Microwave-Supported Leaching of Electric Arc Furnace (EAF) Slag by Ammonium Salts
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
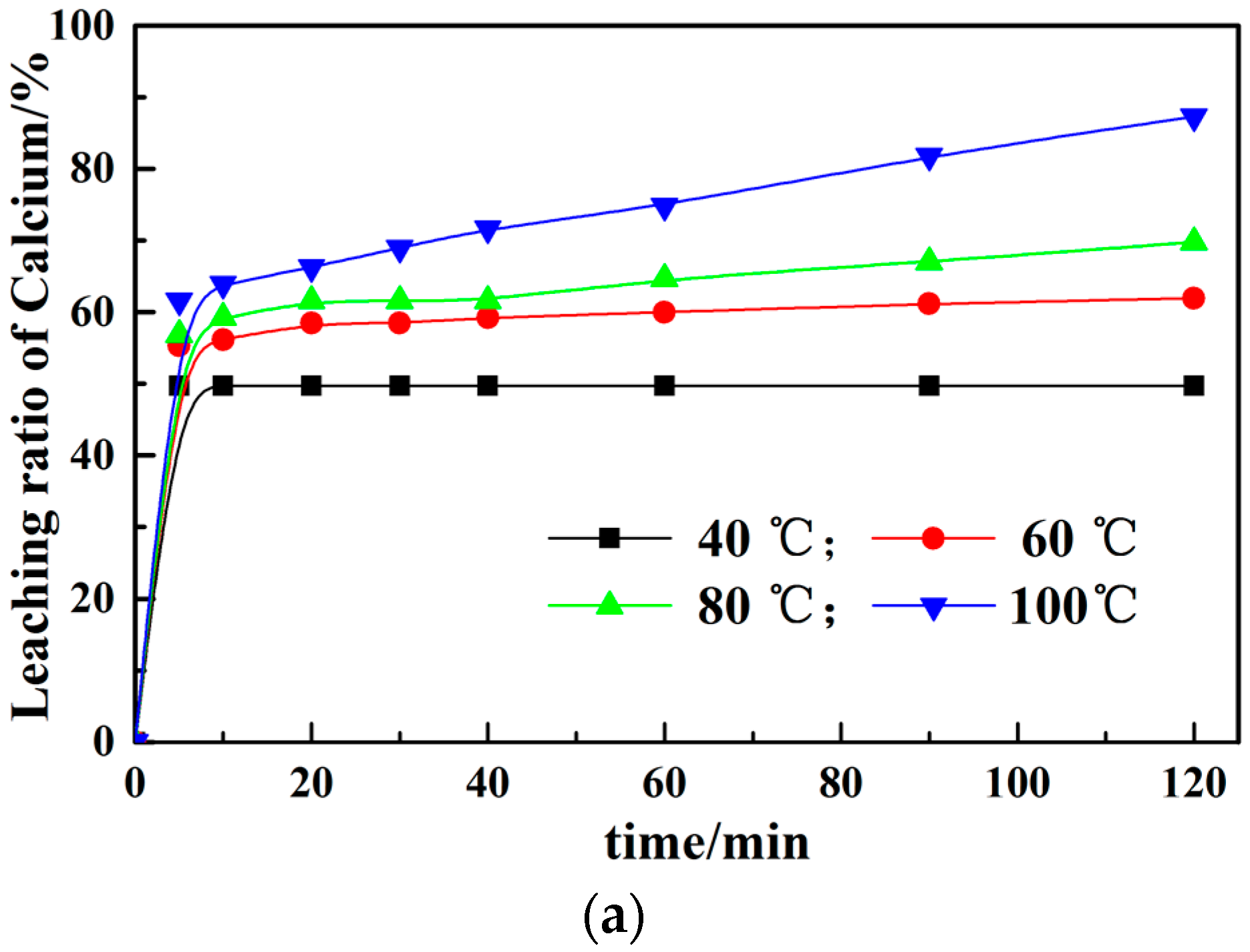
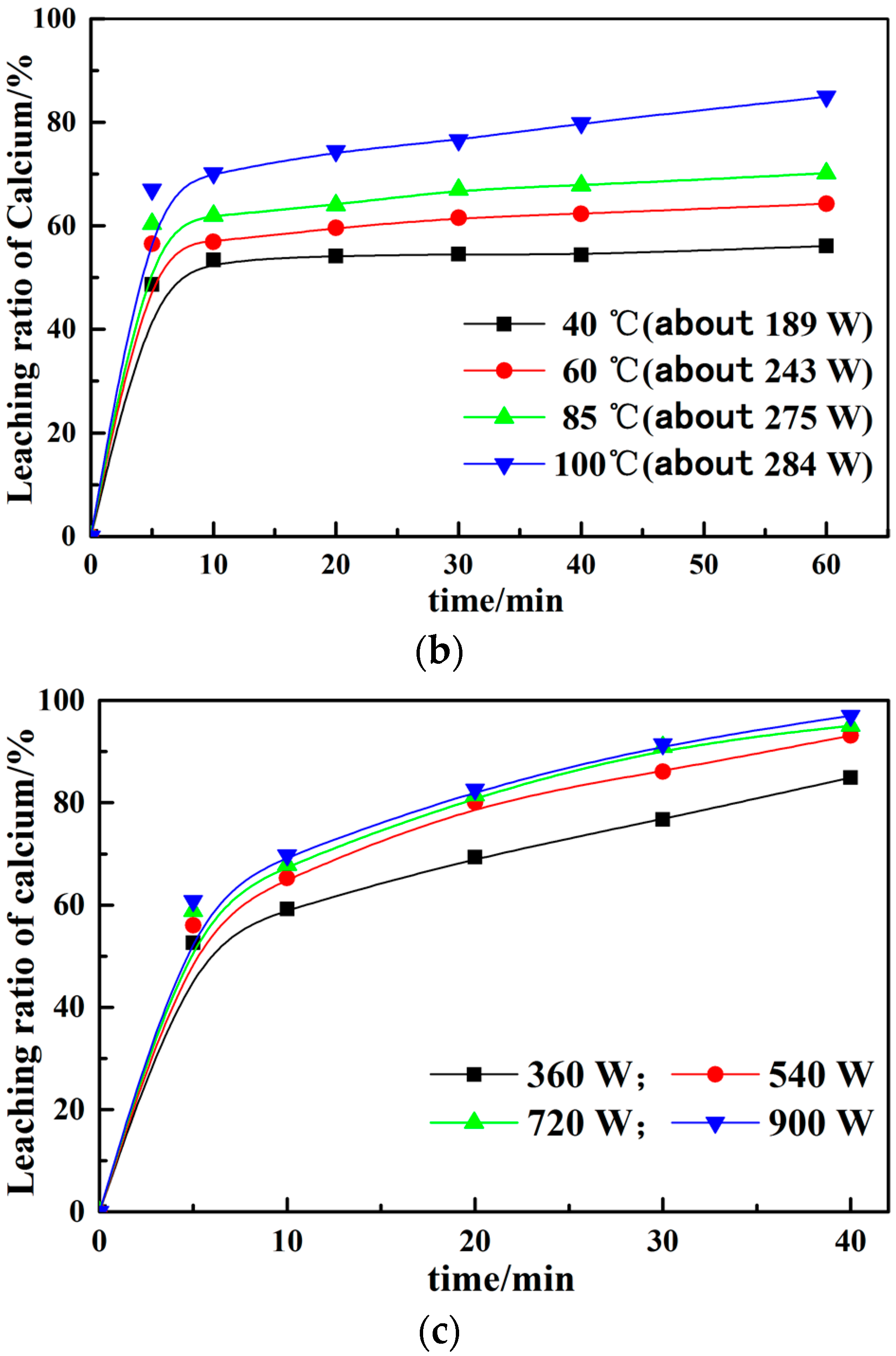
3.1. Calcium Leaching by NH4Cl with Conventional and Microwave Leaching Processes
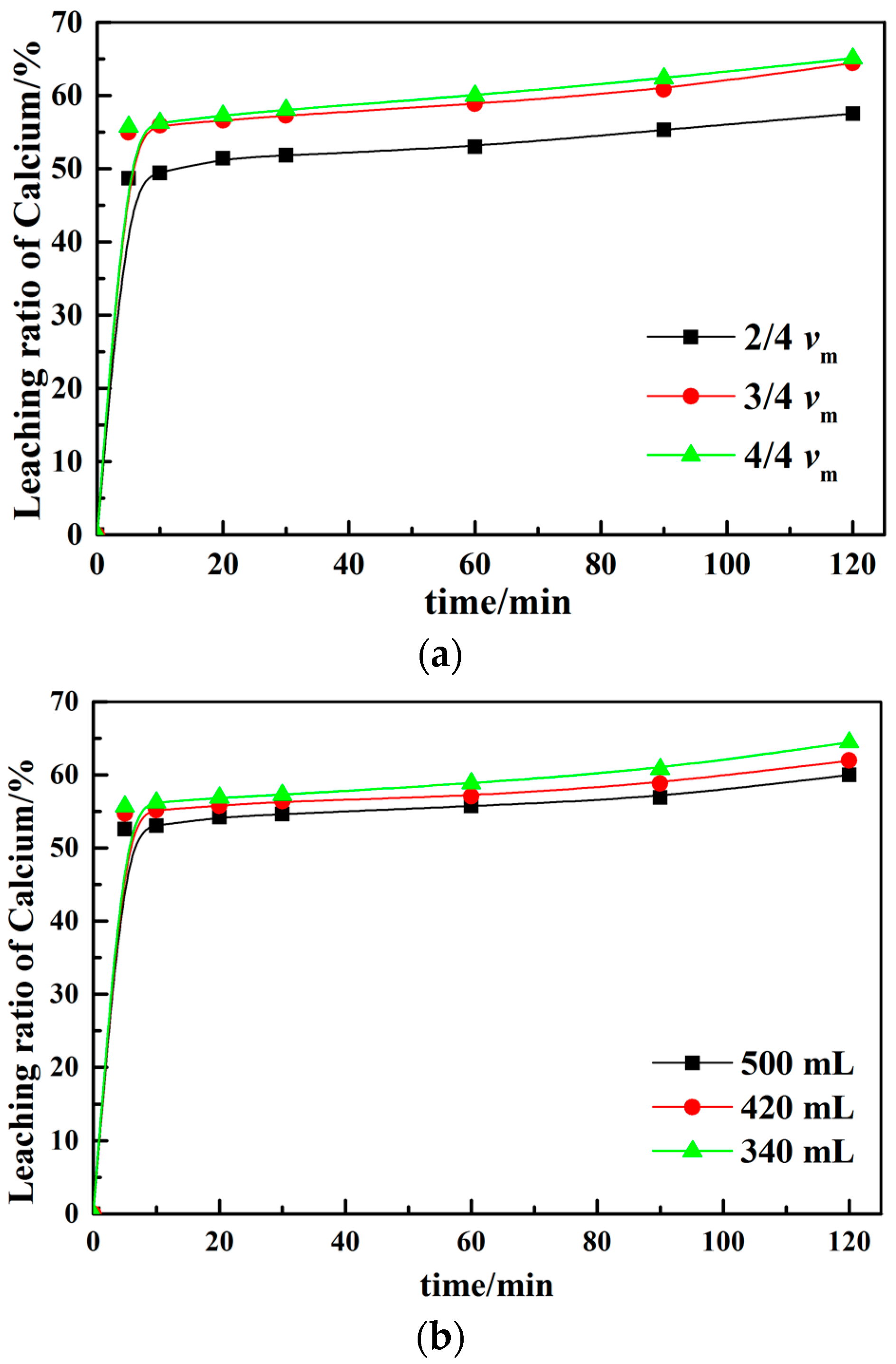
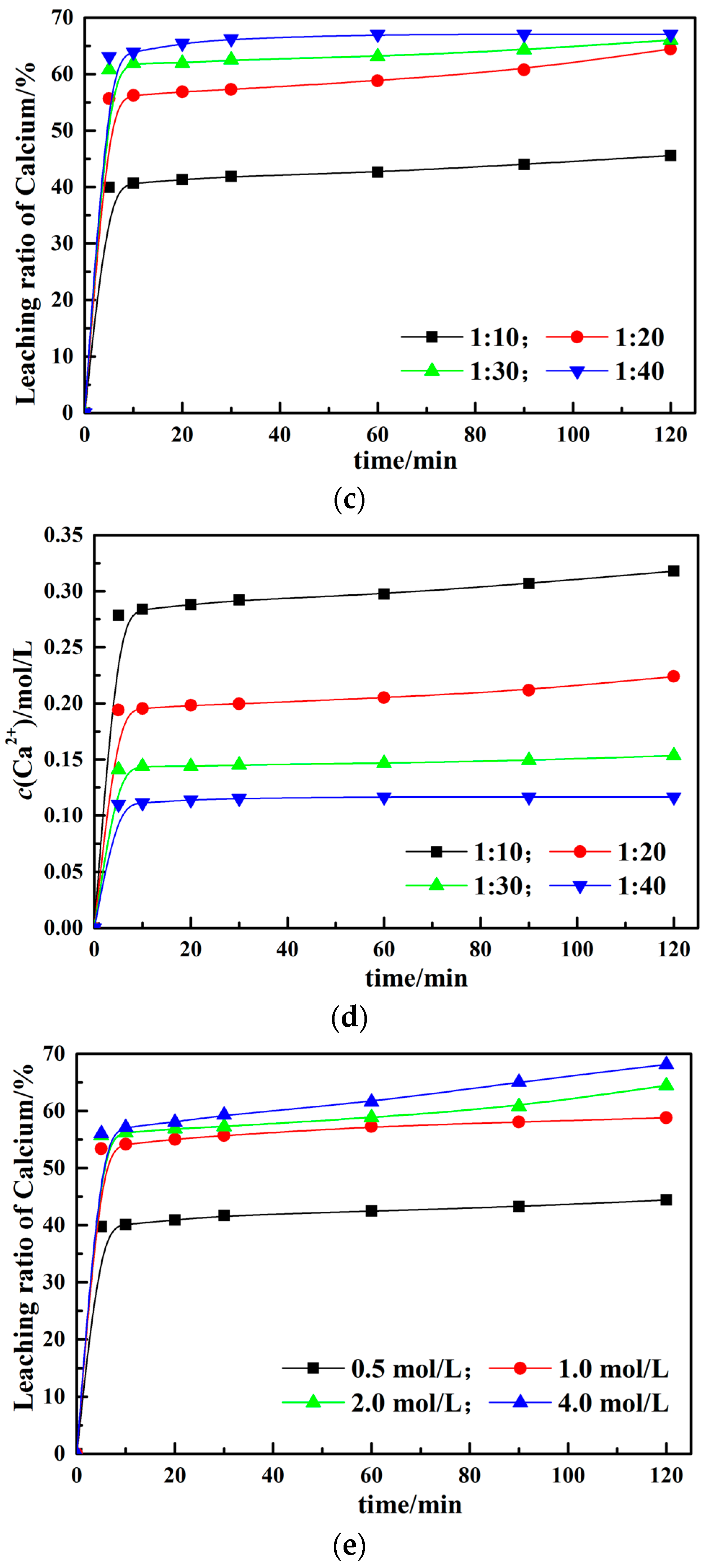
3.2. Effect of Different Leaching Parameters on Calcium Leaching Ratio from EAF Slag
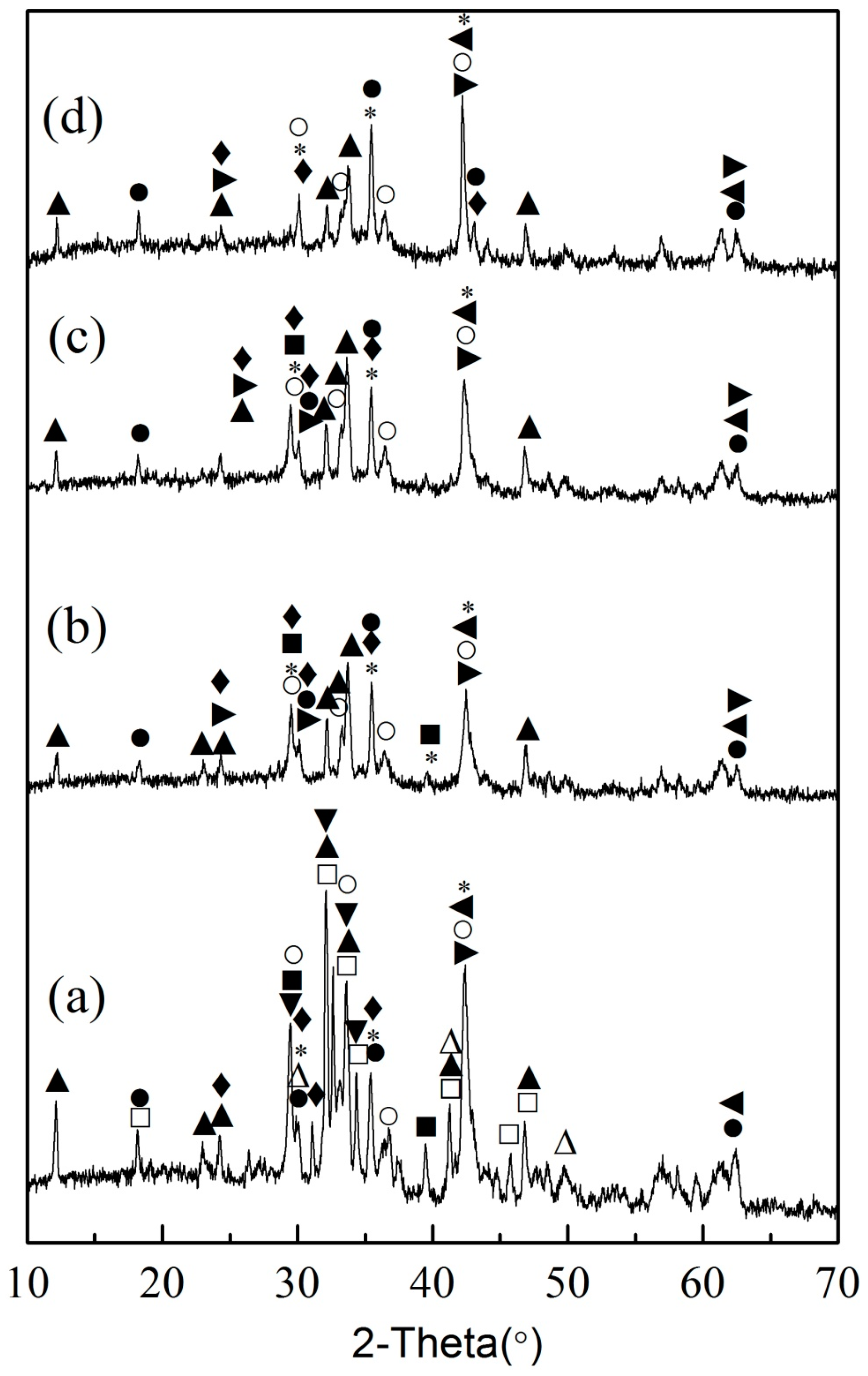
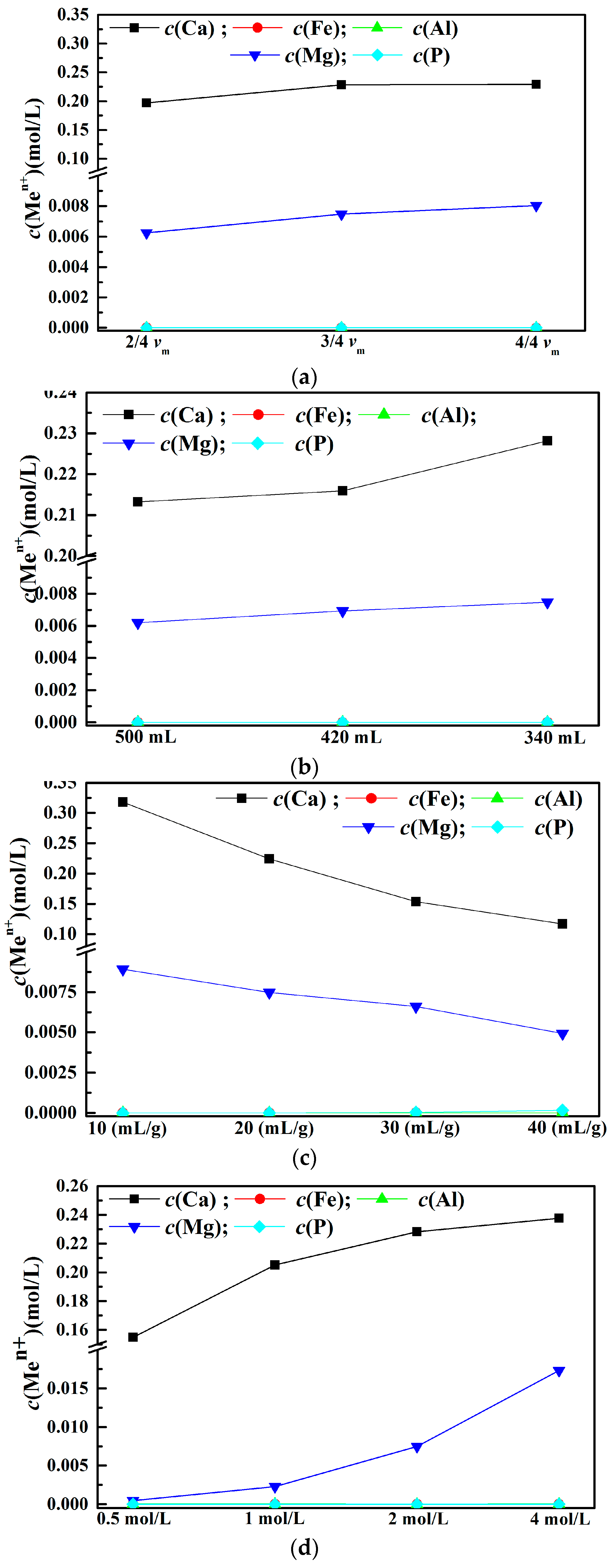
3.3. Leaching Behaviors of Impurity Ions from EAF Slag
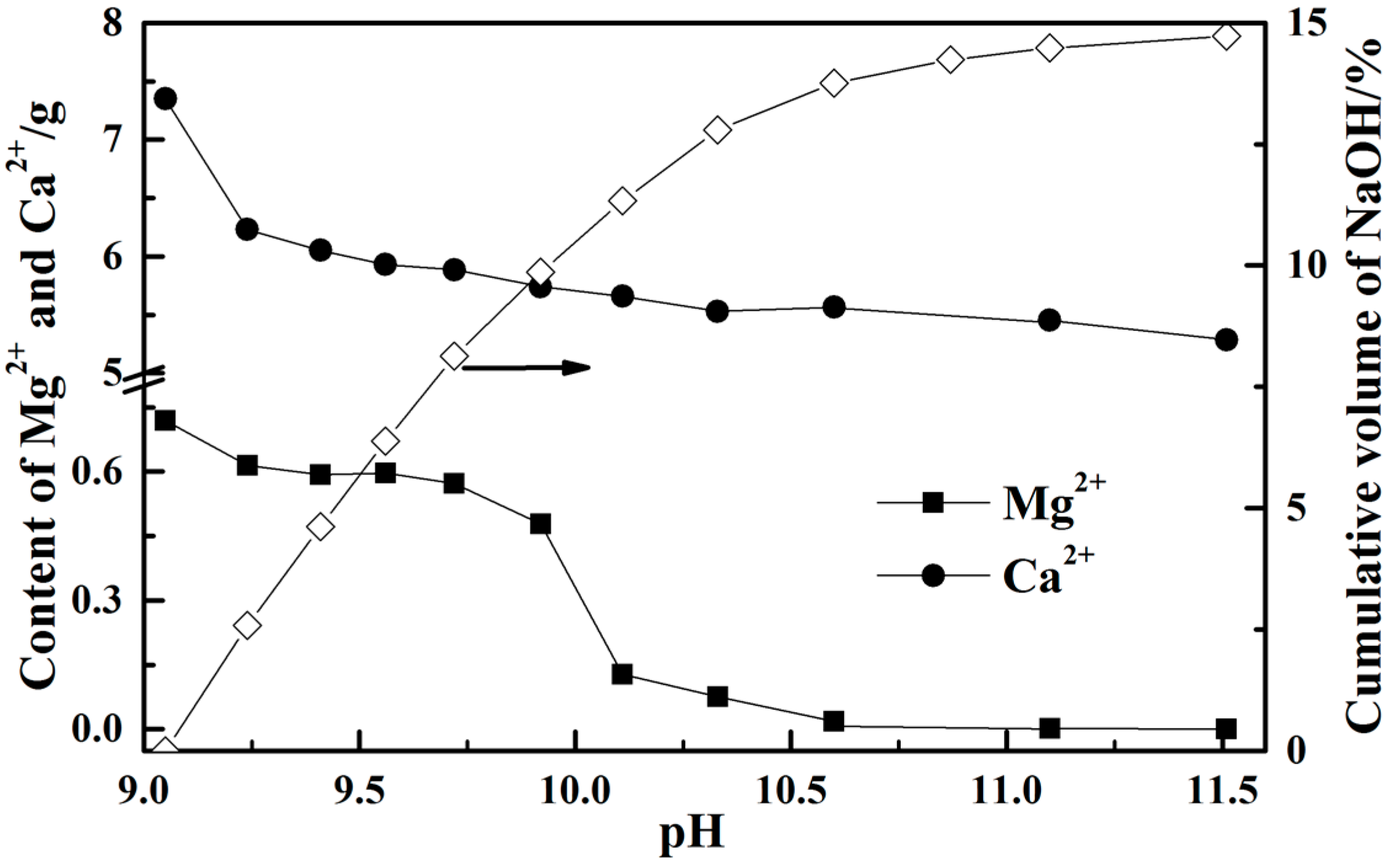
3.4. Removal of Impurity Ions from the Leachate
4. Conclusions
- (1)
- The calcium leaching ratio at the constant temperature in the microwave field increases about 10% than that under the water bath at the same time. The greater the microwave power, the higher the impact of calcium leaching ratio, which proves that microwave treatment can improve the leaching ratio. Meanwhile, the leaching solutions are boiling within several minutes at constant power in the microwave field.
- (2)
