The Effect of Conditioning on the Flotation of Pyrrhotite in the Presence of Chlorite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Experiments
2.2.1. Flotation Tests
2.2.2. Sedimentation Tests
2.2.3. XPS Analysis
3. Results and Discussion
4. Conclusions
- In pyrrhotite flotation, chlorite slimes impair pyrrhotite flotation performance due to the surface coating.
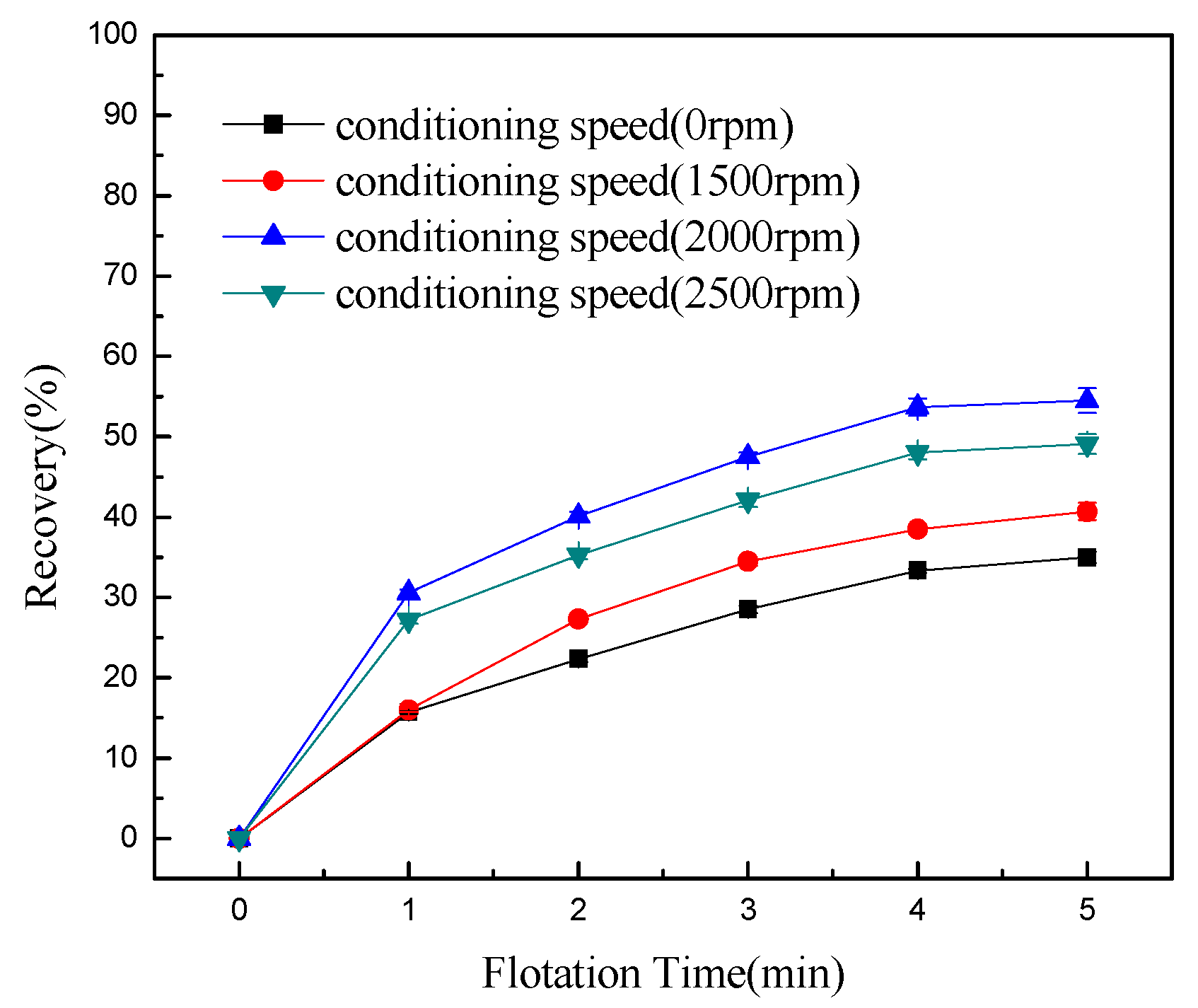
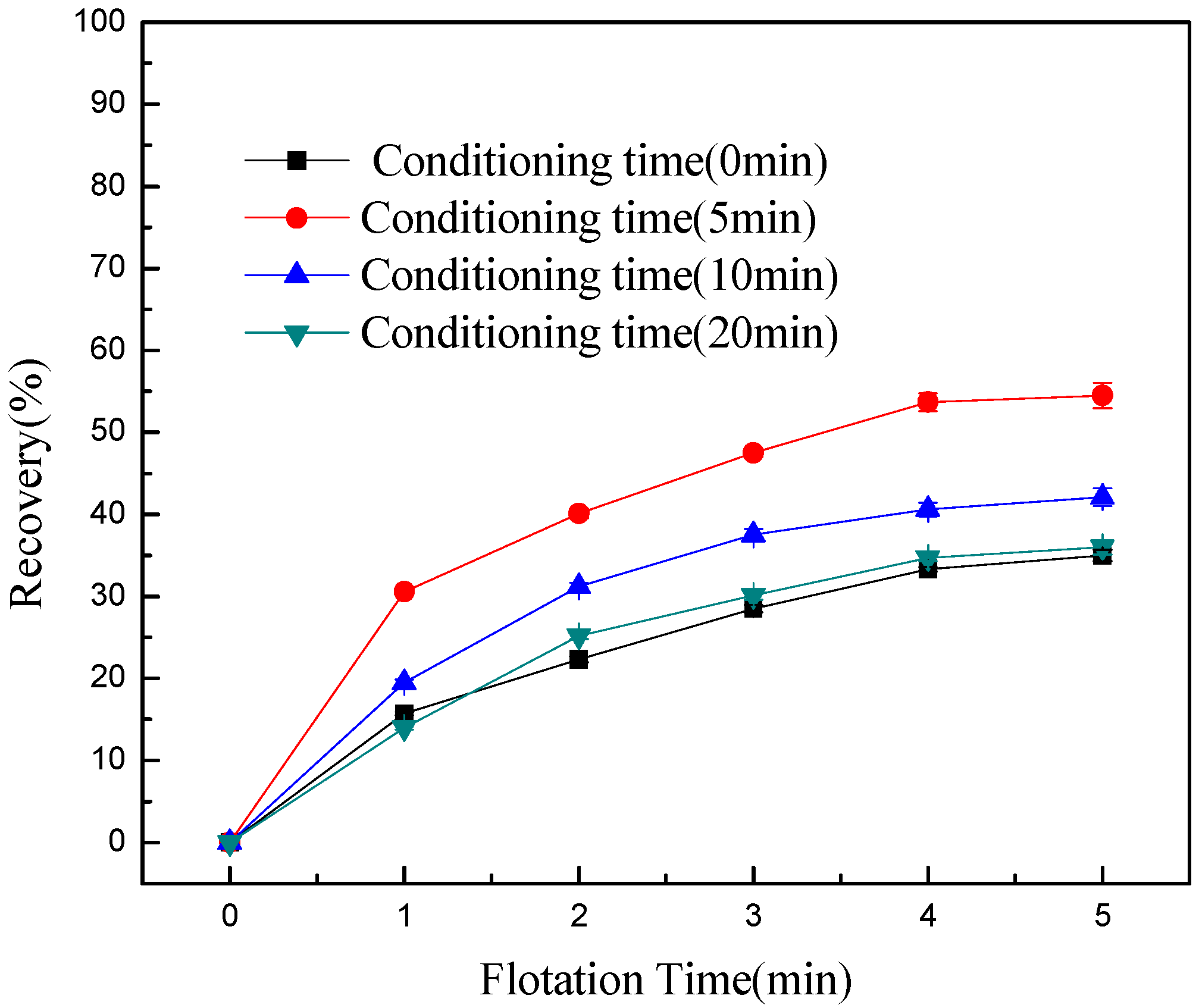
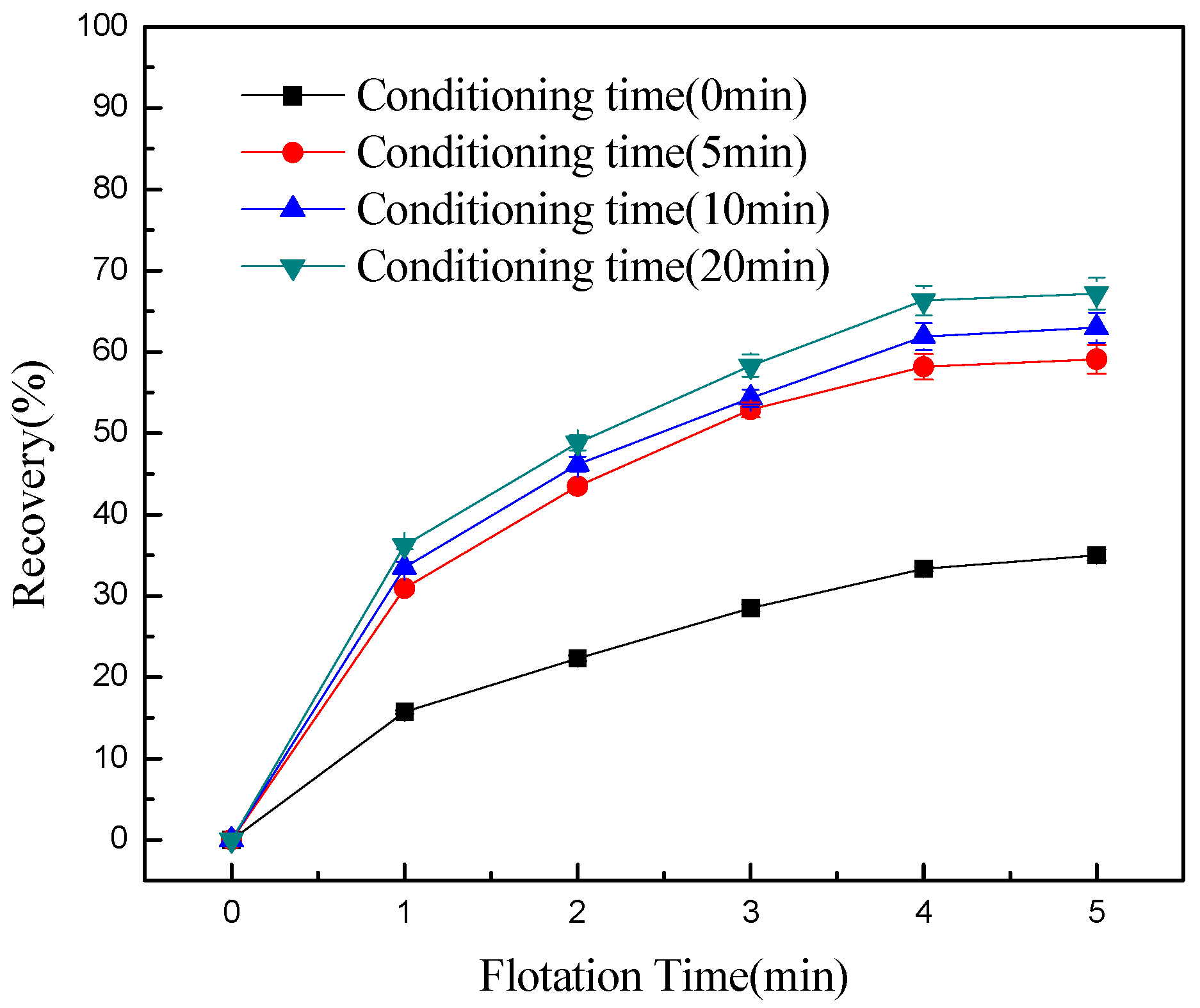
- Conditioning can effectively detach chlorite slimes from pyrrhotite surfaces, resulting in enhanced flotation recovery of pyrrhotite. When mixed minerals were conditioned under the natural atmosphere, a faster conditioning speed and longer conditioning time decreased pyrrhotite flotation recovery to some degree. However, when mixed minerals were conditioned under a nitrogen atmosphere, a faster conditioning speed and longer conditioning time gave better flotation results.
