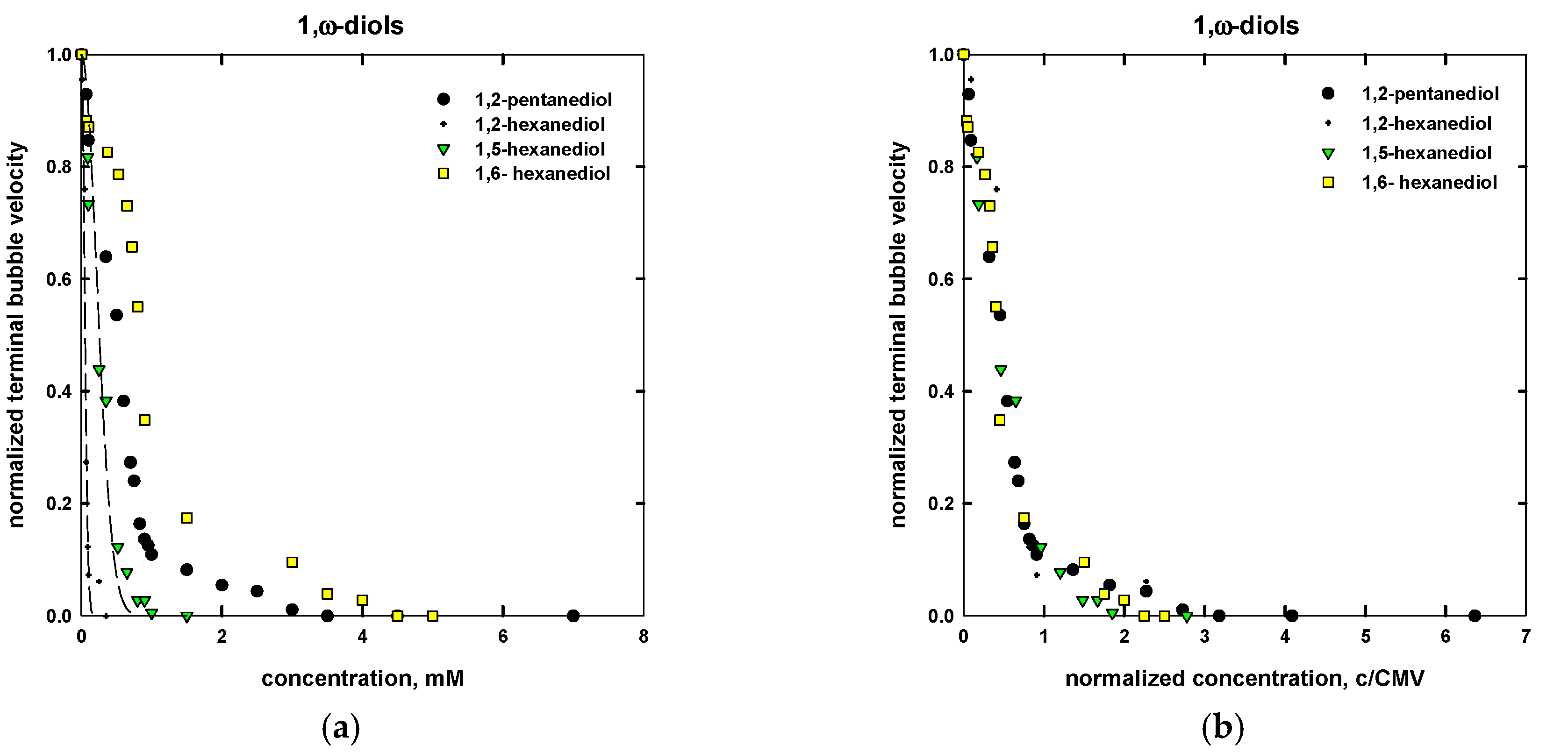
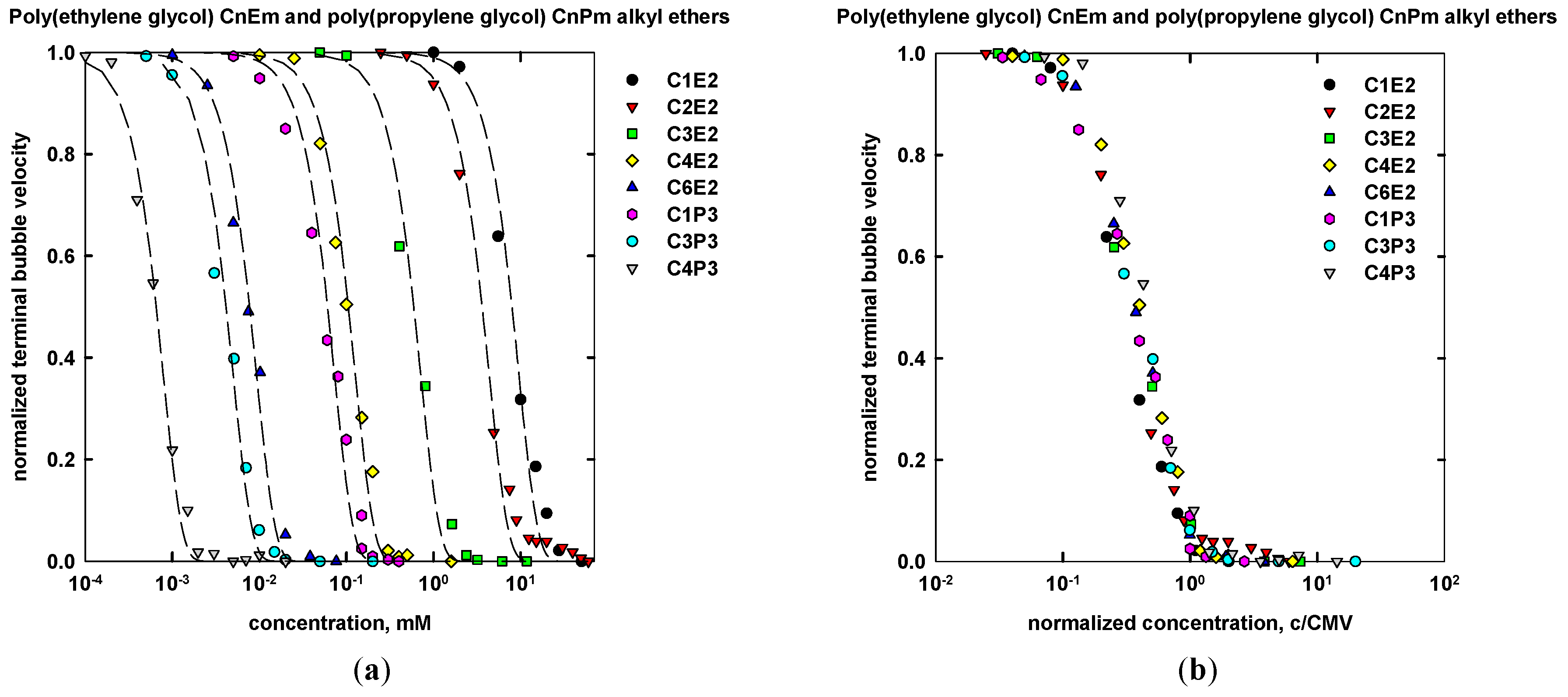
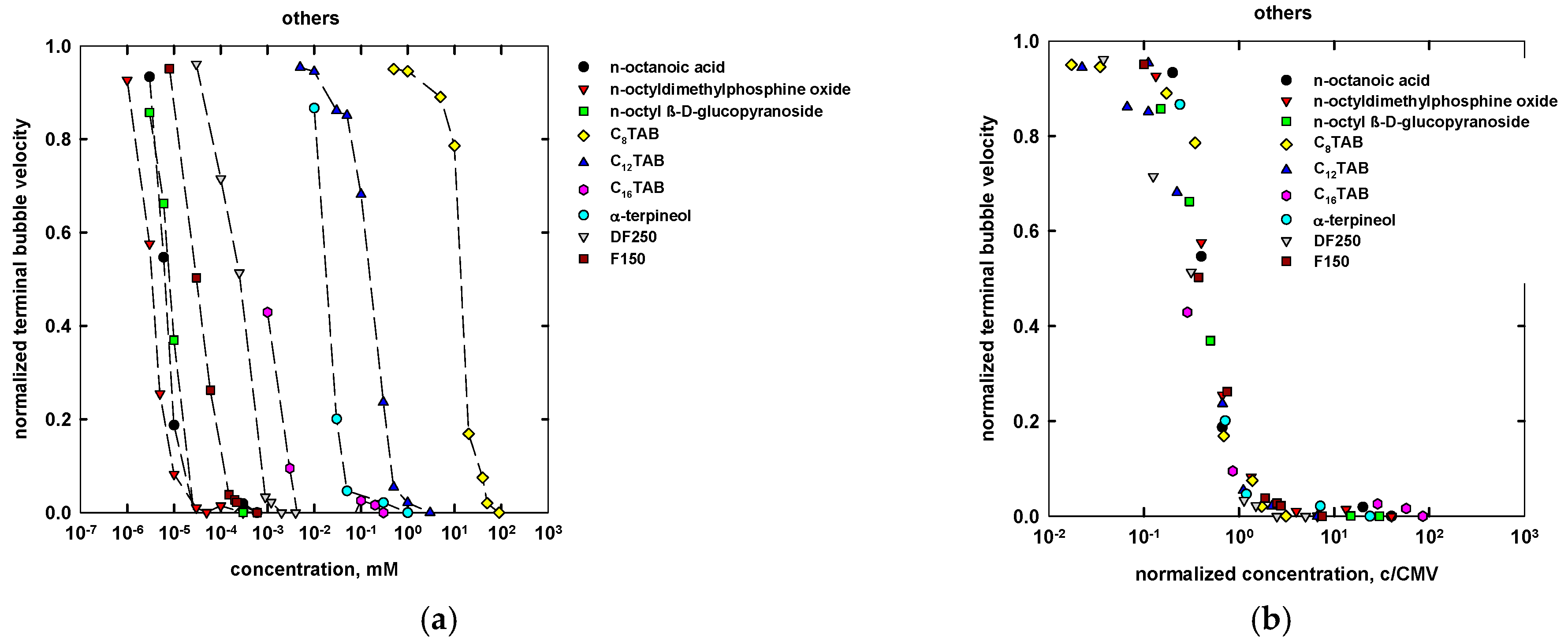

| Name | Chemical Formula | n | m | MW (g/mol) | CMVexp (mM) | CMVcalc (mM) |
|---|---|---|---|---|---|---|
| Aliphatic alcohols, Cn | ||||||
| straight alkyl chain | ||||||
| 1-butanol | CH3(CH2)3OH | 4 | - | 74.12 | 20 a | 24 |
| 1-pentanol | CH3(CH2)4OH | 5 | - | 88.15 | 2.3 a | 2.2 |
| 1-hexanol | CH3(CH2)5OH | 6 | - | 102.17 | 0.27 a | 0.30 |
| 1-heptanol | CH3(CH2)6OH | 7 | - | 116.20 | 0.06 a | 0.05 |
| 1-octanol | CH3(CH2)7OH | 8 | - | 130.23 | 0.013 b | 0.013 |
| 1-nonanol | CH3(CH2)8OH | 9 | - | 144.25 | 0.007 c | 0.007 |
| 2-pentanol | CH3(CH2)2CHOHCH3 | 5 | - | 88.15 | 2.65 d | 2.75 |
| 3-pentanol | CH3CH2CHOHCH2CH3 | 5 | - | 88.15 | 3.05 d | 3.3 |
| 2-hexanol | CH3(CH2)3CHOHCH3 | 6 | - | 102.17 | 0.30 d | 0.31 |
| 3-hexanol | CH3(CH2)2CHOHCH2CH3 | 6 | - | 102.17 | 0.36 d | 0.32 |
| branched alkyl chain | ||||||
| 2-methyl-1-butanol | CH3CH2CHCH3CH2OH | 5 | - | 88.15 | 1.95 d | 3.0 |
| 2-methyl-2-butanol | CH3CH2CH3COHCH3 | 5 | - | 88.15 | 3.5 d | n/d |
| 3-methyl-1-butanol | CH3CHCH3(CH2)2OH | 5 | - | 88.15 | 1.2 d | 1.30 |
| 3-methyl-2-butanol | CH3CHCH3CHOHCH3 | 5 | - | 88.15 | 3.2 d | 3.0 |
| 2-methyl-1-pentanol | CH3(CH2)2CHCH3CH2OH | 6 | - | 102.17 | 0.23 d | 0.22 |
