The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Zeta Potential Measurements
2.4. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometer (SEM-EDS) Analyses
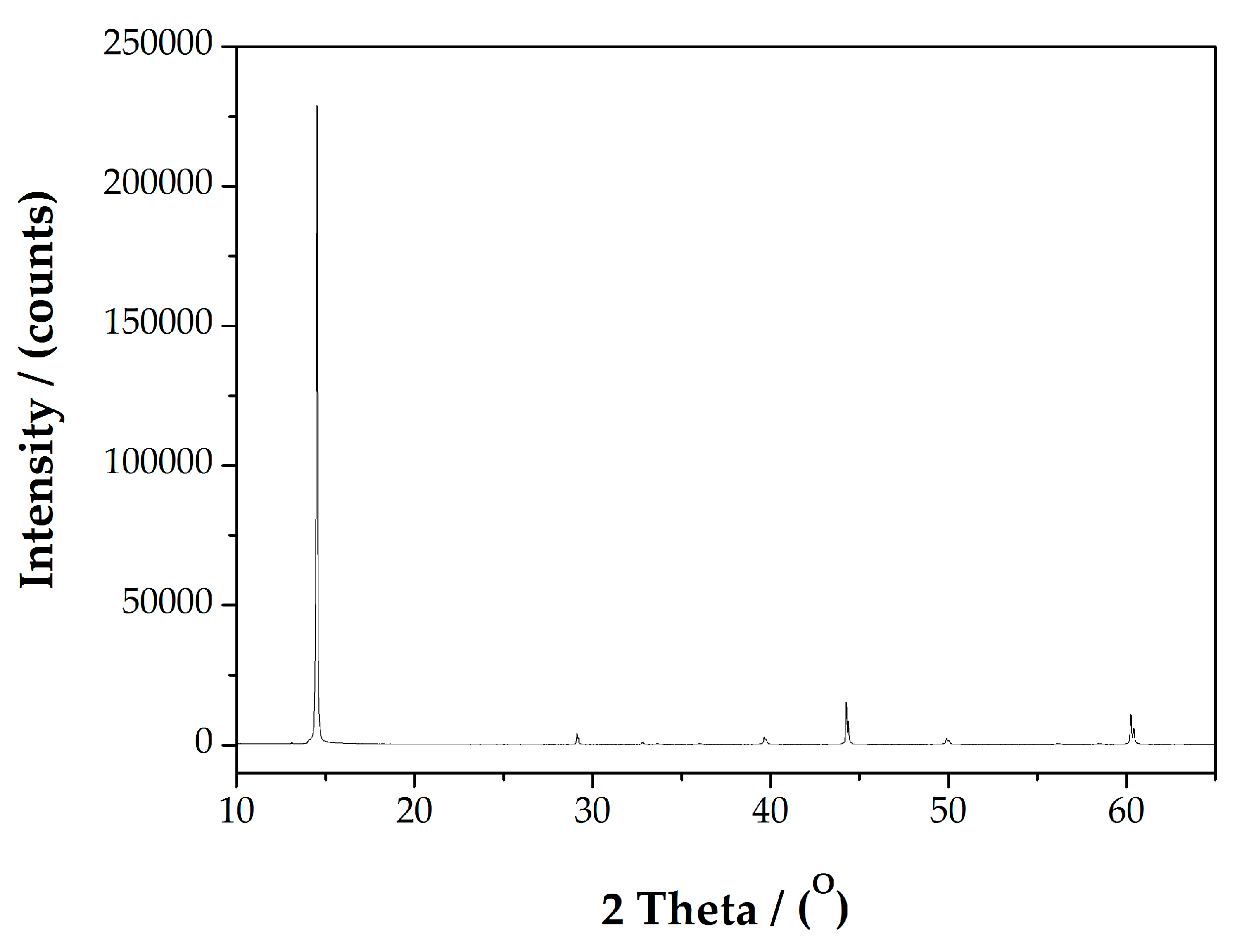
2.5. The Analysis of Molybdenum Phases