- The rapid calcium leaching step (up to 5 min) is possibly due to the easy reaction of calcium silicate and the slower calcium leaching step (after 5 min) is owing to the difficult reaction of calcium ferroaluminates for the hydrolysis of iron and aluminum.
- (3)
- The leaching behaviors of magnesium and calcium ions affected by different leaching parameters are similar and the concentration of aluminum, iron and phosphorus can be neglected.
- (4)
- Calcium ion is probably not precipitated in the real leaching solution from steel slag by NH4Cl solution as its concentration is less than 0.32 mol/L. However, the concentration of magnesium ion starts to drop sharply when the pH value is higher than 10 and it has precipitated completely at pH value of 11.6.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edwin, B. World Steel in Figures 2017; World Steel Association: Brussels, Belgium, 2017. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Effects of thin-film accelerated carbonation on steel slag leaching. J. Hazard. Mater. 2015, 286, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, Y.; Sevinç, N.; Günaydın, A. Slag treatment at kardemir integrated iron and steel works. Int. J. Miner. Process. 2004, 74, 31–39. [Google Scholar] [CrossRef]
- Tong, Z.B.; Ma, G.J.; Cai, X.; Xue, Z.L.; Wang, W.; Zhang, X. Characterization and valorization of kanbara reactor desulfurization Waste Slag of Hot Metal Pretreatment. Waste Biomass Valoriz. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Lai, D.; Wang, C.; Zeng, C.; Tong, C.; Liang, Y.; Peñuelas, J. Effects of steel slag application on greenhouse gas emissions and crop yield over multiple growing seasons in a subtropical paddy field in China. Field Crops Res. 2015, 171, 146–156. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.-S. Steel slag as an iron fertilizer for corn growth and soil improvement in a pot experiment. Pedosphere 2006, 16, 519–524. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.; Misra, V. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Kang, H.; An, K.; Kim, D. Utilization of steel slag as an adsorbent of ionic lead in wastewater. J. Environ. SCI Health 2004, 39, 3015–3028. [Google Scholar] [CrossRef]