| 2-methyl-2-pentanol | CH3(CH2)2CCH3OHCH3 | 6 | - | 102.17 | 0.40 d | n/d |
| 2-methyl-3-pentanol | CH3CH2CHOHCH(CH3)2 | 6 | - | 102.17 | 0.65 d | n/d |
| 3-methyl-1-pentanol | CH3CH2CHCH3(CH2)2OH | 6 | - | 102.17 | 0.14 d | 0.05 |
| 3-methyl-2-pentanol | CH3CH2CHCH3CHOHCH3 | 6 | - | 102.17 | 0.37 d | 0.40 |
| 3-methyl-3-pentanol | CH3CH2COHCH3CH2CH3 | 6 | - | 102.17 | 0.60 d | 0.60 |
| 4-methyl-1-pentanol | (CH3)2CH(CH2)3OH | 6 | - | 102.17 | 0.11 d | 0.05 |
| 4-methyl-2-pentanol (MIBC) | (CH3)2CHCH2CHOHCH3 | 6 | - | 102.17 | 0.4 f | 0.25 |
| 1,ω-diol | ||||||
| 1,2-butanediol | C2H5CHOHCH2OH | 4 | - | 90.12 | 20 a | n/d |
| 1,2-pentanediol | C3H7CHOHCH2OH | 5 | - | 104.15 | 1.8 a | 1.1 |
| 1,2-hexanediol | C4H9CHOHCH2OH | 6 | - | 118.17 | 0.18 a | 0.11 |
| 1,5-hexanediol | CH3CHOH(CH2)4OH | 6 | - | 118.17 | 0.40 a | 0.60 |
| 1,6-hexanediol | HO(CH2)6OH | 6 | - | 118.17 | 2.5 a | 2.0 |
| Poly(ethylene glycol) alkyl ethers, CnEm | ||||||
| Ethylene glycol monoethyl ether | C2H5(OC2H4)OH | 2 | 1 | 90.12 | 20 a | n/d |
| Ethylene glycol monopropyl ether | C3H7(OC2H4)OH | 3 | 1 | 104.15 | 6.5 a | n/d |
| Ethylene glycol monobutyl ether | C4H9(OC2H4)OH | 4 | 1 | 118.17 | 1.1 a | n/d |
| Di(Ethylene glycol) monomethyl ether | CH3(OC2H4)2OH | 1 | 2 | 120.15 | 25 a | 25 |
