A New Zincian Greenockite Occurrence in the Saishitang Cu Skarn Deposit, Qinghai Province, Northwest China
Abstract
:1. Introduction
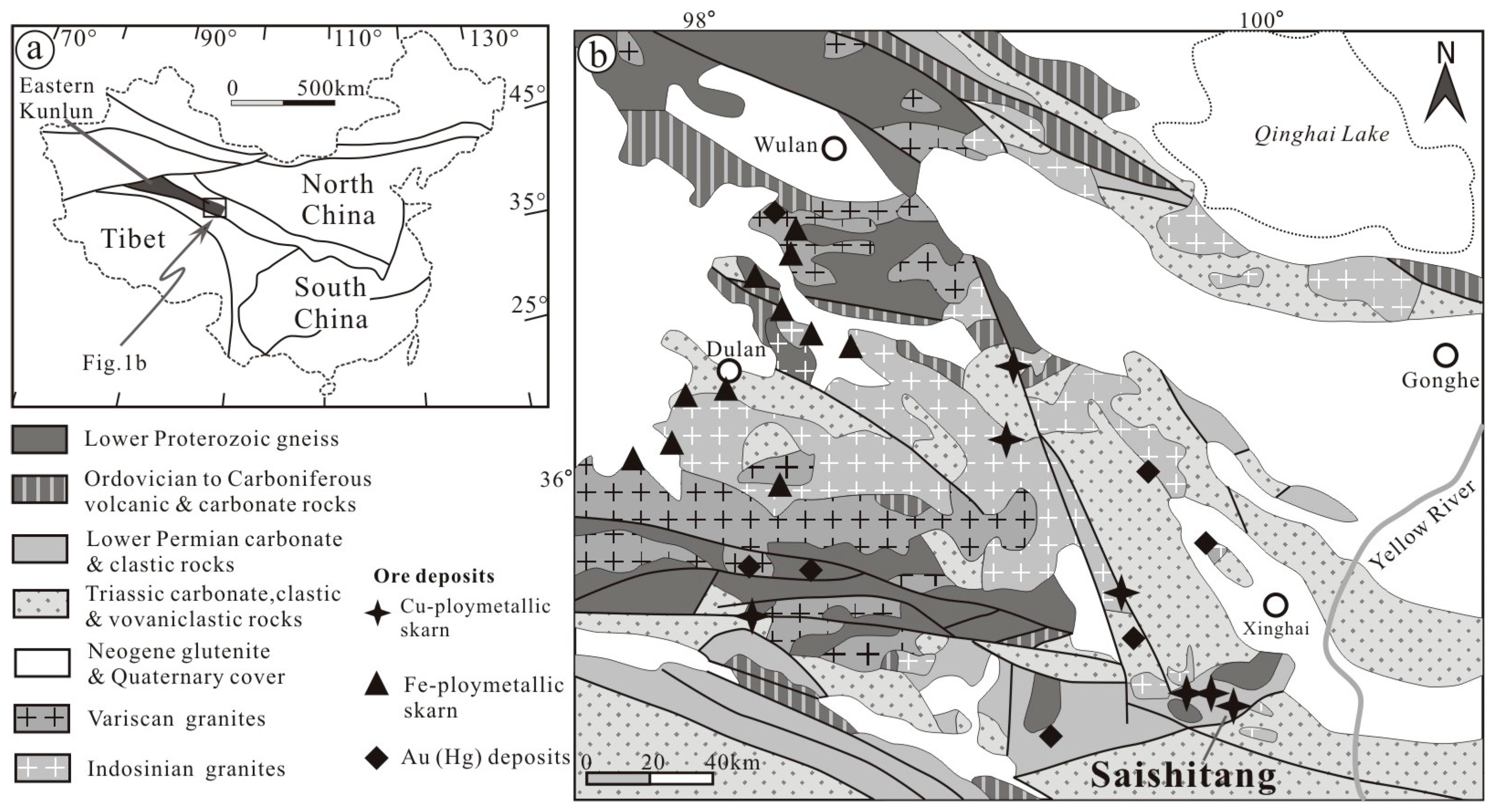
2. Geological Setting
2.1. Regional Geology of the Elashan Region
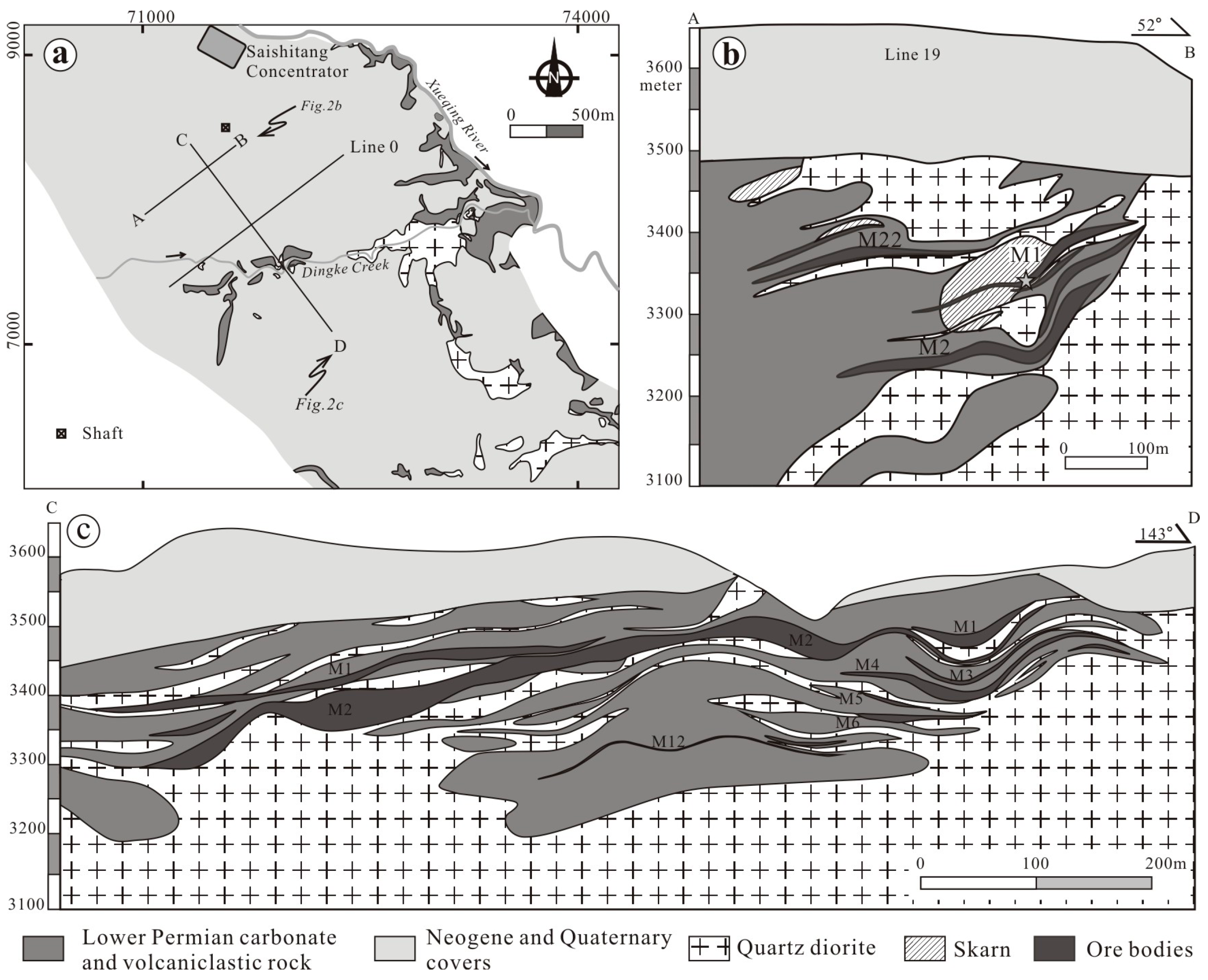
2.2. Geology of the Saishitang Cu Deposit
2.2.1. Stratigraphy
2.2.2. Intrusive Rocks
2.2.3. Orebodies
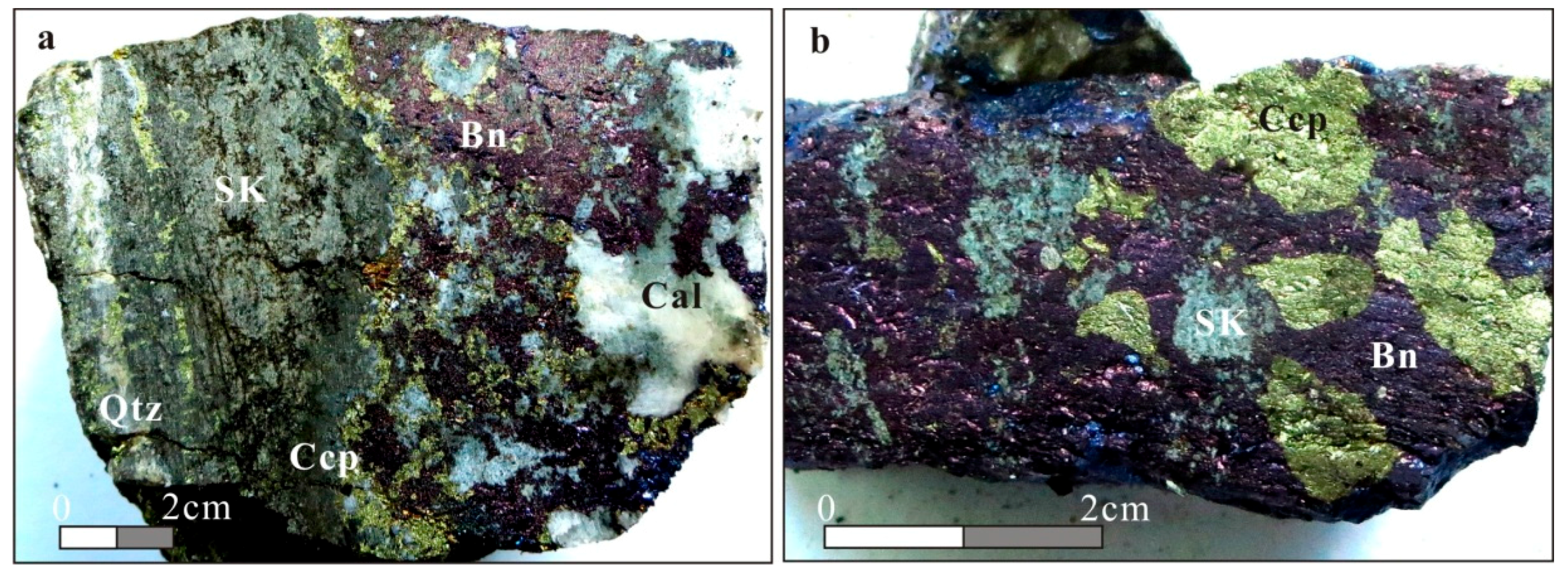
