About the Genetic Mechanisms of Apatites: A Survey on the Methodological Approaches
Abstract
:1. Introduction
- (1)
- A sketch of the apatite bulk structure, drawn according the most realistic related symmetry space groups, focusing mainly on Hydroxyapatite (HAp) and, secondarily, on its structurally derivated, the Carbonated apatite (CAp).
- (2)
- A comparison between the stable surface profiles of the main crystallographic {hkl} forms, i.e., the hexagonal prism and pinacoids, and the monoclinic pinacoids.
- (3)
- The ambiguities arising in interpreting the growth morphology, when the hexagonal symmetry of apatite is assumed “a priori” and the twinning occurrence is not considered as a “habit modifier”.
- (4)
- The role of the epitaxy when another phosphate works as a precursor of the apatite (e.g., the case of the Monetite assisted growth of micrometric HAp crystals).
- (5)
- The growth of nanosized vs. micrometric HAp crystals.
2. Crystal-Chemistry and Bulk Structure of Apatites: Hydroxy-Apatite as a “Case Study”
2.1. The Hexagonal Setting
2.2. The Monoclinic Setting
2.3. The Complexity of Carbonated Apatites
3. Solved and Unsolved Problems about the Relationship between Monoclinic and Hexagonal HAp Growth Behavior
- The first paper in which the surface profiles of HAp were systematically examined and growth morphology was predicted and compared with the observed one, appeared on 1986. Terpstra et al. [13] applied the “connected net” method, based on the morphological theory by Hartman-Perdok [63] to the hexagonal (P63/m) HAp structure. Starting from a qualitative evaluation of the Ca–PO4 and Ca–OH bonds, and assuming that the ordering of the OH dipoles has any influence on the Ca–OH bonds, the Authors argued that one can expect that “…monoclinic HAp should have the same morphological appearance of the hexagonal HAp”; accordingly, “…the lowering of the apatite symmetry (P63/m → P21/b) due to the ordering of OH is not likely to be the cause of the occurrence of the plate-like apatitic crystals in calcified tissues”.
- Surface relaxation on two opposite (100) free surfaces of a P63/m HAp crystal, was invoked to explain the “… symmetry breaking effect on growth morphologies, producing equilibrium platelet morphologies even when these are inconsistent with the symmetry of the crystal” [14,64]. However, the Authors are conscious that platelet growth morphology does not occur when HAp is precipitated in vitro. Hence they speculated that “…it may be possible that in vivo growth of apatite the body may manipulate the growth conditions (carbonate impurities, organic molecules found in bones) so as to allow the platelet growth mechanism to work”.
- An interesting attempt was performed by De Leeuw and Rabone [17], who used molecular dynamics simulations to evaluate the interaction of citric acid with the hexagonal (P63/m) prismatic (01.0) and pinacoidal (00.1) surfaces in an aqueous environment. The prismatic surfaces were assumed as terminated by PO4 groups (nothing being revealed about the termination of the pinacoid). Surface energies were calculated for dehydrated (in vacuum) surfaces, followed by those calculated in aqueous environment and, finally, with citric acid in an aqueous environment. The large difference in the adsorption energies for these two kind of surfaces indicates that “…citric acid would be a much better growth inhibitor of the {01.0} prismatic form, slowing down its growth rate by binding to surface growth sites”. As a consequence, the crystal shape of HAp grown in the presence of citric acid would become along [001], the direction of the OH channels, with expression of the prismatic form, as compared to the crystal morphology in the absence of citric acid. It is worth noting that this work, published on 2007, allowed outlining, for the first time, the influence of the different surface structure of two important HAp forms, even if the molecular adsorption is assumed within the “large constraints” of the Molecular Dynamic Simulation.
- Astala and Stott [61] carried out a first-principles study about water adsorption on the {01.0}, {00.1} and {10.1} forms of hexagonal (P63/m) HAp. In particular, they determined the surface energy in vacuum of the pinacoid {00.1} having considered the approach to modeling the surface profiles proposed by Rulis et al. [62]. In this model, the sole constraint imposed was that “… surface must be constructed so that the corresponding slabs were charge neutral”. Moreover, they considered that, in HAp, the orientation of the OH group along the [001] channels (c axis) reduces the symmetry such that the top and bottom of the 001 slab surfaces are no longer symmetric. Accordingly, these Authors introduced the term “symmetry breaking” limited to the form {00.1}. As concerns the other important form, the hexagonal {01.0} prism, the problem of the “symmetry breaking” does not longer exist since the OH dipoles lie parallel to the {01.0} surfaces. However, this form can terminate with three different profiles, according to the geometric 01.0 cut applied to the bulk of the crystal. One of them corresponds to a stoichiometric slab (Ca/P ratio being 1.67); the two out of three are non-stoichiometric: a Ca-rich slab (Ca/P ratio = 1.75) and a PO4 rich slab (Ca/P ratio = 1.5). This is surely interesting, but something was wrong, not only from a crystallographic point of view but also because the growth mechanism of the flat faces (F-faces, in the sense of Hartman-Perdok [63]) was not respected. In fact, the space group (P63/m) implies that the slices of thickness d002 and d010 should be centrosymmetric and hence their total dipole moment should vanish (owing to the electrical stability of the slice). Hence, the symmetry breaking attributed to the steps growing on both sides of the {00.1} form is not self-consistent.