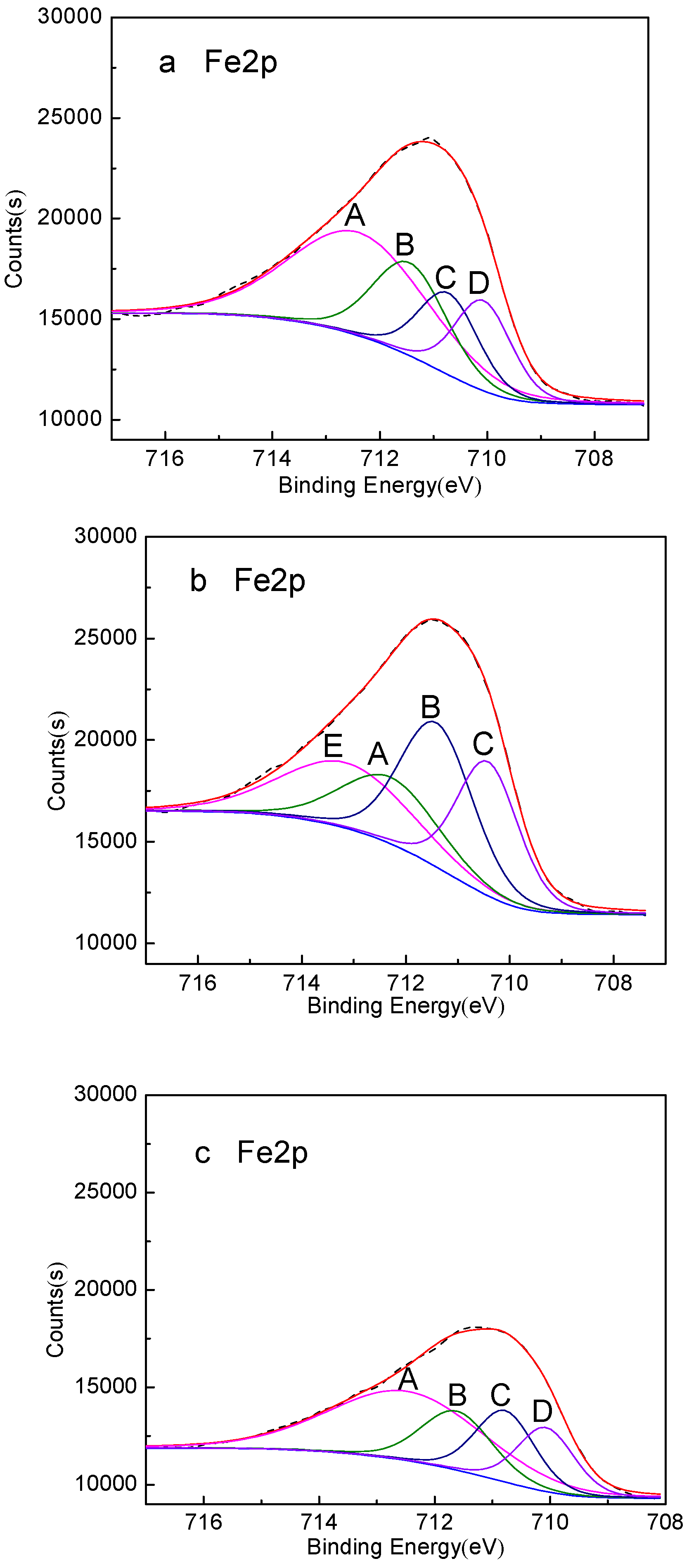
- XPS analysis shows that when mixed minerals were conditioned under the natural atmosphere, a faster conditioning speed and longer conditioning time accelerates the pyrrhotite surface oxidization, which results in a decrease in pyrrhotite flotation recovery.