- Yi, H.; Chen, H. An overview of utilization of steel slag. Int. Conf. Waste Manag. Technol. Chin. Soc. Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.; Zevenhoven, R. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 2007, 32, 528–539. [Google Scholar] [CrossRef]
- Hall, C.; Large, D.; Adderley, B.; West, H. Calcium leaching from waste steelmaking slag: Significance of leachate chemistry and effects on slag grain mineralogy. Miner. Eng. 2014, 65, 156–162. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, M.; Zhang, J.; Yang, G. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem. Eng. J. 2011, 173, 437–445. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Fagerlund, J.; Nduagu, E.; Romão, I.; Zevenhoven, R. CO2 fixation using magnesium silicate minerals part 1: Process description and performance. Energy 2012, 41, 184–191. [Google Scholar] [CrossRef]
- Doucet, F. Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner. Eng. 2010, 23, 262–269. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.; Zevenhoven, R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Chiang, Y.; Santos, R.; Elsen, J.; Meesschaert, B.; Martens, J.A.; Gerven, T. Towards zero-waste mineral carbon sequestration via two-way valorization of ironmaking slag. Chem. Eng. J. 2014, 249, 260–269. [Google Scholar] [CrossRef]
- Kakizawa, M.; Yamasaki, A.; Yanagisawa, Y. A new CO2 disposal process via artificial weathering of calcium silicate accelerated by acetic acid. Energy 2001, 26, 341–354. [Google Scholar] [CrossRef]
- CN-GB. GB/T 15452–2009 Industrial Circulating Cooling Water-Determination of Calcium and Magnesium-EDTA Titration Method; Standardization Administration of China: Beijing, China, 2009. [Google Scholar]
- Al-Harahsheh, M.; Kingman, S. Microwave-assisted leaching-A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Bayca, S. Microwave radiation leaching of colemanite in sulfuric acid solutions. Sep. Purif. Technol. 2013, 105, 24–32. [Google Scholar] [CrossRef]
- Madakkaruppan, V.; Pius, A.; Sreenivas, T.; Giri, N.; Sarbajna, C. Influence of microwaves on the leaching kinetics of uraninite from a low grade ore in dilute sulfuric acid. J. Hazard. Mater. 2016, 313, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, G. A study of the mineral phases of oxygen converter slag and their cementious properties. J. Chin. Ceram. Soc. 1981, 9, 302–308. [Google Scholar]