| Di(Ethylene glycol) monoethyl ether | C2H5(OC2H4)2OH | 2 | 2 | 134.15 | 10 a | 10 |
| Di(Ethylene glycol) monopropyl ether | C3H7(OC2H4)2OH | 3 | 2 | 148.20 | 1.8 a | 1.6 |
| Di(Ethylene glycol) monobutyl ether | C4H9(OC2H4)2OH | 4 | 2 | 162.23 | 0.4 a | 0.25 |
| Di(Ethylene glycol) monohexyl ether | C6H13(OC2H4)2OH | 6 | 2 | 190.28 | 0.025 a | 0.020 |
| Tri(Ethylene glycol) monomethyl ether | CH3(OC2H4)3OH | 1 | 3 | 164.20 | 7 a | n/d |
| Tri(Ethylene glycol) monoethyl ether | C2H5(OC2H4)3OH | 2 | 3 | 178.23 | 1.4 a | n/d |
| Tri(Ethylene glycol) monobutyl ether | C4H9(OC2H4)3OH | 4 | 3 | 206.28 | 0.08 a | n/d |
| Poly(propylene glycol) alkyl ethers, CnPm | ||||||
| Propylene glycol propyl ether | C3H7(OC3H6)OH | 3 | 1 | 118.17 | 3 a | n/d |
| Propylene glycol butyl ether | C4H9(OC3H6)OH | 4 | 1 | 132.20 | 0.5 a | n/d |
| Di(propylene glycol) methyl ether | CH3(OC3H6)2OH | 1 | 2 | 148.20 | 3.5 a | n/d |
| Di(propylene glycol) propyl ether | C3H7(OC3H6)2OH | 3 | 2 | 176.25 | 0.14 a | n/d |
| Di(propylene glycol) bytul ether | C4H9(OC3H6)2OH | 4 | 2 | 190.28 | 0.024 a | n/d |
| Tri(propylene glycol) methyl ether | CH3(OC3H6)3OH | 1 | 3 | 206.28 | 0.2 a | 0.15 |
| Tri(propylene glycol) propyl ether | C3H7(OC3H6)3OH | 3 | 3 | 234.33 | 0.01 a | 0.01 |