- A sensible step forward was taken by the Ugliengo et al. [19], who carried out a periodic B3LYP study of hexagonal P63 HAp (00.1) surface modelled by thin layer slabs [19]. The (00.1) surface coming out from P63 group is intrinsically polar, owing to the OH dipoles, all iso-oriented perpendicularly to the 00.1 planes. Nevertheless, the convergence of , the specific surface energy value for the hexagonal (00.1) form, has been ascertained for a slab thickness varying from a minimum of one layer (~7Å) to a maximum of 9 layers (~60Å). For the homologous monoclinic (non-polar) P21/b HAp (00.1) surface the convergence is, obviously, more rapidly obtained. For the sake of comparison, the two asymptotic values are: = 1107 and = 1337 erg cm−2. In a successive paper [26], it has been explained why the right choice of the P63 group has been made: as a matter of fact, “… the quantum-mechanical simulation of the hexagonal HAp cannot be performed within P63/m space group because of the unphysical duplication of each OH group by the m mirror plane”. In the same paper, the surface profiles of hexagonal prismatic {01.0} and pyramidal {10.1} forms were investigated as well. On the ground of Astala and Stott [61] and according to the Authors way of thinking, the {01.0} non-polar surfaces can be imagined as a stacking of electro-neutral layers …-ABA-ABA-…where the A-type has the composition Ca3(PO4)2 while the B-type corresponds to the composition Ca4(PO4)2(OH)2. The specific surface energy related to these “stoichiometric” surfaces is = 1709 erg cm−2. On the ground of recent findings [59,61], two other “non-stoichiometric” {01.0} surfaces were also considered: [B-AA-B-AA-B], Ca-rich, being Ca/P = 1.71 and [AA-B-AA-B-AA], P-rich, being Ca/P = 1.62. Regrettably, the corresponding values were not calculated, since thesurface energy cannot be evaluated using the standard formula, adopted by the Authors, that is valid for stoichiometric slab only.
- It is worth recollecting as well a remarkable paper recently publishedby Putnis et al. [65] about the growth kinetics measured by AFM on a (010) prismatic face of a synthetic HAp crystal growing from aqueous solution [65]. It was observed that HAp crystallization occurred by either classical spiral growth or non-classical particle-attachment from various supersaturated solutions at near-physiological conditions, suggesting these mechanisms do not need to be mutually exclusive. Moreover, this work represents “… the first evidence of time-resolved morphology evolution during precursor-particle attachment processes, ranging from primary spheroidal particles of different sizes to triangular and hexagonal solids formed by kinetically accessible organized assembly and aggregation”.
4. Approaching the Morphology of the Monoclinic HAp through the Hartman-Perdok Method
4.1. The Surface Profiles of the Faces in the [010] Zone, i.e., Parallel to the HAp Channels
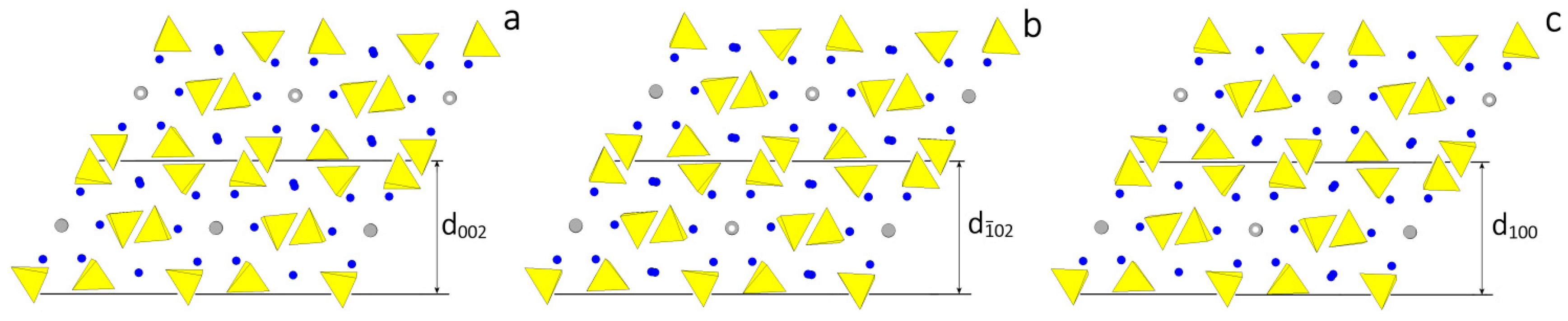
- Neither PO4 tetrahedra nor Ca ions lie on the ideal separation surface between two adjacent slices and hence both ions are not shared between adjacent slices. Further, the , and slices do not show dipole moments perpendicular to their surfaces. Accordingly, the surface profiles of the three pinacoids {100}, {} and {001} do not have to be reconstructed.
- The OH ions in the channels are screened from the mother phase by the outmost layers of each slice populated by PO4 and Ca ions not directly bound to the OH in the channels.
- The and slices are center-symmetric, while the slice contains only the 21 screw axes parallel to the [010] channels: this implies that a growing HAp monoclinic crystal shows two types of slices, according to whether the OH dipoles lying in the middle of the slice are oriented along the positive or negative sense of the y axis. On the contrary, and slices do not show polarity parallel to the slice itself, which could unavoidably affect the difference of both surface and attachment energies of {100} and {} with respect to the {001} form.