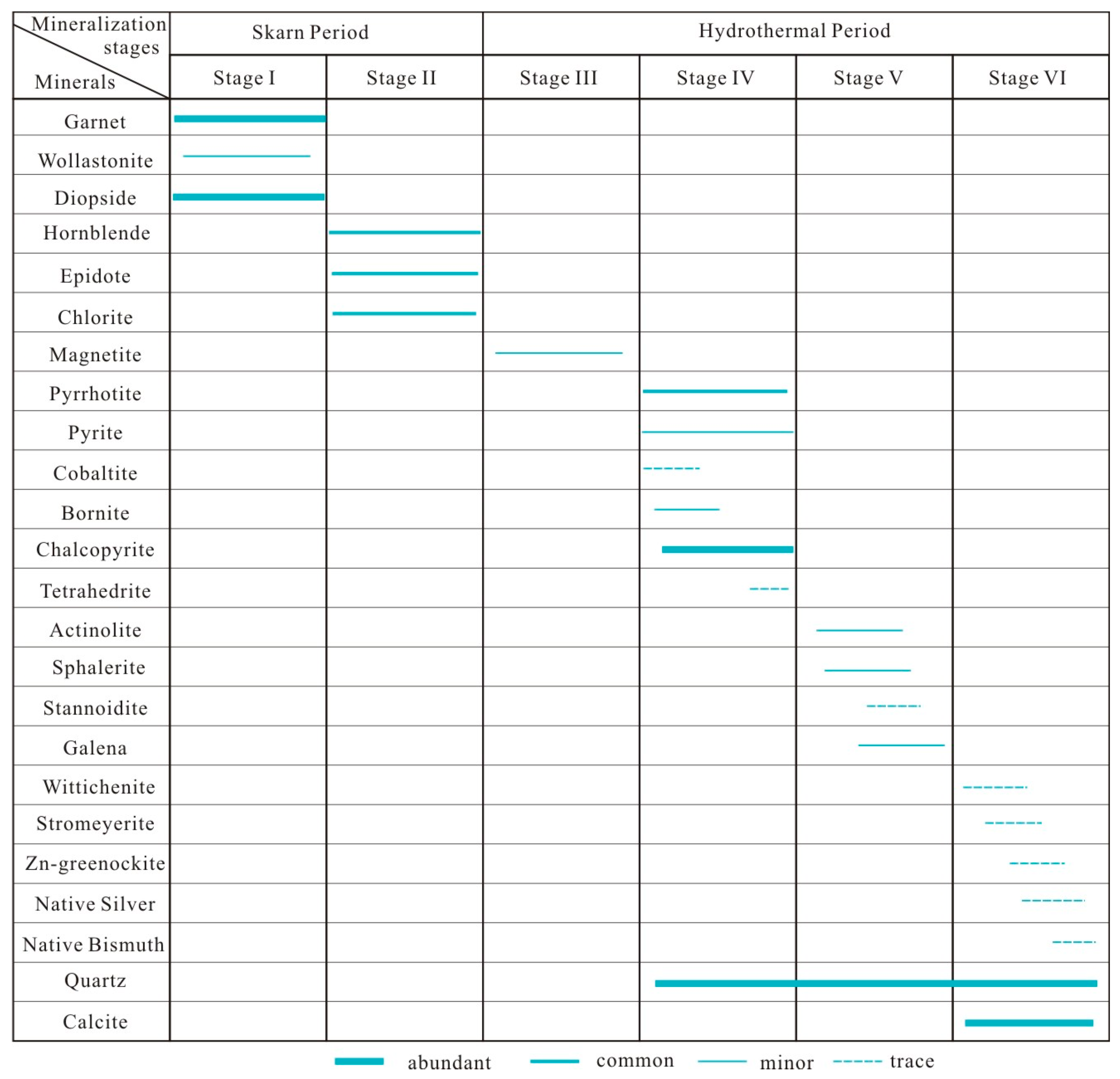
2.2.4. Ore Types and Mineral Paragenetic Sequence
3. Sampling and Analytical Methods
3.1. Sampling Site
3.2. Electron-Probe Microanalyses
3.3. X-ray Diffraction Analysis
4. Results
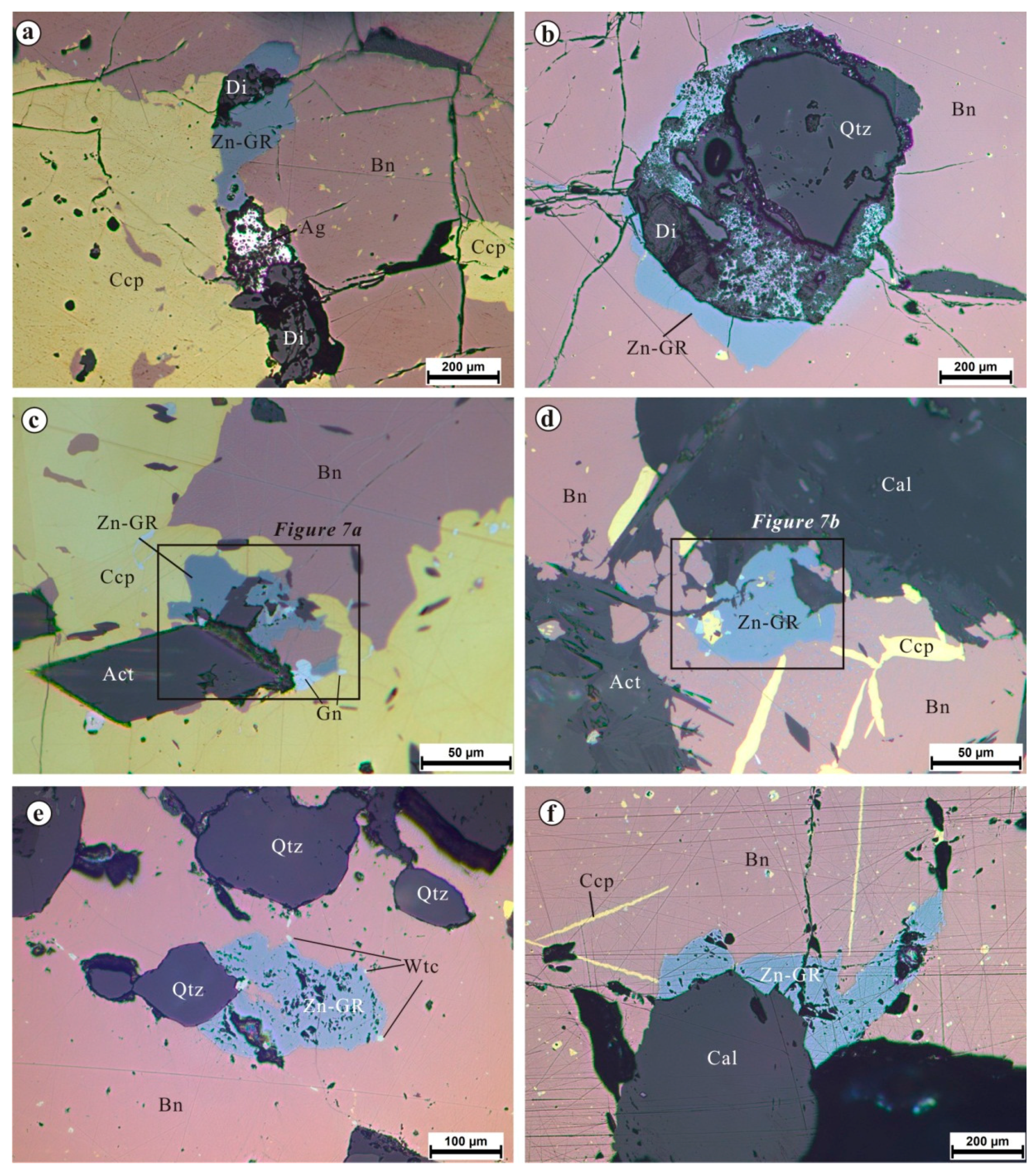
4.1. Optical Properties and Textural Relations for Zincian Greenockite
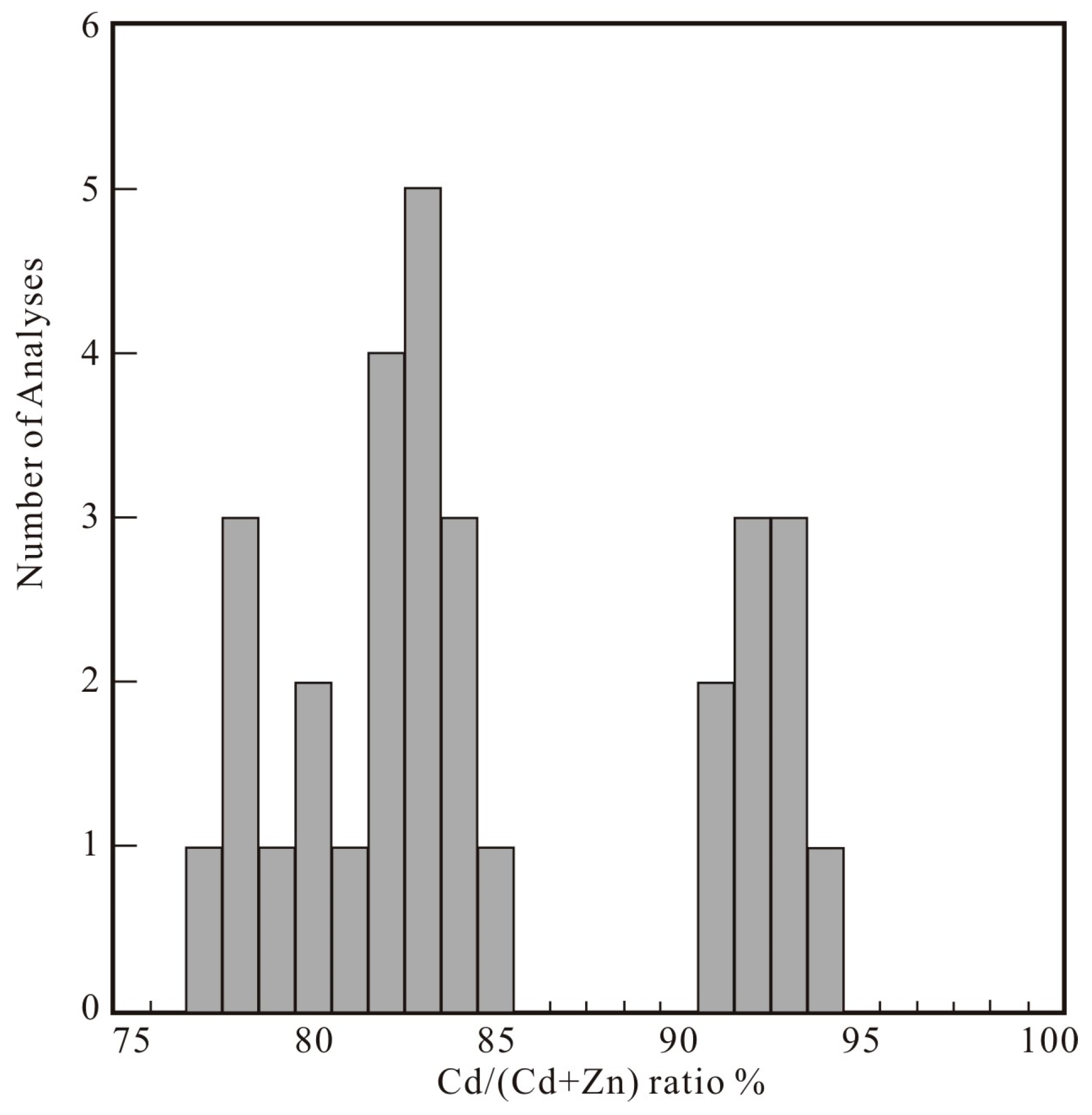
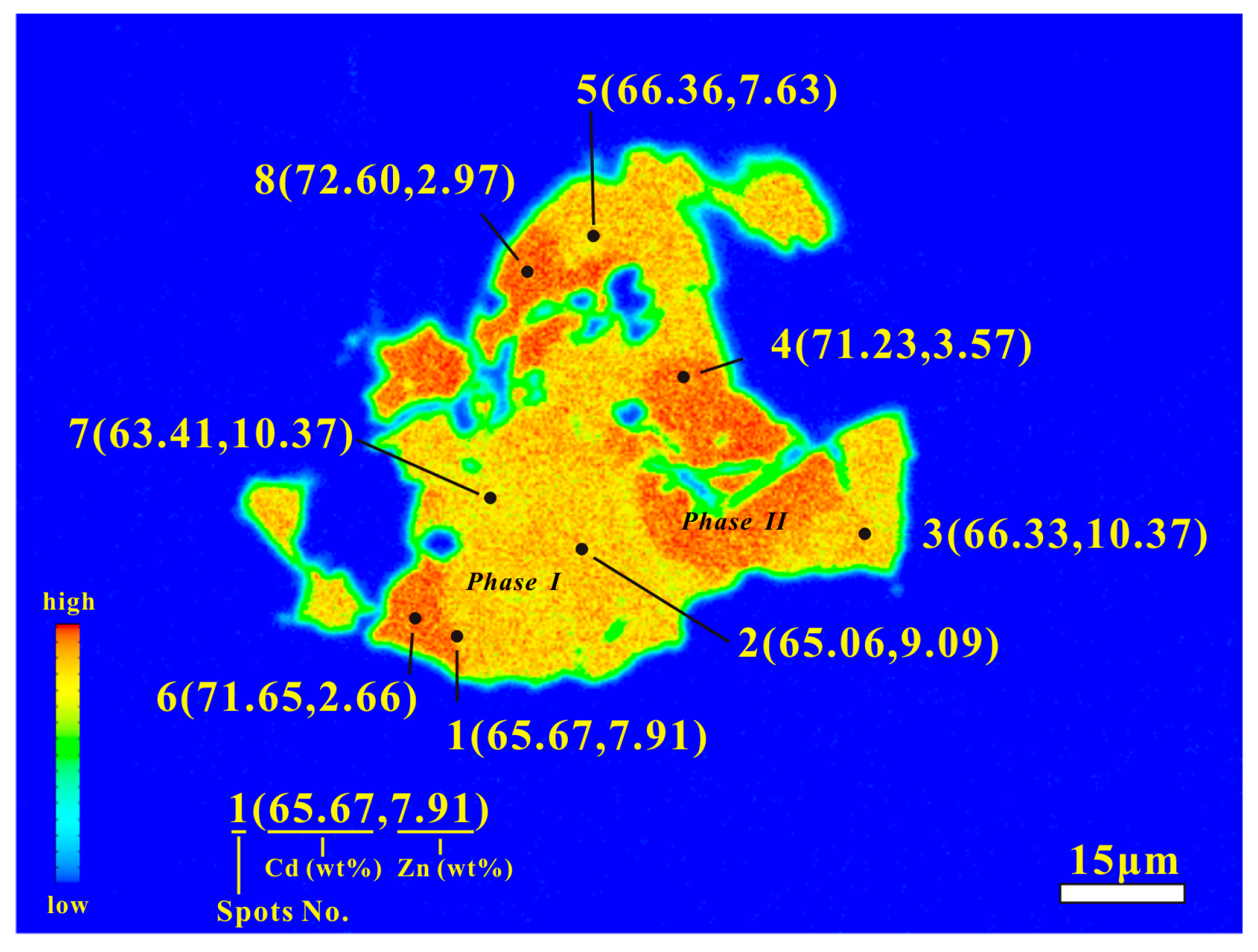