| Tri(propylene glycol) bytul ether | C4H9(OC3H6)3OH | 4 | 3 | 248.36 | 0.0014 a | 0.0014 |
| n-alkyltrimethylammonium bromides, CnTAB | ||||||
| n-octyltrimethylammonium bromide | CH3(CH2)7N(CH3)3Br | 8 | - | 252.24 | 29 b | 29 |
| n-dodecytrimethylammonium bromide | CH3(CH2)11N(CH3)3Br | 12 | - | 308.34 | 0.45 b | 0.45 |
| n-cetyltrimethylammonium bromide | CH3(CH2)15N(CH3)3Br | 16 | - | 364.45 | 0.0035 b | 0.0035 |
| Commercial frothers | ||||||
| DF250 | CH3(C3H6O)4OH | 1 | 4 | 264.35 | 0.002 f | 0.002 |
| F150 | H(C3H6O)7OH | - | 7 | 425.00 | 8 × 10−5 f | 8·× 10−5 |
| Others | ||||||
| n-octanoic acid | CH3(CH2)6COOH | 8 | - | 144.21 | 1.5·× 10−5 g | 1.5·× 10−5 g |
| n-octyl β-d-glucopyranoside | C14H28O6 | 8 | - | 292.37 | 2.0·× 10−5 g | 2.0·× 0−5 g |
| n-octyldimethylphosphine oxide | CH3(CH2)7P(O)(CH3)2 | 8 | - | 190.26 | 7.5·× 10−6 g | 7.5·× 10−5 g |
| α-terpineol | C10H18O | - | - | 154.25 | 0.042 e | 0.042 |
| Surfactant Family | α | β | Determination Coefficient R2 | Standard Error of Estimate σest | |
|---|---|---|---|---|---|
| Aliphatic alcohols, Cn | 1.6424 | 9.0449 | 0.9722 | 0.4491 | |
| Poly(ethylene glycol) alkyl ethers, CnEm | m = 1 | 1.4505 | 6.0049 | 0.9834 | 0.2664 |
| m = 2 | 1.4205 | 4.8466 | 0.9954 | 0.2144 | |
| m = 3 | 1.4821 | 3.3770 | 0.9991 | 0.0953 | |