- the outmost layers of the new surfaces are populated not only by the PO4 tetrahedra, but also by Ca ions and OH dipoles;
- the occupancies of the outmost layers must be reduced by 50%, as follows from the constraints (symmetry, charge, stoichiometry) imposed by the frontiers between adjacent , and slices. In fact, these frontiers pass through the centers of mass of PO4, Ca and OH ions.
4.2. The Specific Surface Energies of the Monoclinic HAp and Its Equilibrium Shape (ES) Calculated at 0 K
- The surfaces of the three pinacoids {001}, {} and {100}, parallel to the OH channels, can be terminated either by only PO4 or by the complete set composed by PO4, Ca and OH ions.
- The basal {010} pinacoid, which is perpendicular to the OH channels, can be terminated either by only Ca or by the set made by PO4, Ca and OH ions.
- The three monoclinic prisms {012}, {110} and {} can terminate either by Ca ions (as for the {012} prism) or by PO4 ions (as for the {110} and {} prisms). It follows that the terms like “Ca rich” and “P rich” along with the terms like “stoichiometric” and “non-stoichiometric” lose meaning. On the contrary, “reconstructed, or non-reconstructed, outmost layer”, “polar, or non-polar, dhkl slice”, “relative disposition of ions in a given reconstruction”, assume a precise crystallographic meaning, unambiguous in order to describe both equilibrium and growth crystal properties.
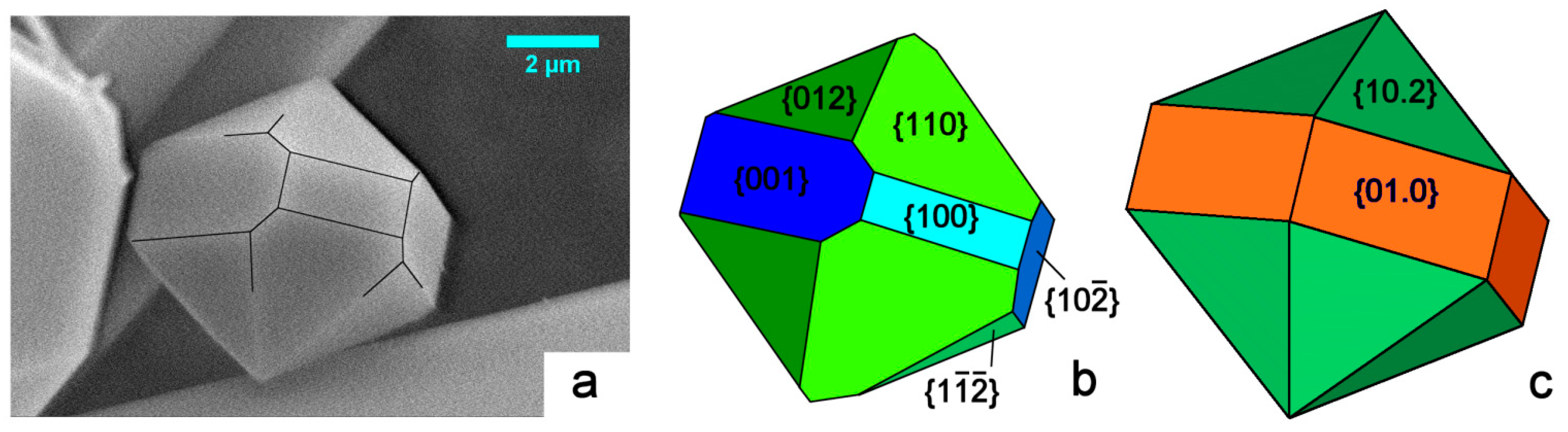
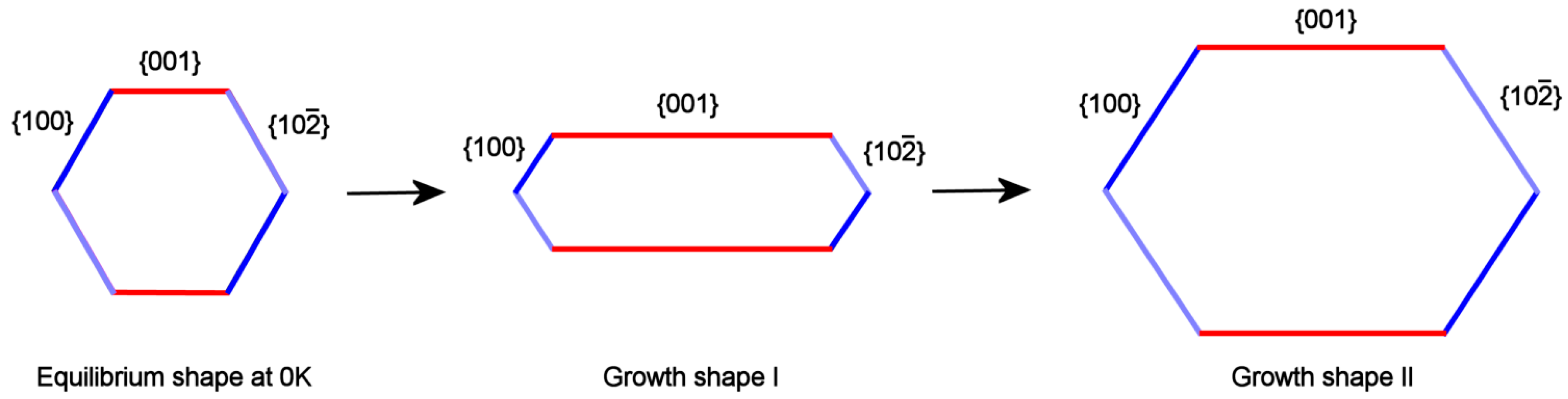
- The lowest values of the calculated specific surface energies, for every {hkl} form, was used to draw the ES of HAp at 0K. As shown in Figure 7 of reference [38], the monoclinic ES can be described by a pseudo-hexagonal “prism” truncated by the {010} pinacoid, which reaches the lowest γ value of the entire crystal when its surface is Ca-terminated ( = 1041 erg cm−2). The pseudo-hexagonality of the “prism” arises from the closeness of the lowest γ values of the three pinacoids: = 1546 erg cm−2; = 1525 erg cm−2; = 1515 erg cm−2, all these values being obtained when the outmost layers of these forms are reconstructed and exhibit an outmost population made by PO4, Ca and OH ions. The ES is completed by the presence of the small–sized quasi-equivalent prisms {012}, {110} and {}.