4.2. Composition of Zincian Greenockite and Associated Minerals
4.2.1. Zincian Greenockite
4.2.2. Associated Minerals
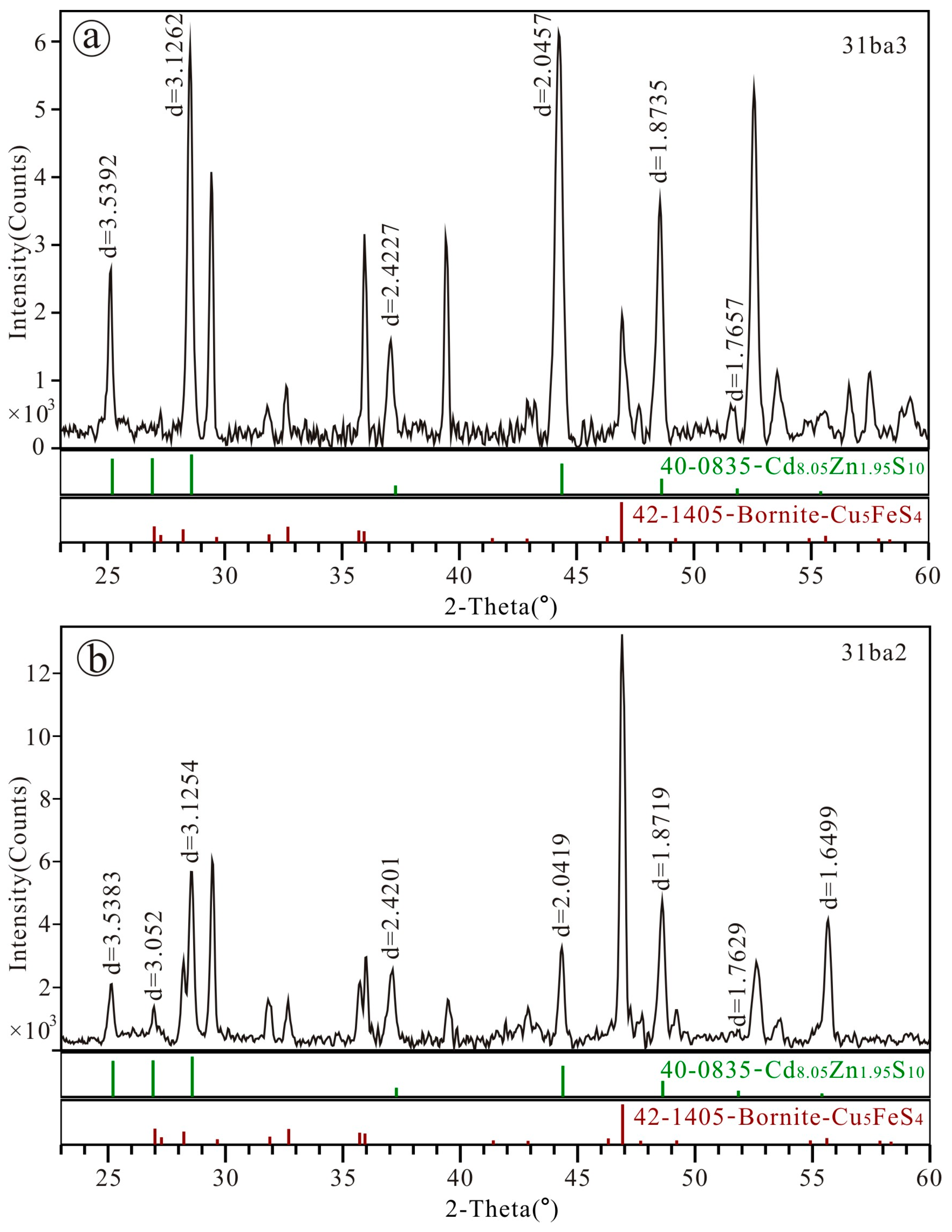
4.3. XRD Data of Zinc Greenockite
5. Discussion
5.1. Factors Influencing Composition of Zincian Greenockite
5.2. Zincian Greenockite Formed during the Skarn Mineraization Processes
6. Conclusions
- (1)
- The zincian greenockite of the Saishitang skarn deposit occurs in the chalcopyrite-bornite ores and was formed during the sulfide-sulfosalt stage of the mineralization process. The chemical composition shows that it contains two phases (I and II). Phase I contains high Zn (14.6 to 21.7 mol % ZnS) and low Cd (72.4 to 82.2 mol % CdS), while Phase II contains low Zn (5.6 to 9.1 mol % ZnS) and high Cd (85.4 to 89.9 mol % CdS). The XRD data indicate that the zincian is hexagonal.