| Poly(propylene glycol) alkyl ethers, CnPm | m = 2 | 1.6535 | 2.9283 | 0.9995 | 0.0824 |
| m = 3 | 1.6317 | 0.0891 | 0.9950 | 0.2503 | |
| n-alkyltrimethylammonium bromides, CnTAB | 1.1278 | 12.5047 | 0.9981 | 0.280 | |
| 1,2-diols | 2.3553 | 12.3992 | 0.9998 | 0.0430 | |
| Surfactant | α | β | Data Points, N | Determination Coefficient R2 | Standard Error of Estimate σest |
|---|---|---|---|---|---|
| Aliphatic alcohols, Cn | 0.4594 | 1.5232 | 8 | 0.9742 | 0.4194 |
| Poly(ethylene glycol) alkyl ethers, CnEm | 0.3026 | 1.6329 | 11 | 0.7640 | 0.3951 |
| Poly(propylene glycol) alkyl ethers, CnPm | 0.2486 | 1.8593 | 8 | 0.9193 | 0.2183 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, P.B.; Zawala, J.; Drzymala, J. Concentration at the Minimum Bubble Velocity (CMV) for Various Types of Flotation Frothers. Minerals 2017, 7, 118. https://doi.org/10.3390/min7070118
Kowalczuk PB, Zawala J, Drzymala J. Concentration at the Minimum Bubble Velocity (CMV) for Various Types of Flotation Frothers. Minerals. 2017; 7(7):118. https://doi.org/10.3390/min7070118
Chicago/Turabian StyleKowalczuk, Przemyslaw B., Jan Zawala, and Jan Drzymala. 2017. "Concentration at the Minimum Bubble Velocity (CMV) for Various Types of Flotation Frothers" Minerals 7, no. 7: 118. https://doi.org/10.3390/min7070118