4.3. About the Growth Morphology of the Monoclinic HAp: A Comparison with the Hexagonal One
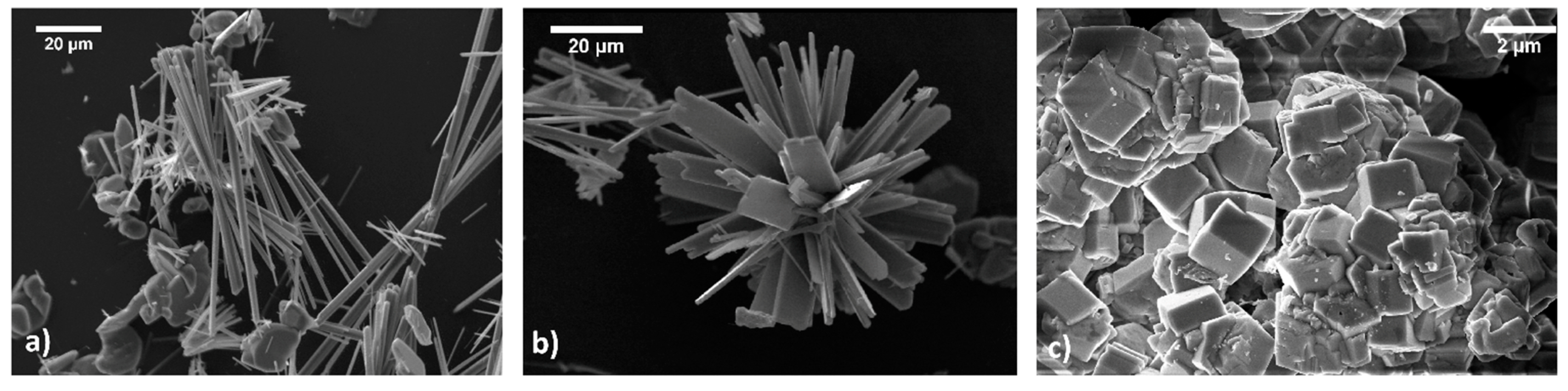
5. The Growth of Nanosized vs. Micrometric HAp Crystals, in the Frame of the Experimental Methods
5.1. The Role of the Epitaxy on the Growth of HAp from a Phosphate Precursor and the Control of Nucleation Frequency and Growth Rate by Supersaturation
5.2. The Issue of the Solubility and Its Consequences on the Nucleation Frequency
5.3. The Effect of the Presence of Carbonate Ions in Solution
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M. The many facets of apatite. Am. Mineral. 2015, 100, 1033–1039. [Google Scholar] [CrossRef]
- Kern, R. The equilibrium form of a crystal. In Morphology of Crystals: Part A: Fundamentals; Sunagawa, I., Ed.; Terra Scientific Publishing Company/Tokyo; D. Reidel Publishing Company: Tokyo, Japan, 1987; pp. 77–206. [Google Scholar]
- Kern, R.; Métois, J.J.; Le Lay, G. Basic mechanisms in the early stages of epitaxy. In Current Topics in Materials Science; Kaldis, E., Ed.; North-Holland Publishing Company: Amsterdam, The Netherland, 1979; Volume 3, pp. 131–419. [Google Scholar]
- Kern, R. Adsorption, absorption, versus crystal growth. Cryst. Res. Technol. 2013, 48, 727–782. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Crystallogr. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Eysel, W.; Roy, D.M. Hydrothermal flux growth of hydroxyapatites by temperature oscillation. J. Cryst. Growth 1973, 20, 245–250. [Google Scholar] [CrossRef]
- Arends, J.; Schuthof, J.; van der Lindwen, W.H.; Bennema, P.; van der Berg, P.J. Preparation of pure hydroxyapatite single crystals by hydrothermal recrystallization. J. Cryst. Growth 1979, 46, 213–220. [Google Scholar] [CrossRef]
- Mengeot, M.; Harvill, M.L.; Gilliam, O.R. Hydrothermal growth of calcium hydroxyapatite single crystals. J. Cryst. Growth 1973, 19, 199–203. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural variations in natural F, OH, and Cl apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Terpstra, R.A.; Bennema, P.; Hartman, P.; Woensdregt, C.F.; Perdok, W.G.; Senechal, M.L. F faces of apatite and its morphology: Theory and observation. J. Cryst. Growth 1986, 78, 468–478. [Google Scholar] [CrossRef]
- Lee, W.T.; Dove, M.T.; Salje, E.K.H. Surface relaxations in hydroxyapatite. J. Phys. Condens. Matter 2000, 12, 9829–9841. [Google Scholar] [CrossRef]
- De Leeuw, N.H. Molecular Dynamics Simulations of the Growth Inhibiting Effect of Fe2+, Mg2+, Cd2+, and Sr2+ on Calcite Crystal Growth. J. Phys. Chem. B 2002, 106, 5241–5249. [Google Scholar] [CrossRef]
- Filgueiras, M.R.T.; Mkhonto, D.; de Leeuw, N.H. Computer simulations of the adsorption of citric acid at hydroxyapatite surfaces. J. Cryst. Growth 2006, 294, 60–68. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Rabone, J.A.L. Molecular dynamics simulations of the interaction of citric acid with the hydroxyapatite (0001) and (011ˉ0) surfaces in an aqueous environment. CrystEngComm 2007, 9, 1178. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H. Formation Energies of Substitutional Sodium and Potassium in Hydroxyapatite. Mater. Trans. 2009, 50, 1041–1045. [Google Scholar] [CrossRef] [Green Version]
- Corno, M.; Orlando, R.; Civalleri, B.; Ugliengo, P. Periodic B3LYP study of hydroxyapatite (001) surface modelled by thin layer slabs. Eur. J. Mineral. 2007, 19, 757–767. [Google Scholar] [CrossRef]
- Elliott, J.C. Monoclinic Space Group of Hydroxyapatite. Nature 1971, 230, 72. [Google Scholar] [CrossRef]
- Elliott, J.C.; Young, R.A. Conversion of Single Crystals of Chlorapatite into Single Crystals of Hydroxyapatite. Nature 1967, 214, 904–906. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Dong, Z.L. Structural derivation and crystal chemistry of apatites. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 1–16. [Google Scholar] [CrossRef]