- The industrial tests of nickel sulfide ores under nitrogen atmosphere have not been performed, which will be the focus of the following study.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fornasiero, D.; Ralston, J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite. Int. J. Miner. Process. 2005, 76, 75–81. [Google Scholar] [CrossRef]
- Peng, Y.; Bradshaw, D. Mechanisms for the improved flotation of ultrafine pentlandite and its separation from lizardite in saline water. Miner. Eng. 2012, 36–38, 284–290. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.; Lu, Y. The effect of lizardite surface characteristics on pyrite flotation. Appl. Surf. Sci. 2012, 259, 153–158. [Google Scholar] [CrossRef]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Senior, G.D.; Trahar, W.J. The influence of metal hydroxides and collector on the flotation of chalcopyrite. Int. J. Miner. Process. 1991, 33, 321–341. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.; Cai, M.; Li, Y. A review on pyrrhotite oxidation. J. Geochem. Explor. 2004, 84, 65–76. [Google Scholar] [CrossRef]
- Allison, S.A.; O’Connor, C.T. An investigation into the flotation behaviour of pyrrhotite. Int. J. Miner. Process. 2011, 98, 202–207. [Google Scholar] [CrossRef]
- Engel, M.D.; Middlebrook, P.D.; Jameson, G.J. Advances in the study of high intensity conditioning as a means of improving mineral flotation performance. Miner. Eng. 1997, 10, 55–68. [Google Scholar] [CrossRef]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. The effect of high intensity conditioning on the flotation of a nickel ore. Part 1: Size-by-size analysis. Miner. Eng. 1999, 12, 1185–1200. [Google Scholar] [CrossRef]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. The effect of high intensity conditioning on the flotation of a nickel ore, part 2: Mechanisms. Miner. Eng. 1999, 12, 1359–1373. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Studies on carbon flotation from fly ash. Fuel Process. Technol. 2015, 139, 236–241. [Google Scholar] [CrossRef]
- Bulatovic, S.M.; Salter, R.S. High-intensity conditioning—A new approach to improving flotation of mineral slimes. In Proceedings of the Complex Ores. Mineral Processing and Extractive Metallurgy, Halifax, NS, Canada, 20–24 August 1989. [Google Scholar]
- Bulatovic, S.M.; Wyslouzil, D.M. Research and development in selective froth flotation of mineral fines from polymetallic refractory sulfide ores. In Proceedings of the Second International Conference on Mineral Processing and Extractive Metallurgy, Beijing, China, 23–27 July 1992. [Google Scholar]
- Feng, B.; Feng, Q.; Lu, Y.; Lv, P. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Miner. Eng. 2012, 39, 48–50. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.; Lu, Y. Mechanism of hetero-aggregation of chlorite and pyrite. J. Cent. South Univ. 2015, 46, 14–19. [Google Scholar]