- Dong, O.; Yuping, X.; Junyuan, H. Composition, mineral morphology and cementitious properties of converter slag. J. Chin. Ceram. Soc. 1991, 19, 488–494. [Google Scholar]
- Wu, X.; Wang, P.; Li, L.; Wu, Z.; Chen, R. Distribution and enrichment of phosphorus in solidified BOF steelmaking slag. Ironmak. Steelmak. 2013, 38, 185–188. [Google Scholar] [CrossRef]
- Li, G.H.; Wu, B.; Zhang, Y.B.; Zhang, K.C.; Chen, Y.L.; Jiang, T. Mineralogical characteristics and comprehensive utilization of converter steel slag. J. Cent. South Univ. 2010, 41, 2065–2071. [Google Scholar]
- Zhang, X.; Ma, G.J.; Tong, Z.B.; Xue, Z.L. Microwave-assisted selective leaching behavior of calcium from Basic Oxygen Furnace (BOF) slag with ammonium chloride solution. J. Min. Metall. B. 2017, 2, 139–146. [Google Scholar] [CrossRef]
- Tong, Z.B. Basic research of microwave strengthening calcium leaching from EAF steelmaking slag by ammonium salts and its fixation of carbon dioxide. Wuhan Univ. Sci. Technol. 2017, in press. [Google Scholar]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L., Jr.