- Mathew, M.; Takagi, S. Structures of biological minerals in dental research. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Ortophosphates; Elsevier: Amsterdam, The Netherland, 1994; ISBN 9781483290317. [Google Scholar]
- Corno, M.; Rimola, A.; Bolis, V.; Ugliengo, P. Hydroxyapatite as a key biomaterial: Quantum-mechanical simulation of its surfaces in interaction with biomolecules. Phys. Chem. Chem. Phys. 2010, 12, 6309. [Google Scholar] [CrossRef] [PubMed]
- Bolis, V.; Busco, C.; Martra, G.; Bertinetti, L.; Sakhno, Y.; Ugliengo, P.; Chiatti, F.; Corno, M.; Roveri, N. Coordination chemistry of Ca sites at the surface of nanosized hydroxyapatite: Interaction with H2O and CO. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2012, 370, 1313–1336. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic Hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, T.; Yamazaki, A.; Nakamura, S.; Akao, M. Preparation and Structure Refinement of Monoclinic Hydroxyapatite. J. Solid State Chem. 1999, 144, 272–276. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Tanaka, J. Crystal growth and structure analysis of twin-free monoclinic hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Treboux, G.; Layrolle, P.; Kanzaki, N.; Onuma, K.; Ito, A. Symmetry of posner’s cluster. J. Am. Chem. Soc. 2000, 122, 8323–8324. [Google Scholar] [CrossRef]
- Hochrein, O.; Kniep, R.; Zahn, D. Atomistic simulation study of the order/disorder (monoclinic to hexagonal) phase transition of hydroxyapatite. Chem. Mater. 2005, 17, 1978–1981. [Google Scholar] [CrossRef]
- Haverty, D.; Tofail, S.A.M.; Stanton, K.T.; McMonagle, J.B. Structure and stability of hydroxyapatite: Density functional calculation and Rietveld analysis. Phys. Rev. B 2005, 71, 94103. [Google Scholar] [CrossRef]
- Corno, M.; Busco, C.; Civalleri, B.; Ugliengo, P. Periodic ab initio study of structural and vibrational features of hexagonal hydroxyapatite Ca10(PO4)6(OH)2. Phys. Chem. Chem. Phys. 2006, 8, 2464. [Google Scholar] [CrossRef] [PubMed]
- Suda, H.; Yashima, M.; Kakihana, M.; Yoshimura, M. Monoclinic ↔ Hexagonal Phase Transition in Hydroxyapatite Studied by X-ray Powder Diffraction and Differential Scanning Calorimeter Techniques. J. Phys. Chem. 1995, 99, 6752–6754. [Google Scholar] [CrossRef]
- Ikoma, T.; Yamazaki, A.; Nakamura, S.; Masaru, A. Phase Transition of Monoclinic Hydroxyapatite. Netsu Sokutei 1998, 25, 141–149. [Google Scholar]
- Aquilano, D.; Bruno, M.; Rubbo, M.; Massaro, F.R.; Pastero, L. Low Symmetry Polymorph of Hydroxyapatite. Theoretical Equilibrium Morphology of the Monoclinic Ca5(OH)(PO4)3. Cryst. Growth Des. 2014, 14, 2846–2852. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Location of type B carbonate ion in type A–B carbonate apatite synthesized at high pressure. J. Solid State Chem. 2004, 177, 3174–3182. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Local structure of channel ions in carbonate apatite. Biomaterials 2005, 26, 7548–7554. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, Y.; Takahashi, Y.; Okamura, F.P.; Tanaka, J. Structure Analysis of A-Type Carbonate Apatite by a Single-Crystal X-Ray Diffraction Method. J. Solid State Chem. 2000, 155, 292–297. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martinéz-Piñeiro, E.L.; Brès, É.F. Crystallographic structure of human tooth enamel by electron microscopy and X-ray diffraction: Hexagonal or monoclinic? J. Microsc. 2012, 248, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mugnaioli, E.; Reyes-Gasga, J.; Kolb, U.; Hemmerlé, J.; Brès, E.F. Evidence of noncentrosymmetry of human tooth hydroxyapatite crystals. Chem. A Eur. J. 2014, 20, 6849–6852. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Dolhaine, H.; Du Chesne, A.; Heinz, S.; Hochrein, O.; Laeri, F.; Podebrad, O.; Vietze, U.; Weiland, T.; Kniep, R. Biomimetic Morphogenesis of Fluorapatite-Gelatin Composites: Fractal Growth, the Question of Intrinsic Electric Fields, Core/Shell Assemblies, Hollow Spheres and Reorganization of Denatured Collagen. Eur. J. Inorg. Chem. 1999, 1999, 1643–1653. [Google Scholar] [CrossRef]