- Tsai, C.; Pui, D.Y.H.; Liu, B.Y.H. Particle detachment from disk surfaces of computer disk drives. J. Aerosol Sci. 1991, 22, 737–746. [Google Scholar] [CrossRef]
- Phares, D.J.; Smedley, G.T.; Flagan, R.C. Effect of particle size and material properties on aerodynamic resuspension from surfaces. J. Aerosol Sci. 2000, 31, 1335–1353. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Aguado, A.; Chinchón, S. A comparative investigation of the degradation of pyrite and pyrrhotite under simulated laboratory conditions. Eng. Geol. 2012, 127, 75–80. [Google Scholar] [CrossRef]
- Becker, M.; Villiers, J.D.; Bradshaw, D. The flotation of magnetic and non-magnetic pyrrhotite from selected nickel ore deposits. Miner. Eng. 2010, 23, 1045–1052. [Google Scholar] [CrossRef]
- Chiriţă, P.; Descostes, M.; Schlegel, M.L. Oxidation of FeS by oxygen-bearing acidic solutions. J. Colloid Interface Sci. 2008, 321, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite reaction kinetics: Reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim. Cosmochim. Acta 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Miller, J.D.; Li, J.; Davidtz, J.C.; Vos, F. A review of pyrrhotite flotation chemistry in the processing of PGM ores. Miner. Eng. 2005, 18, 855–865. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Dzhurinskii, B.F.; Gati, D.; Sergushin, N.P. An X-ray photoelectron spectroscopic study of certain oxides. Russ. J. Inorg. Chem. 1975, 20, 2307–2314. [Google Scholar]