- (2)
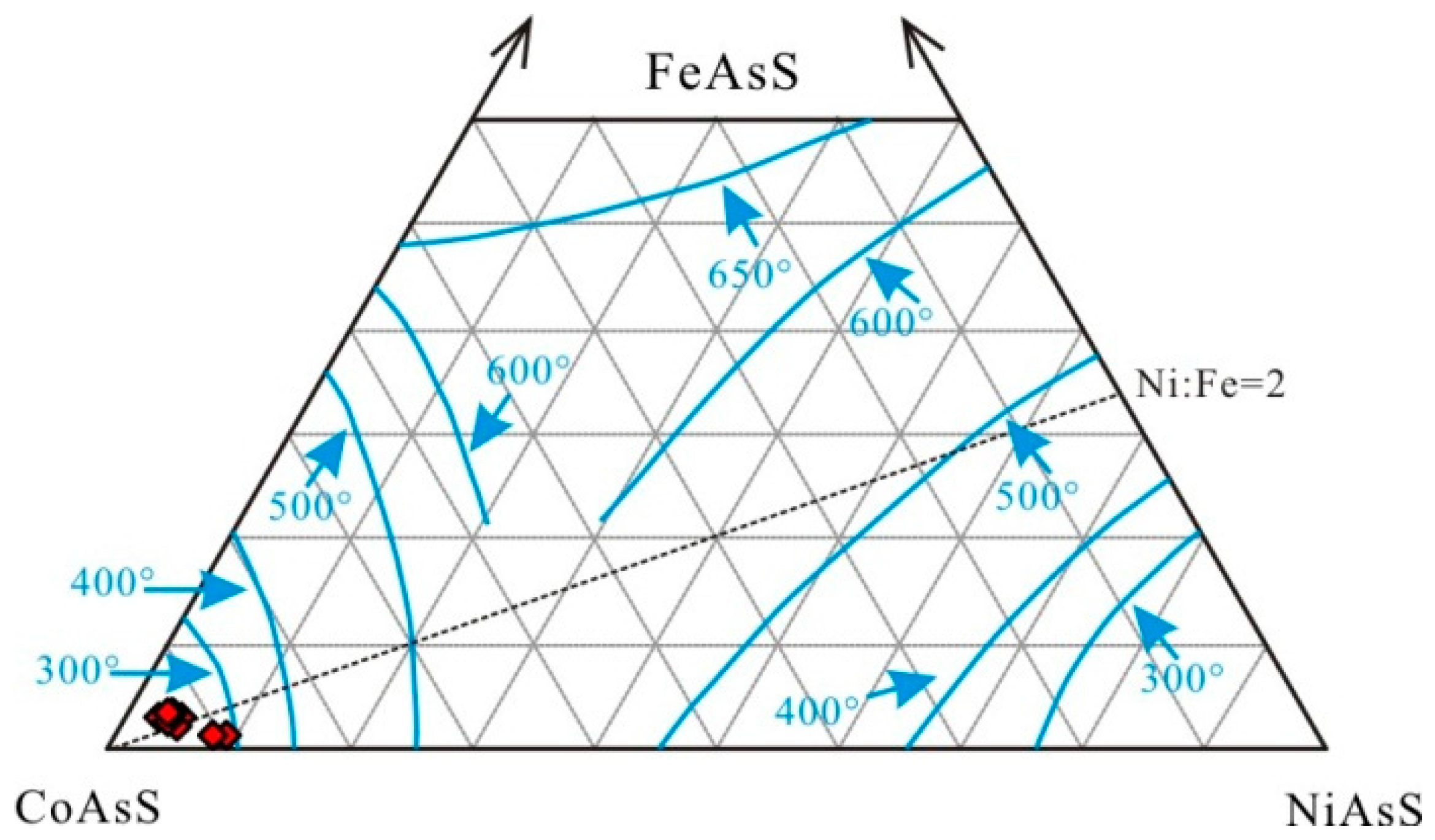
- Zincian greenockite was formed at temperatures of 300–270 °C in a reducing to oxidizing environment during the late mineralization stage of the Saishitang skarn Cu deposit.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.W.; Zhang, J.M.; Ardell, A.J.; Dunn, B. Solid state phase equilibria in the ZnS-CdS system. Mater. Res. Bull. 1988, 23, 1667–1673. [Google Scholar] [CrossRef]
- Chaplygin, I.V.; Mozgova, N.N.; Mokhov, A.V.; Koporulina, E.V.; Bernhardt, H.J.; Bryzgalov, I.A. Minerals of the system ZnS-CdS from fumaroles of the Kudriavy volcano, Iturup island, Kuriles, Russia. Can. Mineral. 2007, 45, 709–722. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L. Paragenesis of Cu-Fe ores from Ocna de Fier-Dognecea (Romania), typifying fluid plume mineralization in a proximal skarn setting. Mineral. Mag. 2001, 65, 351–372. [Google Scholar] [CrossRef]
- Tombros, S.; Seymour, K.S.; Spry, P.G.; Williams-Jones, A. Greenockite and zincian greenockite in epithermal polymetallic Ag-Au-Te mineralization, Tinos Island, Hellas: Description and conditions of formation. Neues Jahrb. Mineral. Abh. 2005, 182, 1–9. [Google Scholar] [CrossRef]
- Skinner, B.J.; Bethke, P.M. The relationship between unit-cell edges and compositions of synthetic wurtzites. Am. Mineral. 1961, 46, 1382–1398. [Google Scholar]
- Cherin, P.; Lind, E.L.; Davis, E.A. The preparation and crystallography of cadmium zinc sulfide solid solutions. J. Electrochem. Soc. 1970, 117, 233–236. [Google Scholar] [CrossRef]
- Tauson, V.L.; Chernyshev, L.V. Phase relationships and structural features of the ZnS-CdS mixed crystals. Geochem. Int. 1977, 44, 11–22. [Google Scholar]
- Osadchii, E.G. Solid solutions and phase relations in the system Cu2SnS3-ZnS-CdS at 850 °C and 700 °C. Neues Jahrb. Mineral. Abh. 1986, 155, 23–38. [Google Scholar]
- Osadchii, E.G. The kësterite–černýite and sphalerite-greenockite solid-solutions in the system Cu2SnS3-ZnS-CdS at 400 °C and 101.3 MPa. Neues Jahrb. Miner. Mon. 1991, 10, 457–463. [Google Scholar]
- Osadchii, E.G.; Lunin, S. Determination of activity-composition relations in (FexZn1-x) solid solutions at 850–1020 K by solid state EMF measurements. Exper. Geosci. 1994, 4, 48–50. [Google Scholar]
- Mogessie, A.; Gallien, F.; Bernhard, F.; Bauer, C.; Castro De Machuca, B.; Meissl, E.; Bjerg, E.; Delpino, S. Greenockite and associated sulfide mineralization from the Caledonia Group mines, Blanca Creek, La Huerta Range, San Juan province, Argentina. Can. Mineral. 2009, 47, 129–141. [Google Scholar] [CrossRef]
- Mishra, B.; Hazarika, P. Rare greenockite (CdS) within the chromite-PGE association in the Bangur Gabbro, Baula-Nuasahi Complex, Eastern India. Ore Geol. Rev. 2016, 72, 1327–1334. [Google Scholar] [CrossRef]
- Černŷ, P.; Harris, D.C. The Tanco pegmatite at Bernic Lake, Manitoba. XI. Native elements, alloys, sulfides and sulfosalts. Can. Mineral. 1978, 16, 625–640. [Google Scholar]
- Butler, J.R.; Thompson, A.J. Cadmium and zinc in some alkali acidic rocks. Geochim. Cosmochim. Acta 1967, 31, 97–105. [Google Scholar] [CrossRef]
- Burianova, E.Z. On the mineralogy and geochemistry of cadmium in sedimentary rocks. Geokhimiya 1960, 2, 177–182. [Google Scholar]
- Davies, C.R.J.; Nelson, P.O.; Williamson, K.J. Sulfide control of cadmium and copper concentrations in anaerobic estuarine sediments. Mar. Chem. 1985, 16, 173–186. [Google Scholar] [CrossRef]
- Hurlbut, S.C.J. The wurtzite-greenockite series. Am. Mineral. 1957, 42, 184–190. [Google Scholar]
- Cornwall, H.B. Occurrence of greenockite on calcite from Joplin, Missouri. Am. J. Sci. 1902, 14, 7–8. [Google Scholar] [CrossRef]
- Patterson, D.J. Zincian greenockite in stratiform lead-zinc-silver mineralization at Lady Loretta, northwest Queensland. Can. Mineral. 1985, 23, 89–94. [Google Scholar]
- Oen, I.S.; Kager, P.; Kieft, C. Hawleyite and greenockite in ores from Los Blancos, Sierra de Cartagena, Spain. Neues Jahrb. Miner. Mon. 1974, 507–513. [Google Scholar]
- Tarkian, M.; Breskovska, V. Greenockite from the Madjarovo Pb-Zn ore district, eastern Rhodope, Bulgaria. Mineral. Petrol. 1989, 40, 137–144. [Google Scholar] [CrossRef]
- Iizasa, K.; Yuasa, M.; Yokota, S. Mineralogy and geochemistry of volcanogenic sulfides from the Myojinsho submarine caldera, the Shichito-Iwojima Ridge, Izu-Ogasawara Arc, northwestern Pacific. Mar. Geol. 1992, 108, 39–58. [Google Scholar] [CrossRef]
- Golding, L.Y. Mineralogy, Geochemistry and Origin of the Kalgoorlie Gold Deposits, Western Australia. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 1978. [Google Scholar]
- Varas, G.; Menzies, A.; Peralta, A.; Sola, S.; Pizarro, H.; Sola, S.; Barraza, M. A rare occurrence of betekhtinite [Cu10(Fe,Pb)S6] and greenockite [CdS] at La Platina deposit, Chile. In Proceedings of the XIV Congreso Geológico Chileno, La Serena, Chile, 4–8 October 2015. [Google Scholar]