- Tao, J.; Battle, K.C.; Pan, H.; Salter, E.A.; Chien, Y.-C.; Wierzbicki, A.; De Yoreo, J.J. Energetic basis for the molecular-scale organization of bone. Proc. Natl. Acad. Sci. USA 2015, 112, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, Y.; Ivanchenko, P.; Iafisco, M.; Tampieri, A.; Martra, G. A step toward control of the surface structure of biomimetic hydroxyapatite nanoparticles: Effect of carboxylates on the {010} P-rich/Ca-rich facets ratio. J. Phys. Chem. C 2015, 119, 5928–5937. [Google Scholar] [CrossRef]
- Yao, X.; Yao, H.; Li, G.; Li, Y. Biomimetic synthesis of needle-like nano-hydroxyapatite templated by double-hydrophilic block copolymer. J. Mater. Sci. 2010, 45, 1930–1936. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Nie, Y.; Hu, H.; Liu, J.; Peng, S. Nano-hydroxyapatite improves the properties of β-tricalcium phosphate bone scaffolds. Int. J. Appl. Ceram. Technol. 2013, 10, 1003–1013. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhang, L.; Li, Y.; Wei, S. In situ synthesis and in vitro biocompatibility of needle-like nano-hydroxyapatite in agar-gelatin co-hydrogel. Mater. Lett. 2013, 104, 8–12. [Google Scholar] [CrossRef]
- Ito, H.; Oaki, Y.; Imai, H. Selective synthesis of various nanoscale morphologies of hydroxyapatite via an intermediate phase. Cryst. Growth Des. 2008, 8, 1055–1059. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ono, S.; Hirakura, S.; Oaki, Y.; Imai, H. Morphological variation of hydroxyapatite grown in aqueous solution based on simulated body fluid. CrystEngComm 2012, 14, 1143–1149. [Google Scholar] [CrossRef]
- Bertinetti, L.; Tampieri, A.; Landi, E.; Ducati, C.; Midgley, P.A.; Coluccia, S.; Martra, G. Surface Structure, Hydration, and Cationic Sites of Nanohydroxyapatite: UHR-TEM, IR, and Microgravimetric Studies. J. Phys. Chem. C 2007, 111, 4027–4035. [Google Scholar] [CrossRef]
- Sakhno, Y.; Bertinetti, L.; Iafisco, M.; Tampieri, A.; Roveri, N.; Martra, G. Surface Hydration and Cationic Sites of Nanohydroxyapatites with Amorphous or Crystalline Surfaces: A Comparative Study. J. Phys. Chem. C 2010, 114, 16640–16648. [Google Scholar] [CrossRef]
- Eppell, S.J.; Tong, W.; Lawrence Katz, J.; Kuhn, L.; Glimcher, M.J. Shape and size of isolated bone mineralites measured using atomic force microscopy. J. Orthop. Res. 2001, 19, 1027–1034. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Delgado-López, J.M.; Frison, R.; Cervellino, A.; Gómez-Morales, J.; Guagliardi, A.; Masciocchi, N. Crystal Size, Morphology, and Growth Mechanism in Bio-Inspired Apatite Nanocrystals. Adv. Funct. Mater. 2014, 24, 1090–1099. [Google Scholar] [CrossRef]
- Sato, K.; Kogure, T.; Iwai, H.; Tanaka, J. Atomic-Scale {101ˉ0} Interfacial Structure in Hydroxyapatite Determined by High-Resolution Transmission Electron Microscopy. J. Am. Ceram. Soc. 2002, 85, 3054–3058. [Google Scholar] [CrossRef]
- Ospina, C.A.; Terra, J.; Ramirez, A.J.; Farina, M.; Ellis, D.E.; Rossi, A.M. Experimental evidence and structural modeling of nonstoichiometric (010) surfaces coexisting in hydroxyapatite nano-crystals. Colloids Surf. B Biointerfaces 2012, 89, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Astala, R.; Stott, M.J. First-principles study of hydroxyapatite surfaces and water adsorption. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 78, 75427. [Google Scholar] [CrossRef]
- Rulis, P.; Yao, H.; Ouyang, L.; Ching, W.Y. Electronic structure, bonding, charge distribution, and X-ray absorption spectra of the (001) surfaces of fluorapatite and hydroxyapatite from first principles. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 245410. [Google Scholar] [CrossRef]
- Hartman, P. Structure and morphology. In Crystal Growth: An Introduction; Hartman, P., Ed.; North-Holland Publishing Company: Amsterdam, The Netherland, 1973; pp. 367–402. [Google Scholar]