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Alamdari, A.; Alamdari, A.; Mowla, D. Kinetics of calcium carbonate precipitation through CO2 absorption from flue gas into distiller waste of soda ash plant. J. Ind. Eng. Chem. 2014, 20, 3480–3486. [Google Scholar] [CrossRef]

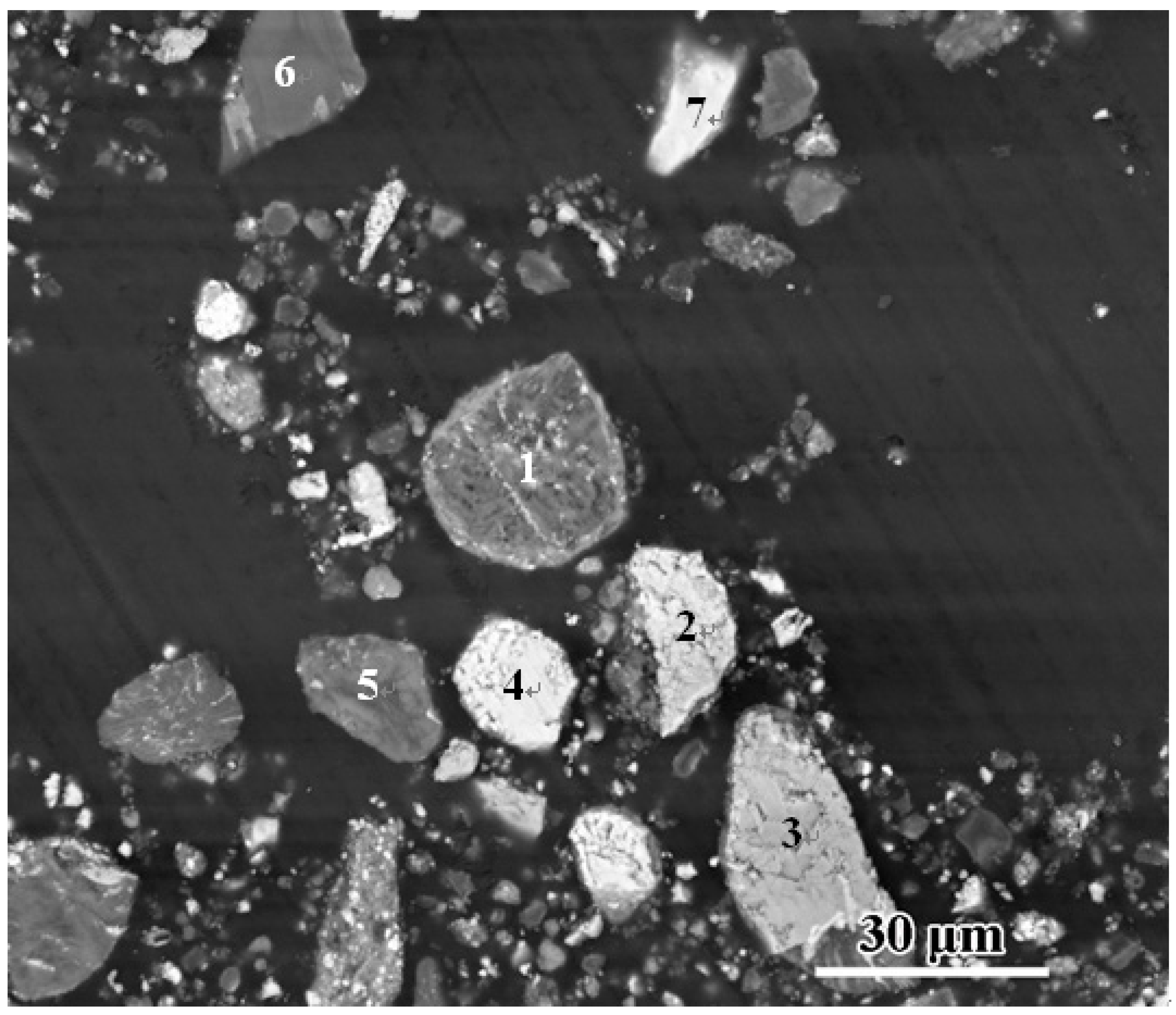

| Spectrum | Mg | Al | Si | P | Ca | Mn | Fe | Possible Phase | |
|---|---|---|---|---|---|---|---|---|---|
| Figure 3 | 1 | 33.31 | 65.3 | 1.36 | Ca2SiO4(C/S = 1.96) | ||||
| 2 | 54 | 1.58 | 3.23 | 41.22 | RO phase | ||||
| 3 | 32.54 | 66.2 | 1.29 | Ca2SiO4(C/S = 2.03) | |||||
| 4 | 75.4 | 1.79 | 22.8 | RO phase | |||||
| 5 | 76.8 | 0.84 | 2.32 | 20.04 | RO phase | ||||
| 6 | 31.98 | 68 | Ca2SiO4(C/S = 2.13) | ||||||
| 7 | 62.6 | 2.03 | 35.4 | RO phase | |||||
| 8 | 59 | 3.64 | 37.34 | RO phase | |||||
| 9 | 6.55 | 5.59 | 66.9 | 4.34 | 16.66 | - | |||
| 10 | 32.24 | 66.3 | 1.42 | Ca2SiO4(C/S = 2.07) | |||||
| 11 | 31.88 | 67.2 | 0.9 | Ca2SiO4(C/S = 2.11) | |||||
| 12 | 13.37 | 50.5 | 36.18 | CaFA(C/(A + F) = 1.02) | |||||
| 13 | 59.1 | 5 | 35.94 | RO phase | |||||