- Fu, C.; Xiao, H.; Lang, K. Geological Report on the Prospecting and Exploration of the Saishitang Deposit and Surrounding Area in Xinghai County, Qinghai Province; The 3rd Geological Team of Qinghai Province: Xining, China, 1993. (In Chinese) [Google Scholar]
- Liu, J.P.; Gu, X.P.; Shao, Y.J.; Feng, Y.Z.; Lai, J.Q. Indium mineralization in copper-tin stratiform skarn ores at the Saishitang-Rilonggou Ore Field, Qinghai, Northwest China. Resour. Geol. 2016, 66, 351–367. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Song, Z.; Su, Z.; Liu, R.; Li, W. The Ngola Shan Region Paleo-Thermal Mineralization Pattern; Xi’an Jiaotong University Press: Xi’an, China, 1993. (In Chinese) [Google Scholar]
- Wang, H.; Feng, C.Y.; Li, D.X.; Li, C.; Ding, T.Z.; Liao, F.Z. Geology, geochronology and geochemistry of the Saishitang Cu deposit, East Kunlun Mountains, NW China: Constraints on ore genesis and tectonic setting. Ore Geol. Rev. 2016, 72, 43–59. [Google Scholar] [CrossRef]
- Lu, Y.C.; Liu, J.J.; Zhang, D.; Carranza, E.J.M.; Zhai, D.G.; Ge, L.S.; Sun, H.; Wang, B.; Chen, Y.F.; Liu, P. Genesis of the Saishitang skarn type copper deposit, west Qinling, Qinghai Province: Evidence from fluid inclusions and stable isotopes. Ore Geol. Rev. 2016, 75, 268–283. [Google Scholar] [CrossRef]
- Mo, X.X.; Luo, Z.H.; Deng, J.F.; Yu, X.H.; Liu, C.D.; Chen, H.W.; Yuan, W.M.; Liu, Y.H. Granitoids and crustal growth in the east Kunlun orogenic belt. Geosci. J. China Univ. 2007, 13, 403–414. (In Chinese) [Google Scholar]
- Dai, J.G.; Wang, C.S.; Hourigan, J.; Santosh, M. Multi-stage tectono-magmatic events of the Eastern Kunlun Range, northern Tibet: Insights from U-Pb geochronology and (U-Th)/He thermochronology. Tectonophysics 2013, 599, 97–106. [Google Scholar] [CrossRef]
- Wang, B.Z.; Chen, J.; Luo, Z.H.; Chen, F.B.; Wang, T.; Guo, G.E. Spatial and temporal distribution of Late Permian-Early Jurassic intrusion assemblages in eastern Qimantag, East Kunlun, and their tectonic settings. Acta Petrol. Sin. 2014, 30, 3213–3228. (In Chinese) [Google Scholar]
- Ma, L.F. The Geological Atlas of China; Geological Publishing House: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Li, W.L.; Xia, R.; Qing, M.; Li, C.; Zhang, D.; Sun, H.; Lu, Y.C.; Liu, P.; Zhou, A.B. Re-Os molybdenite ages of the Shenduolong skarn Mo-Pb-Zn deposit and geodynamic framework, Qinghai Province. Rock Miner. Anal. 2014, 33, 900–907. (In Chinese) [Google Scholar]
- Song, Z.; Zhang, H.; Li, W.; Zhang, X.; Wang, W. Metallogenic conditions and model of copper multi-metal deposits in the Ngola Shan region. Qinghai Province. Northwest Geosci. 1995, 16, 134–144. (In Chinese) [Google Scholar]
- Gu, X.P.; Xie, X.D.; Wu, X.B.; Zhu, G.C.; Lai, J.Q.; Hoshino, K.; Huang, J.W. Ferrisepiolite: A new mineral from Saishitang copper skarn deposit in Xinghai County, Qinghai Province, China. Eur. J. Mineral. 2013, 25, 177–186. [Google Scholar]
- Lai, J.Q.; Ju, P.J.; Mao, Y.; An, J.H.; Wang, X.J. Ore-forming fluids characteristics of the Saishitang Cu-polymetallic deposit in Qinghai, China. Acta Geol. Sin. 2015, 89, 485–504. [Google Scholar]
- Qiu, F.; Dong, J. Characters of magma and its mineralization in the Saishitang copper deposit in Qinghai Province. Qinghai Geol. 1978, 1, 1–19. (In Chinese) [Google Scholar]
- Liu, J.P.; Lai, J.Q.; Gu, X.P.; Wang, X.J.; Mao, Y.; Song, W.B. Geochemistry and zircon LA-ICPMS U-Pb geochronology of intrusive body in Saishitang copper deposit, Qinghai Province, China. Chin. J. Nonferrous Met. 2012, 22, 622–632. (In Chinese) [Google Scholar]
- Liu, Z.; Jiang, W.; Deng, A. Geological Report on the Preliminary Exploration of the Saishitang Cu Deposit in Xinghai County, Qinghai Province; The 3rd Geological Team of Qinghai Province: Xining, China, 1983. (In Chinese) [Google Scholar]
- Wang, H.; Feng, C.Y.; Li, D.X.; Ding, T.Z.; Wang, H.Q.; Liu, J.N.; Zhou, J.H. Mineralogical characteristics and geological significance of skarn in the Saishitang copper deposit, Xinghai county, Qinghai Province. Acta Geosci. Sin. 2015, 36, 313–322. (In Chinese) [Google Scholar]
- Wright, K.; Gale, J. Interatomic potentials for simulation of the zinc blende and wurtzite forms of ZnS and CdS: Bulk structure, properties, and phase stability. Phys. Rev. B 2004, 70, 35–41. [Google Scholar] [CrossRef]
- He, P.; Yan, G.S.; Zhu, X.Y.; Zhang, Z.Y.; Wang, Y.L.; Cheng, X.Y.; Li, Y.S.; Zhen, S.M.; Du, Z.Z.; Jia, D.L.; et al. Fluid inclusion study of the Saishitang Cu deposit in Qinghai. Geol. China 2015, 40, 580–593. (In Chinese) [Google Scholar]
- Klemm, D. Synthesen und Analysen in den Dreiecksdiagrammen FeAsS-CoAsS-NiAsS und FeS2-CoS2-NiS2. Neues Jahrb. Mineral. Abh. 1965, 103, 205–255. [Google Scholar]
- Tauson, V.L.; Chernyshov, L.V. Experimental Study on Crystallochemistry and Geochemistry of Zinc Sulfide; Nauka: Novosibirsk, Russia, 1981. (In Russian) [Google Scholar]