- Lee, W.T.; Salje, E.K.H.; Dove, M.T. Effect of surface relaxations on the equilibrium growth morphology of crystals: Platelet formation. J. Phys. Condens. Matter 1999, 11, 7385–7410. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Zhang, W.; Putnis, C.V.; Putnis, A. Direct Observation of Spiral Growth, Particle Attachment, and Morphology Evolution of Hydroxyapatite. Cryst. Growth Des. 2016, 16, 4509–4518. [Google Scholar] [CrossRef]
- Bruno, M. The reconstruction of dipolar surfaces: A preliminary step for adsorption modeling. Cryst. Res. Technol. 2013, 48, 811–818. [Google Scholar] [CrossRef]
- Aquilano, D.; Pastero, L. Anomalous mixed crystals: A peculiar case of adsorption/absorption. Cryst. Res. Technol. 2013, 48, 819–839. [Google Scholar] [CrossRef]
- Aquilano, D.; Bruno, M.; Rubbo, M.; Pastero, L.; Massaro, F.R. Twin Laws and Energy in Monoclinic Hydroxyapatite, Ca5(PO4)3(OH). Cryst. Growth Des. 2015, 15, 411–418. [Google Scholar] [CrossRef]
- Pastero, L.; Aquilano, D. Monetite-Assisted Growth of Micrometric Ca-Hydroxyapatite Crystals from Mild Hydrothermal Conditions. Cryst. Growth Des. 2016, 16, 852–860. [Google Scholar] [CrossRef]
- Jaffe, E.B. Abstracts of the Literature on Synthesis of Apatites and Some Related Phosphates; U.S. Geological Survey: Washington, DC, USA, 1951.
- Perloff, A.; Posner, A.S. Preparation of Pure Hydroxyapatite Crystals. Science 1956, 124, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Schleede, H.A.; Schmidt, W.; Kindt, H. Zur Kenntnis der Calciumphosphate und Apatite. Z. Elektrochem. 1932, 38, 633–641. [Google Scholar]
- Jullman, H.; Mosebach, R. Zur Synthese, Licht- und Doppelbrechung des Hydroxylapatits. Z. Naturf. B 1966, 21, 493–494. [Google Scholar] [CrossRef]
- Kirn, J.F.; Leidheiser, H. Progress in efforts to grow large single crystals of hydroxyapatite. J. Cryst. Growth 1968, 2, 111–112. [Google Scholar] [CrossRef]
- Pastero, L.; Bruno, M.; Rubbo, M.; Camara, F.; Aquilano, D. Growth of large Ca-Hydroxyapatite crystals from aqueous solution. In Proceedings of the IV Meeting of the Italian and Spanish Crystallographic Associations, Tenerife, Spain, 21–25 June 2016. [Google Scholar]
- Van Wazer, J.R. Phosphorus and its Compounds, Bd. 1: Chemistry. Angew. Chem. 1961, 73, 552. [Google Scholar] [CrossRef]
- Clark, J.S. Solubility criteria for the existence of hydroxyapatite. Can. J. Chem. 1955, 33, 1696–1700. [Google Scholar] [CrossRef]
- Larsen, S. Solubility of Hydroxyapatite. Nature 1966, 212, 605. [Google Scholar] [CrossRef]
- Chen, Z.F.; Darvell, B.W.; Leung, V.W.H. Hydroxyapatite solubility in simple inorganic solutions. Arch. Oral Biol. 2004, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.B.; Darvell, B.W. Calcium Phosphate Solubility: The Need for Re-Evaluation. Cryst. Growth Des. 2009, 9, 639–645. [Google Scholar] [CrossRef]
- Neuman, W.F.F.; Neuman, M.W.W. The Nature of the Mineral Phase of Bone. Chem. Rev. 1953, 53, 1–45. [Google Scholar] [CrossRef]
- Levinskas, G.; Neuman, W. The Solubility of Bone Mineral. I. Solubility Studies of Synthetic Hydroxylapatite. J. Phys. Chem. 1955, 59, 164–168. [Google Scholar] [CrossRef]
- Rootare, H.M.; Deitz, V.R.; Carpenter, F.G. Solubility product phenomena in hydroxyapatite-water systems. J. Colloid Sci. 1962, 17, 179–206. [Google Scholar] [CrossRef]
- Bell, L.C.; Mika, H. The pH dependence of the surface concentrations of calcium and phosphorous on hydroxyapatite in aqueous solutions. J. Soil Sci. 1979, 30, 247–258. [Google Scholar] [CrossRef]
- Mika, H.; Bell, L.C.; Kruger, B.J. The role of surface reactions in the dissolution of stoichiometric hydroxyapatite. Arch. Oral Biol. 1976, 21, 697–701. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Inorganic chemistry of the dissolution phenomenon: The dissolution mechanism of calcium apatites at the atomic (ionic) level. Comments Inorg. Chem. 1999, 20, 285–299. [Google Scholar] [CrossRef]
- Dorozhkin, S. V Surface Reactions of Apatite Dissolution. J. Colloid Interface Sci. 1997, 191, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. A review on the dissolution models of calcium apatites. Prog. Cryst. Growth Charact. Mater. 2002, 44, 45–61. [Google Scholar] [CrossRef]