- Liu, X.; Huang, G.; Li, C.; Cheng, R. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. Chemischer Informationsdienst. 1978, 9, 1801–1810. [Google Scholar] [CrossRef]
- Bunkholt, I.; Kleiv, R.A. Pyrrhotite oxidation and its influence on alkaline amine flotation. Miner. Eng. 2015, 71, 65–72. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Becker, M.; Tekes, E.B.; Bradshaw, D. The relationship between the electrochemical, mineralogical and flotation characteristics of pyrrhotite samples from different Ni Ores. J. Electroanal. Chem. 2010, 647, 133–143. [Google Scholar] [CrossRef]
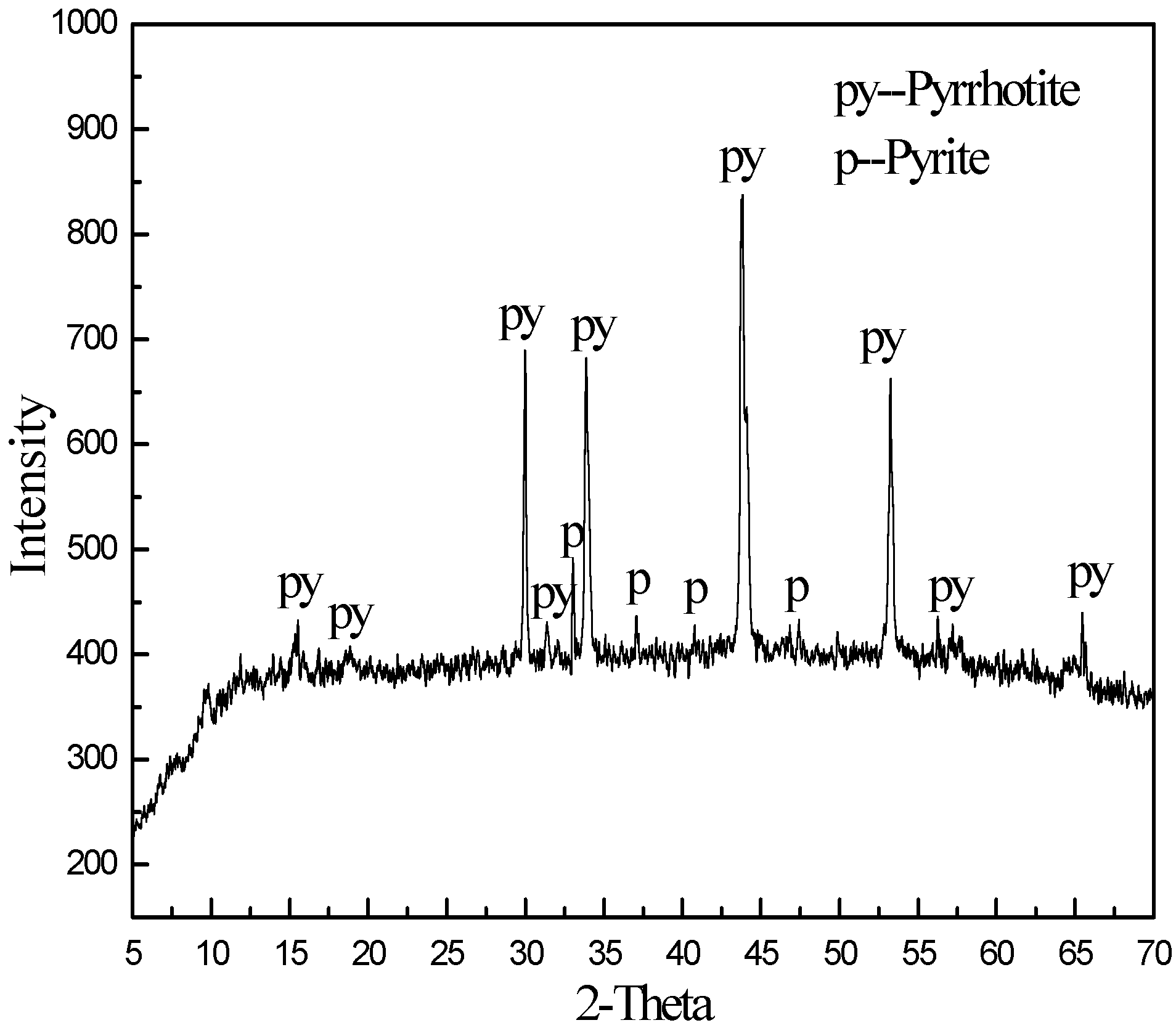
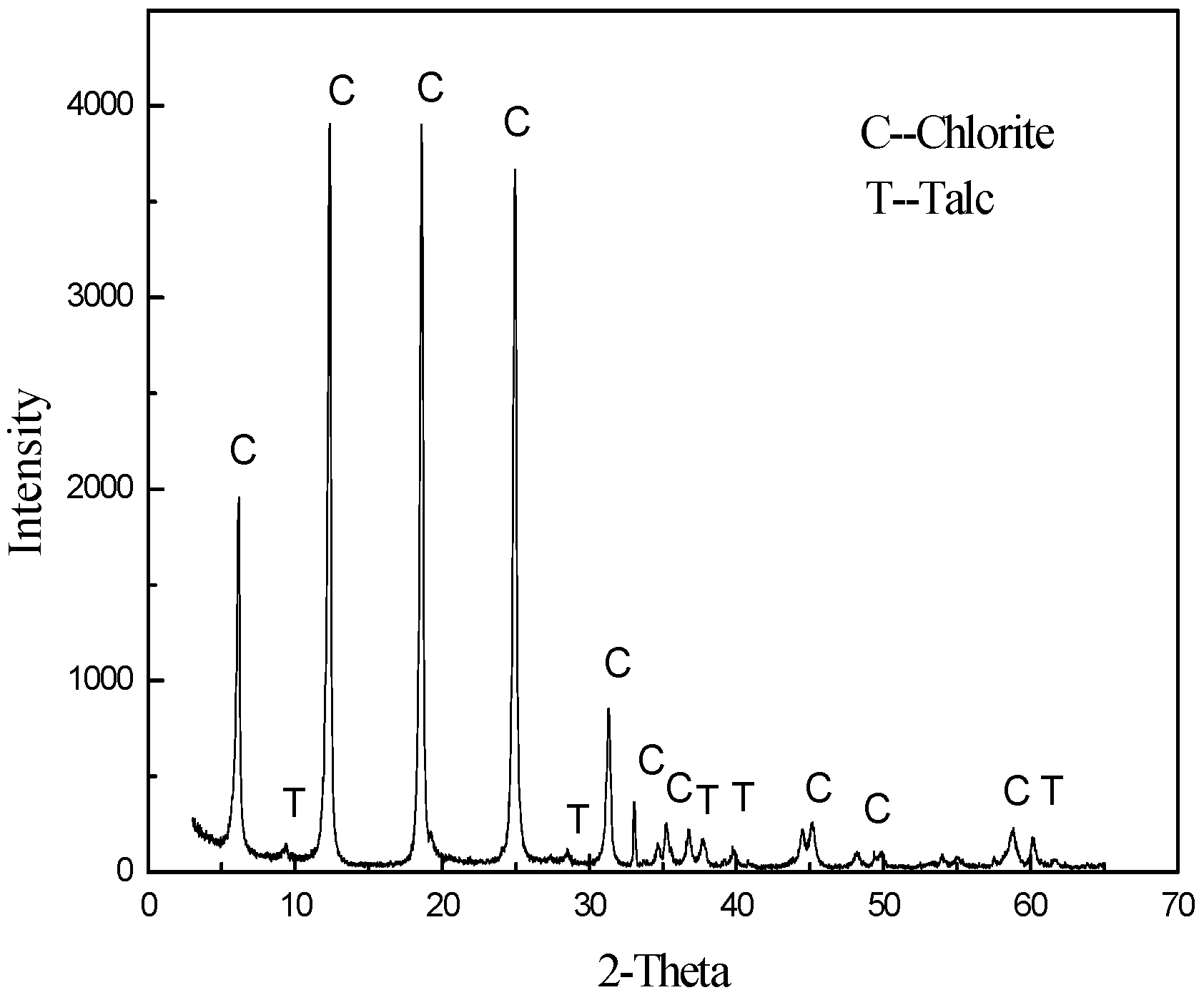
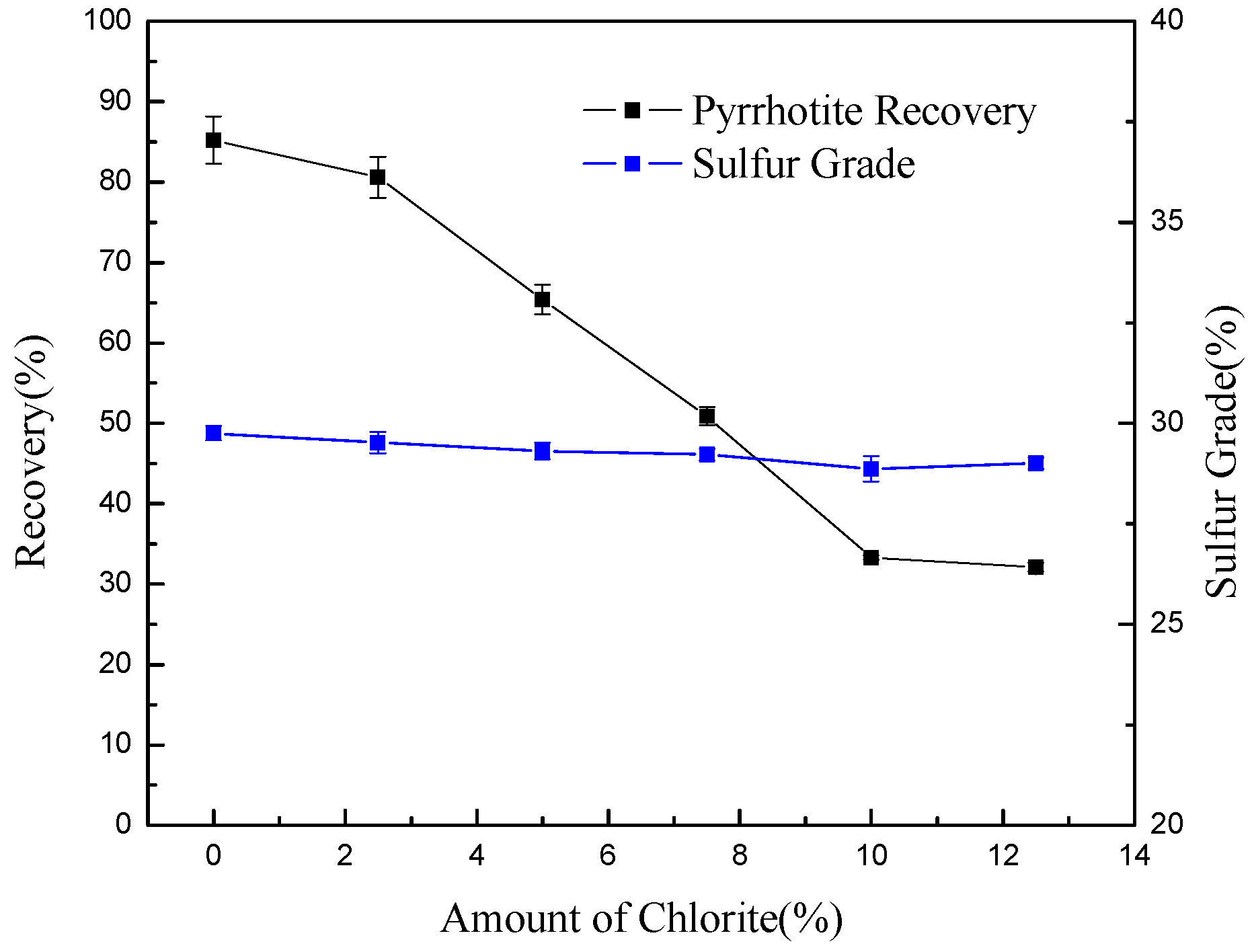
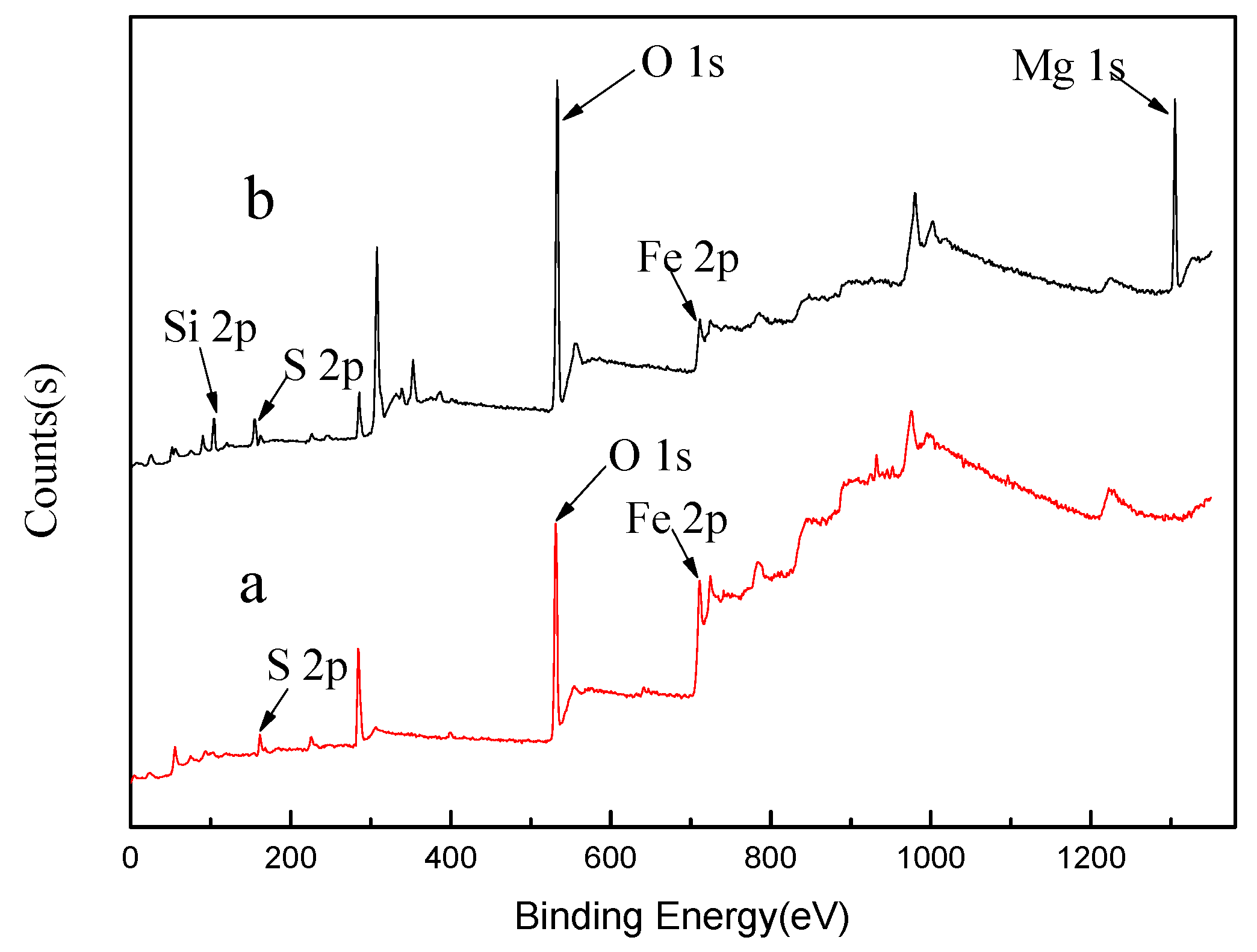


| Composition | MgO | SiO2 | Al2O3 | CaO | TFe | Other |
|---|---|---|---|---|---|---|
| Percentage/% | 25.41 | 34.37 | 15.76 | 0.78 | 3.72 | 19.96 |
| Peak | Fe Species | Without Conditioning | Conditioning under Natural Atmosphere for 20 min | Conditioning under Nitrogen Atmosphere for 20 min | |||
|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | Relative Contents (%) | Binding Energy (eV) | Relative Contents (%) | Binding Energy (eV) | Relative Contents (%) | ||
| A | Fe2(Sx)3 | 712.40 | 45.83 | 712.30 | 19.09 | 712.48 | 47.05 |
| B | FeOOH | 711.45 | 21.95 | 711.41 | 31.20 | 711.62 | 18.37 |
| C | Fe2O3 | 710.72 | 15.78 | 710.42 | 24.43 | 710.78 | 18.62 |