| Sample | 1k31a.5 | 1k31a.6 | k31b.5 | k31b.6 | k31b.15 | ||||||||||
| 1 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | e | d | c | h | f | c | 1 | |
| wt % | |||||||||||||||
| Cd | 64.82 | 68.4 | 64.9 | 68.27 | 64.78 | 66.89 | 67.04 | 63.51 | 62.53 | 63.31 | 70.08 | 71.71 | 70.85 | 65.5 | 65.67 |
| Zn | 8.41 | 7.08 | 9.29 | 7.29 | 9.54 | 7.93 | 7.67 | 10.26 | 10.88 | 10.31 | 3.48 | 3.01 | 3.46 | 7.59 | 7.91 |
| Cu | 1.28 | 0.06 | 0.44 | 0.12 | 0.20 | 0.14 | 0.20 | 0.34 | 1.92 | 1.48 | 2.39 | 0.69 | 1.83 | 0.61 | 2.07 |
| Fe | 0.26 | 0.07 | 0.16 | 0.12 | 0.13 | 0.17 | 0.09 | 0.20 | 0.76 | 0.44 | 0.43 | 0.53 | 0.48 | 0.17 | 0.38 |
| Mn | 0.01 | 0.06 | 0.06 | 0.09 | n/d | n/d | 0.03 | n/d | 0.02 | 0.06 | 0.04 | 0.09 | 0.03 | 0.07 | n/d |
| Ag | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0.04 | 0.05 | 0.08 | 0.01 | 0.07 | 0.03 | n/d |
| S | 24.4 | 24.43 | 24.33 | 24.18 | 24.32 | 24.52 | 24.42 | 24.63 | 24.69 | 24.48 | 23.63 | 23.65 | 23.20 | 24.12 | 24.51 |
| Total | 99.18 | 100.1 | 99.17 | 100.07 | 98.97 | 99.65 | 99.46 | 98.93 | 100.85 | 100.12 | 100.13 | 99.68 | 99.91 | 98.08 | 100.53 |
| apfu | |||||||||||||||
| Cd | 0.77 | 0.82 | 0.78 | 0.82 | 0.78 | 0.80 | 0.81 | 0.75 | 0.72 | 0.74 | 0.85 | 0.89 | 0.87 | 0.80 | 0.77 |
| Zn | 0.17 | 0.15 | 0.19 | 0.15 | 0.20 | 0.16 | 0.16 | 0.21 | 0.22 | 0.21 | 0.07 | 0.06 | 0.07 | 0.16 | 0.16 |
| Cu | 0.03 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.03 | 0.05 | 0.02 | 0.04 | 0.01 | 0.04 |
| Fe | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ag | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
| S | 1.02 | 1.03 | 1.02 | 1.02 | 1.02 | 1.03 | 1.03 | 1.03 | 1.00 | 1.01 | 1.01 | 1.02 | 1.00 | 1.03 | 1.01 |
| Cd/(Cd+Zn) | 0.82 | 0.85 | 0.8 | 0.84 | 0.8 | 0.83 | 0.84 | 0.78 | 0.77 | 0.78 | 0.92 | 0.93 | 0.92 | 0.83 | 0.83 |
| Sample | k31b.15 | k31b.16 | |||||||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| wt % | |||||||||||||||
| Cd | 65.06 | 66.33 | 71.23 | 66.36 | 71.65 | 63.41 | 72.6 | 71.05 | 65.86 | 66.62 | 63.98 | 71.15 | 72.66 | 64.85 | 65.64 |
| Zn | 9.09 | 7.57 | 3.57 | 7.63 | 2.66 | 10.37 | 2.97 | 4.10 | 8.17 | 8.51 | 9.83 | 4.29 | 3.00 | 8.32 | 8.02 |
| Cu | 0.94 | 1.97 | 0.72 | 0.76 | 2.36 | 0.92 | 0.91 | 1.29 | 0.55 | 0.55 | 0.62 | 0.68 | 1.16 | 1.85 | 1.86 |
| Fe | 0.18 | 0.38 | 0.11 | 0.30 | 0.36 | 0.23 | 0.28 | 0.32 | 0.27 | 0.21 | 0.46 | 0.32 | 0.26 | 0.76 | 0.46 |
| Mn | n/d | n/d | 0.03 | n/d | n/d | 0.02 | 0.07 | n/d | 0.01 | 0.03 | n/d | 0.01 | 0.02 | 0.01 | n/d |
| Ag | 0.07 | 0.04 | 0.04 | 0.01 | 0.04 | n/d | n/d | n/d | n/d | 0.03 | 0.07 | 0.02 | n/d | n/d | 0.08 |
| S | 24.13 | 23.9 | 22.71 | 24.29 | 23.57 | 25.05 | 23.76 | 23.83 | 24.25 | 24.07 | 24.57 | 23.08 | 23.19 | 24.64 | 24.72 |
| Total | 99.47 | 100.18 | 98.39 | 99.35 | 100.63 | 100.01 | 100.58 | 100.59 | 99.10 | 100.02 | 99.53 | 99.53 | 100.3 | 100.43 | 100.77 |
| apfu | |||||||||||||||
| Cd | 0.78 | 0.79 | 0.90 | 0.80 | 0.88 | 0.74 | 0.89 | 0.86 | 0.79 | 0.80 | 0.76 | 0.88 | 0.90 | 0.76 | 0.77 |
| Zn | 0.19 | 0.16 | 0.08 | 0.16 | 0.06 | 0.21 | 0.06 | 0.09 | 0.17 | 0.16 | 0.20 | 0.09 | 0.06 | 0.17 | 0.16 |
| Cu | 0.02 | 0.04 | 0.02 | 0.02 | 0.05 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.04 |
| Fe | 0.00 | 0.01 | 0.00 | 0.08 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||
| Ag | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| S | 1.01 | 1.00 | 1.01 | 1.02 | 1.01 | 1.03 | 1.02 | 1.02 | 1.02 | 1.01 | 1.02 | 1.00 | 1.01 | 1.01 | 1.02 |
| Cd/(Cd+Zn) | 0.81 | 0.84 | 0.92 | 0.83 | 0.94 | 0.78 | 0.93 | 0.91 | 0.82 | 0.82 | 0.79 | 0.91 | 0.93 | 0.82 | 0.83 |
| Sample | Fe | Co | Ni | As | S | Total | Fe | Co | Ni | As | S | As/S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt % | apfu | |||||||||||
| 1.2b | 1.00 | 31.90 | 1.50 | 44.79 | 19.54 | 98.73 | 0.03 | 0.91 | 0.04 | 1.00 | 1.02 | 0.98 |
| 1.3 | 1.18 | 32.40 | 1.52 | 44.94 | 19.53 | 99.57 | 0.04 | 0.91 | 0.04 | 1.00 | 1.01 | 0.98 |
| 1.4 | 0.82 | 31.98 | 1.60 | 44.70 | 19.66 | 98.75 | 0.02 | 0.91 | 0.05 | 1.00 | 1.03 | 0.97 |
| 2.1 | 0.50 | 32.80 | 3.35 | 39.56 | 24.48 | 100.68 | 0.01 | 0.87 | 0.09 | 0.83 | 1.20 | 0.69 |
| 3.1 | 0.46 | 33.81 | 2.93 | 36.59 | 25.44 | 99.23 | 0.01 | 0.90 | 0.08 | 0.77 | 1.24 | 0.62 |
| 4.1 | 0.50 | 33.17 | 2.98 | 38.54 | 24.63 | 99.82 | 0.01 | 0.89 | 0.08 | 0.81 | 1.21 | 0.67 |
| 5.1 | 1.07 | 32.73 | 0.87 | 45.57 | 19.72 | 99.96 | 0.03 | 0.92 | 0.02 | 1.01 | 1.02 | 0.99 |
| 5.2 | 0.90 | 32.81 | 1.23 | 45.60 | 19.65 | 100.20 | 0.03 | 0.92 | 0.03 | 1.01 | 1.01 | 0.99 |
| 5.3 | 1.22 | 32.38 | 1.20 | 45.37 | 19.55 | 99.72 | 0.04 | 0.91 | 0.03 | 1.01 | 1.01 | 0.99 |
| 5.4 | 1.28 | 32.11 | 1.15 | 45.64 | 19.81 | 99.97 | 0.04 | 0.90 | 0.03 | 1.01 | 1.02 | 0.99 |
| 6.1 | 1.20 | 32.91 | 1.14 | 45.47 | 19.63 | 100.35 | 0.04 | 0.92 | 0.03 | 1.00 | 1.01 | 0.99 |
| Sample | Bi | Ag | Cu | Co | Cd | Fe | Zn | Sb | As | Sn | S | Total | Bi | Ag | Cu | Co | Cd | Fe | Zn | Sb | As | Sn | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt % | apfu | ||||||||||||||||||||||