- Chuong, R. Experimental Study of Surface and Lattice Effects on the Solubility of Hydroxyapatite. J. Dent. Res. 1973, 52, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, I. The solubility of calcium phosphate. I. The effect of pH and ofamount of solid phase. J. Biol. Chem. 1942, 143, 703–710. [Google Scholar]
- Greenwald, I. The solubility of calcium phosphate: II. The solubility product. J. Biol. Chem. 1942, 143, 711–714. [Google Scholar]
- Greenwald, I. The effect of phosphate on the solubility of calcium carbonate and of bicarbonate on the solubility of calcium and magnesium phosphates. J. Biol. Chem. 1945, 161, 697–704. [Google Scholar] [PubMed]
- Kaufman, H.W.; Kleinberg, I. Studies on the incongruent solubility of hydroxyapatite. Calcif. Tissue Int. 1979, 27, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Peech, M. Solubility Criteria for the Existence of Calcium and Aluminum Phosphates in Soils1. Soil Sci. Soc. Am. J. 1955, 19, 171. [Google Scholar] [CrossRef]
- Skinner, H.C.W. Studies in the basic mineralizing system, CaO-P2O5-H2O. Calcif. Tissue Res. 1974, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.; Ogasawara, T.; Silva, F.T. Hydrothermal Crystallization of HAp. Proc 2nd Int. Symp. Apatite. 1997, 1, 4147. [Google Scholar]
- Byrappa, K.; Yoshimura, M. Hydrothermal growth of some selected crystals. In Handbook of Hydrothermal Technology; Noyes Publications/William Andrew Publishing, LLC: Norwich, NY, USA, 2001; pp. 287–299. ISBN 0-8155-1445-X. [Google Scholar]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5,15,25 and 37 °C. J. Res. Natl. Bur. Stand. Chem. 1977, 81A, 273–281. [Google Scholar] [CrossRef]
- Pastero, L.; Aquilano, D. CaCO3 (Calcite)/Li2CO3 (zabuyelite) anomalous mixed crystals. Sector zoning and growth mechanisms. Cryst. Growth Des. 2008, 8, 3451–3460. [Google Scholar] [CrossRef]

| Ca/P Molar Ratio | Mineral Name | Chemical Composition |
|---|---|---|
| 1.5–1.67 | Calcium-deficient hydroxyapatite (Ca-def HAp) | Ca10−x(HPO4)x(PO4)6−x (OH)2−x(0 < x < 1) |
| 1.67 | Hydroxyapatite (HAp) | Ca10(PO4)6(OH)2 |
| 1.67 | End-member, A-type, carbonated apatite | Ca10(PO4)6CO3 |
| ≥1.67 | End-member, B-type, carbonated hydroxylapatite | Ca10−x[(PO4)6−2x (CO3)2x](OH)2(0 < x < 1) |
| ≥1.67 | Mixed A-type and B-type carbonated apatite | Ca10−x[(PO4)6−2x (CO3)2x]CO3(0 < x < 1) |
| 1.67 | Fluorapatite (FAp) | Ca10(PO4)6F2 |
| 1.67 | Oxyapatite (OAp), mineral voelckerite | Ca10(PO4)6O |
| Structure | Space Group | a0 | b0 | c0 | α | β | γ | Reference |
|---|---|---|---|---|---|---|---|---|
| HAp (Hydroxy-apatite) | ||||||||
| hexagonal | P63/m | 9.4302 | 9.4302 | 6.8911 | 90° | 90° | 120° | [7,8,9,10,11,12,13,14,15,16,17,18,19] |
| monoclinic | P21/b | 9.4214 | 2 × a0 | 6.881 | 90° | 90° | 120° | [20,21,22] |
| FAp (fluor-apatite) | ||||||||
| hexagonal | P63/m | 9.367 | 9.3973 | 6.8782 | 90° | 90° | 120° | [12,23,24,25] |
| OAp (oxy-apatite) | ||||||||
| hexagonal | P | 9.432 | 9.432 | 6.88 | 90.3° | 90° | 119.9° | [1,23,24,25] |
| Monoclinic HApForm | PBC of Reference | Surface Termination | Is the Outmost Layer Shared between Adjacent Slices? | Surface Reconstruction Needed | γ001(erg cm−1) |
|---|---|---|---|---|---|
| {001} | [010]B | PO4, Ca, OH | yes | yes | 1546, 1613, 1666,1691 |
| [010]A | PO4 | no | no | 1712 | |
| [010]B | PO4, Ca, OH | yes | yes | 1738,1741,1742,1793 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastero, L.; Bruno, M.; Aquilano, D. About the Genetic Mechanisms of Apatites: A Survey on the Methodological Approaches. Minerals 2017, 7, 139. https://doi.org/10.3390/min7080139
Pastero L, Bruno M, Aquilano D. About the Genetic Mechanisms of Apatites: A Survey on the Methodological Approaches. Minerals. 2017; 7(8):139. https://doi.org/10.3390/min7080139
Chicago/Turabian StylePastero, Linda, Marco Bruno, and Dino Aquilano. 2017. "About the Genetic Mechanisms of Apatites: A Survey on the Methodological Approaches" Minerals 7, no. 8: 139. https://doi.org/10.3390/min7080139