| Figure 4 | 1 | 1.85 | 1.39 | 63.84 | 4.64 | 23.48 | 1.01 | 3.79 | Calcium silicate after leached |
| 2 | 51.47 | 2.32 | 3.40 | 42.81 | RO phase | ||||
| 3 | 68.96 | 0.92 | 3.15 | 26.98 | RO phase | ||||
| 4 | 33.39 | 0.14 | 1.25 | 5.28 | 59.94 | RO phase | |||
| 5 | 1.12 | 1.90 | 55.37 | 13.43 | 24.99 | 3.19 | Calcium silicate after leached | ||
| 6 | 2.91 | 4.23 | 62.41 | 8.42 | 18.33 | 1.17 | 2.53 | Calcium silicate after leached | |
| 7 | 9.60 | 1.44 | 47.54 | 41.42 | CaFA(C/(A + F) = 0.93) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Ma, G.; Zhang, X.; Cai, Y. Microwave-Supported Leaching of Electric Arc Furnace (EAF) Slag by Ammonium Salts. Minerals 2017, 7, 119. https://doi.org/10.3390/min7070119
Tong Z, Ma G, Zhang X, Cai Y. Microwave-Supported Leaching of Electric Arc Furnace (EAF) Slag by Ammonium Salts. Minerals. 2017; 7(7):119. https://doi.org/10.3390/min7070119
Chicago/Turabian StyleTong, Zhibo, Guojun Ma, Xiang Zhang, and Yongsheng Cai. 2017. "Microwave-Supported Leaching of Electric Arc Furnace (EAF) Slag by Ammonium Salts" Minerals 7, no. 7: 119. https://doi.org/10.3390/min7070119