| D | FeS | 710.07 | 16.44 | – | – | 710.07 | 15.96 |
| E | Fe2(SO4)3 | – | – | 713.15 | 25.28 | – | – |
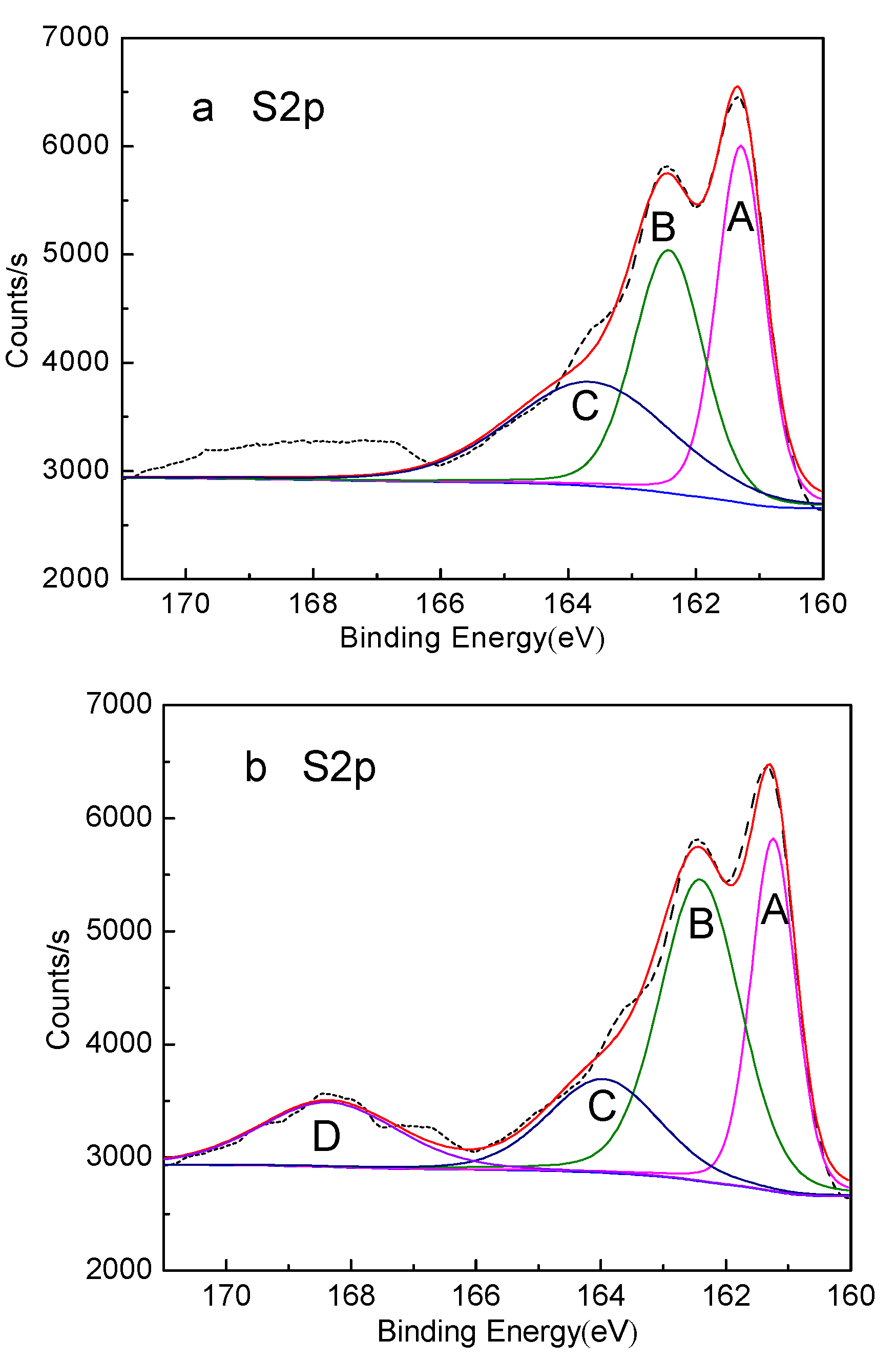
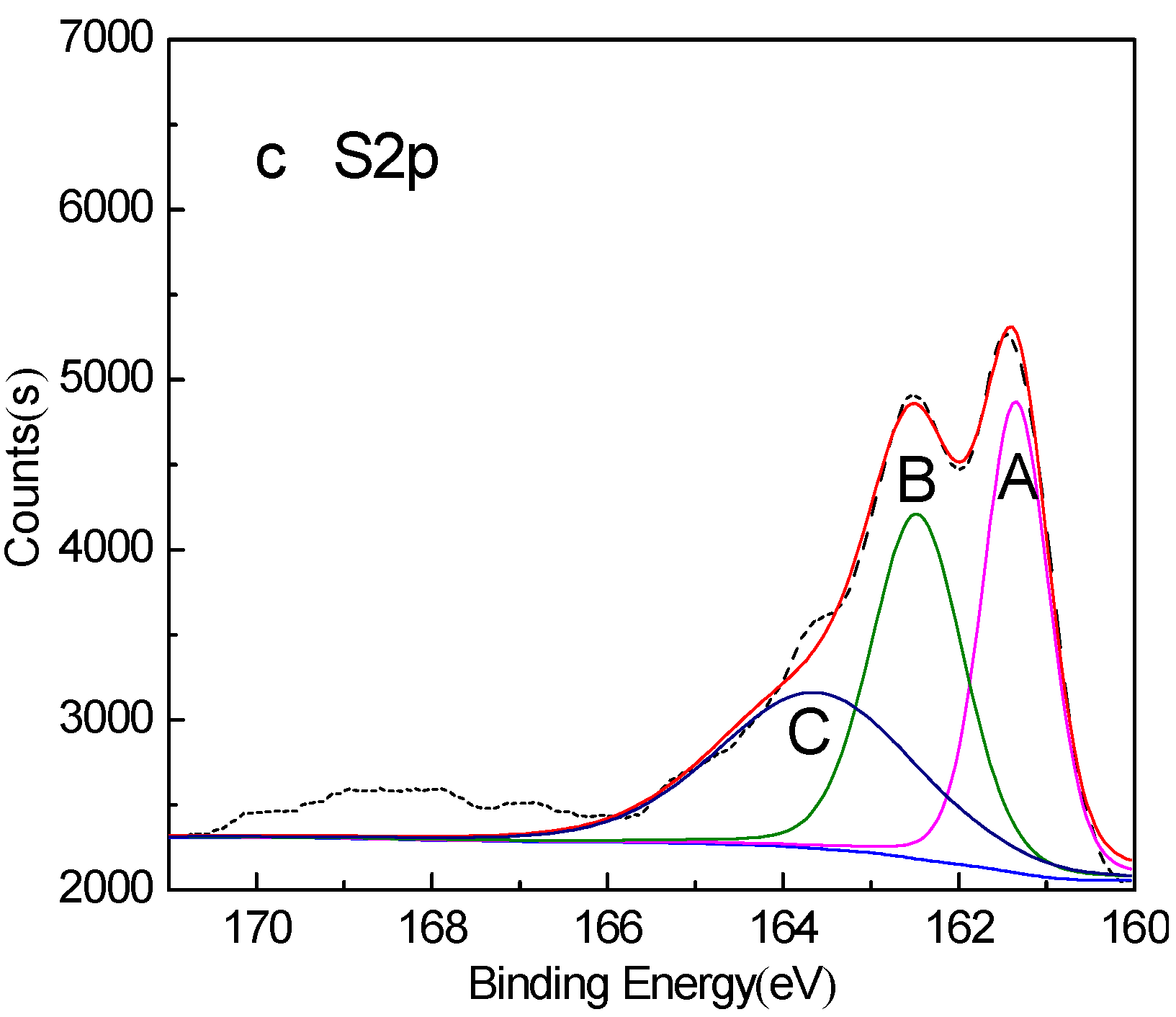
| Peak | S Species | Without Conditioning | Conditioning under Natural Atmosphere for 20min | Conditioning under Nitrogen Atmosphere for 20min | |||
|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | Relative Contents (%) | Binding Energy (eV) | Relative Contents (%) | Binding Energy (eV) | Relative Contents (%) | ||
| A | FeS | 161.29 | 33.80 | 161.24 | 27.15 | 161.35 | 33.30 |
| B | Fe2(Sx)3 | 162.42 | 33.44 | 162.41 | 33.49 | 162.47 | 33.92 |
| C | S0 | 163.65 | 32.76 | 163.96 | 20.98 | 163.62 | 32.78 |
| D | Fe2(SO4)3 | – | – | 168.36 | 15.38 | – | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shi, Q.; Feng, Q.; Lu, Y.; Zhang, W. The Effect of Conditioning on the Flotation of Pyrrhotite in the Presence of Chlorite. Minerals 2017, 7, 125. https://doi.org/10.3390/min7070125
Chen Y, Shi Q, Feng Q, Lu Y, Zhang W. The Effect of Conditioning on the Flotation of Pyrrhotite in the Presence of Chlorite. Minerals. 2017; 7(7):125. https://doi.org/10.3390/min7070125
Chicago/Turabian StyleChen, Yanfei, Qing Shi, Qiming Feng, Yiping Lu, and Wencai Zhang. 2017. "The Effect of Conditioning on the Flotation of Pyrrhotite in the Presence of Chlorite" Minerals 7, no. 7: 125. https://doi.org/10.3390/min7070125