| Bornite | ∑10 atoms | ||||||||||||||||||||||
| bn6a.2 | 0.21 | 0.21 | 62.81 | 0.01 | 0.02 | 11.02 | n/a | n/a | 0.02 | n/a | 25.23 | 99.54 | 0.01 | 0.01 | 5.00 | 0.00 | 0.00 | 1.00 | 0.00 | 3.98 | |||
| bn6a.1 | 0.09 | 0.24 | 63.55 | 0.05 | 0.02 | 10.44 | n/a | n/a | 0.07 | n/a | 24.81 | 99.26 | 0.00 | 0.01 | 5.09 | 0.00 | 0.00 | 0.95 | 0.00 | 3.94 | |||
| bn1.1 | 0.22 | 2.84 | 60.51 | 0.04 | 0.03 | 11.18 | n/a | n/a | 0.08 | n/a | 24.59 | 99.48 | 0.01 | 0.14 | 4.89 | 0.00 | 0.00 | 1.03 | 0.01 | 3.94 | |||
| Wittichenite | ∑7 atoms | ||||||||||||||||||||||
| lbt1.1 | 40.9 | 0.55 | 39.29 | n/a | n/a | 0.33 | n/a | n/a | n/a | n/a | 19.84 | 100.9 | 0.95 | 0.02 | 3.00 | 0.03 | 3.00 | ||||||
| lbt2.1 | 40.8 | 0.42 | 39.63 | n/a | n/a | 0.39 | n/a | n/a | n/a | n/a | 19.80 | 101.04 | 0.94 | 0.02 | 3.02 | 0.03 | 2.99 | ||||||
| lbt3.1 | 40.86 | 0.67 | 39.33 | n/a | n/a | 0.53 | n/a | n/a | n/a | n/a | 19.95 | 101.34 | 0.94 | 0.03 | 2.98 | 0.05 | 3.00 | ||||||
| lbt4.1 | 40.48 | 0.24 | 39.3 | n/a | n/a | 0.74 | n/a | n/a | n/a | n/a | 19.57 | 100.32 | 0.94 | 0.01 | 3.01 | 0.06 | 2.97 | ||||||
| lbt5.1 | 40.1 | 0.28 | 39.36 | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 19.98 | 100.22 | 0.93 | 0.01 | 3.00 | 0.04 | 3.02 | ||||||
| lbt6.1a | 40.6 | 0.21 | 40.23 | n/a | n/a | 0.64 | n/a | n/a | n/a | n/a | 19.87 | 101.55 | 0.93 | 0.01 | 3.03 | 0.05 | 2.97 | ||||||
| Tetrahedrite | ∑29 atoms | ||||||||||||||||||||||
| y6.1 | 2.97 | 0.63 | 39.79 | 0.22 | 1.67 | 0.41 | 5.11 | 14.2 | 7.96 | n/a | 24.98 | 97.93 | 0.24 | 0.10 | 10.36 | 0.06 | 0.25 | 0.12 | 1.29 | 1.93 | 1.76 | 12.89 | |
| y5.1 | 3.14 | 0.5 | 39.68 | 0.29 | 1.77 | 0.39 | 5.06 | 14.73 | 7.55 | n/a | 25.03 | 98.13 | 0.25 | 0.08 | 10.34 | 0.08 | 0.26 | 0.12 | 1.28 | 2.00 | 1.67 | 12.92 | |
| y2.1 | 4.55 | 4.49 | 37.99 | 0.15 | 0.88 | 0.2 | 5.06 | 15.11 | 6.33 | n/a | 24.82 | 99.57 | 0.36 | 0.70 | 9.99 | 0.04 | 0.13 | 0.06 | 1.29 | 2.07 | 1.41 | 12.94 | |
| Stannoidite | ∑12 atoms | ||||||||||||||||||||||
| 3.4 | n/a | 0.02 | 38.11 | 0.21 | 0.05 | 10.89 | 2.47 | n/a | n/a | 17.95 | 28.44 | 98.15 | 0.00 | 3.84 | 0.02 | 0.00 | 1.25 | 0.24 | 0.97 | 5.68 | |||
| 3.5 | n/a | 0.09 | 38.12 | 0.2 | 0.03 | 10.51 | 2.86 | n/a | n/a | 17.72 | 28.27 | 97.79 | 0.01 | 3.86 | 0.02 | 0.00 | 1.21 | 0.28 | 0.96 | 5.67 | |||
| 3.5a | n/a | 0.08 | 38.59 | 0.23 | 0 | 10.34 | 2.6 | n/a | n/a | 17.61 | 29.02 | 98.47 | 0.00 | 3.86 | 0.02 | 0.00 | 1.18 | 0.25 | 0.94 | 5.75 | |||
| 3.6 | n/a | 0.11 | 39.13 | 0.13 | 0 | 10.13 | 2.86 | n/a | n/a | 17.42 | 28.65 | 98.42 | 0.01 | 3.92 | 0.01 | 0.00 | 1.16 | 0.28 | 0.93 | 5.69 | |||
| 2a.3 | n/a | 0.2 | 37.84 | 0.2 | 0.04 | 10.74 | 2.8 | n/a | n/a | 17.66 | 29.07 | 98.55 | 0.01 | 3.78 | 0.02 | 0.00 | 1.22 | 0.27 | 0.94 | 5.75 | |||
| Sphalerite | ∑2 atoms | ||||||||||||||||||||||
| 3.3 | 0.1 | n/d | 0.1 | n/a | 0.56 | 1.07 | 65.73 | n/a | n/a | n/a | 32.09 | 99.65 | 0.00 | 0.00 | 0.00 | 0.02 | 0.99 | 0.99 | |||||
| 3.2 | 0.03 | n/d | 0.07 | n/a | 0.5 | 1.12 | 65.39 | n/a | n/a | n/a | 32.02 | 99.13 | 0.00 | 0.00 | 0.00 | 0.02 | 0.99 | 0.99 | |||||
| 3.1 | 0.01 | n/d | 0.06 | n/a | 0.55 | 0.92 | 66.24 | n/a | n/a | n/a | 31.58 | 99.36 | 0.00 | 0.00 | 0.00 | 0.02 | 1.00 | 0.98 | |||||
| 2a.1 | 0.03 | n/d | 0.17 | n/a | 0.57 | 1.06 | 65.02 | n/a | n/a | n/a | 31.72 | 98.56 | 0.00 | 0.00 | 0.01 | 0.02 | 0.99 | 0.98 | |||||
| 2a.2 | 0.06 | n/d | 0.1 | n/a | 0.56 | 1.18 | 65.66 | n/a | n/a | n/a | 32.13 | 99.68 | 0.00 | 0.00 | 0.00 | 0.02 | 0.99 | 0.99 | |||||
| PDF#40-0835 (Cd8.05Zn1.95)S10 Hex | 31ba3 Hex | 31ba2 Hex | ||||
|---|---|---|---|---|---|---|
| hkl | d | I | deas | Iest | deas | Iest |
| 100 | 3.530 | 89.0 | 3.5392 | 39.9 | 3.5383 | 33.6 |
| 002 | 3.310 | 90.0 | 3.3168 | 2.3 | 3.3052 | 20.7 |
| 101 | 3.120 | 100.0 | 3.1262 | 93.7 | 3.1254 | 100.0 |
| 102 | 2.410 | 18.0 | 2.4227 | 22.3 | 2.4201 | 43.9 |
| 110 | 2.040 | 76.0 | 2.0457 | 100.0 | 2.0419 | 53.9 |
| 103 | 1.871 | 36.0 | 1.8735 | 56.7 | 1.8719 | 77.0 |
| 200 | 1.762 | 10.0 | 1.7657 | 6.8 | 1.7629 | 4.3 |
| 004 | 1.657 | 3.0 | 1.6534 | 5.3 | 1.6499 | 71.1 |
| a, Å | 4.131(1) | 4.086(5) | 4.079(6) | |||
| c, Å | 6.173(5) | 6.628(6) | 6.618(5) | |||
| V, Å3 | 94.94 | 95.85 | 95.37 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, S. A New Zincian Greenockite Occurrence in the Saishitang Cu Skarn Deposit, Qinghai Province, Northwest China. Minerals 2017, 7, 132. https://doi.org/10.3390/min7080132
Liu J, Zhang S. A New Zincian Greenockite Occurrence in the Saishitang Cu Skarn Deposit, Qinghai Province, Northwest China. Minerals. 2017; 7(8):132. https://doi.org/10.3390/min7080132
Chicago/Turabian StyleLiu, Jianping, and Shugen Zhang. 2017. "A New Zincian Greenockite Occurrence in the Saishitang Cu Skarn Deposit, Qinghai Province, Northwest China" Minerals 7, no. 8: 132. https://doi.org/10.3390/min7080132