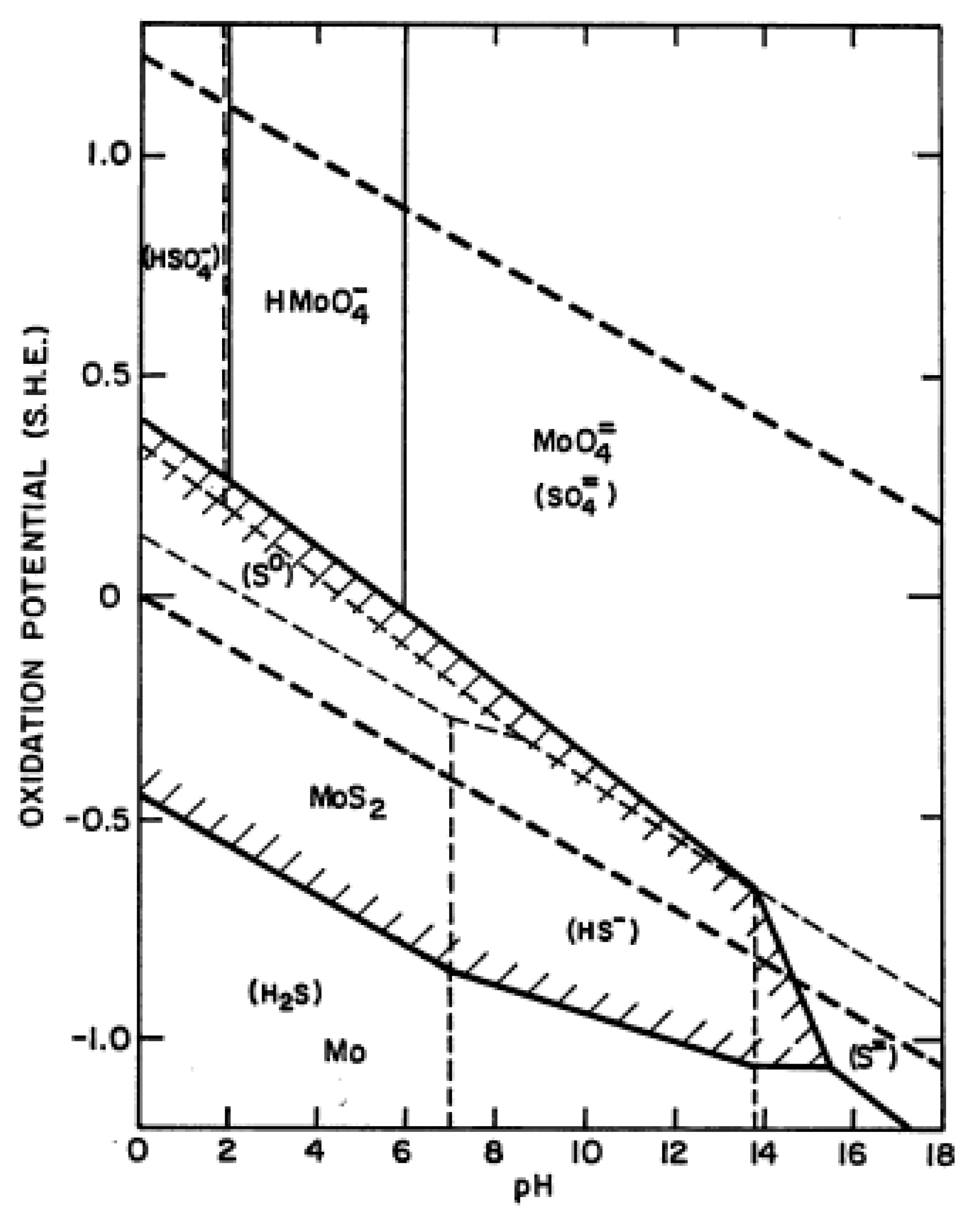
3. Results and Discussion
3.1. Flotation Tests
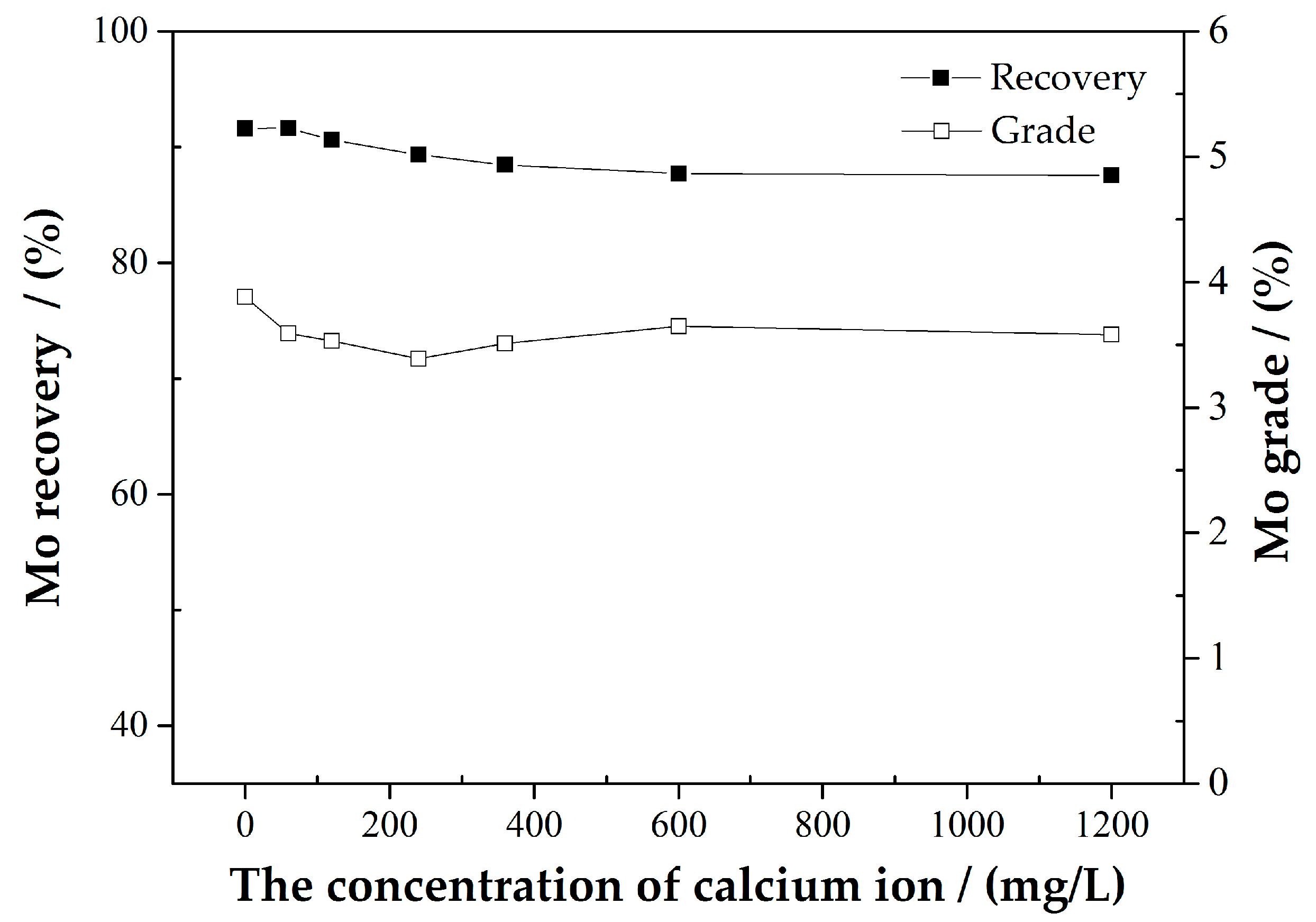
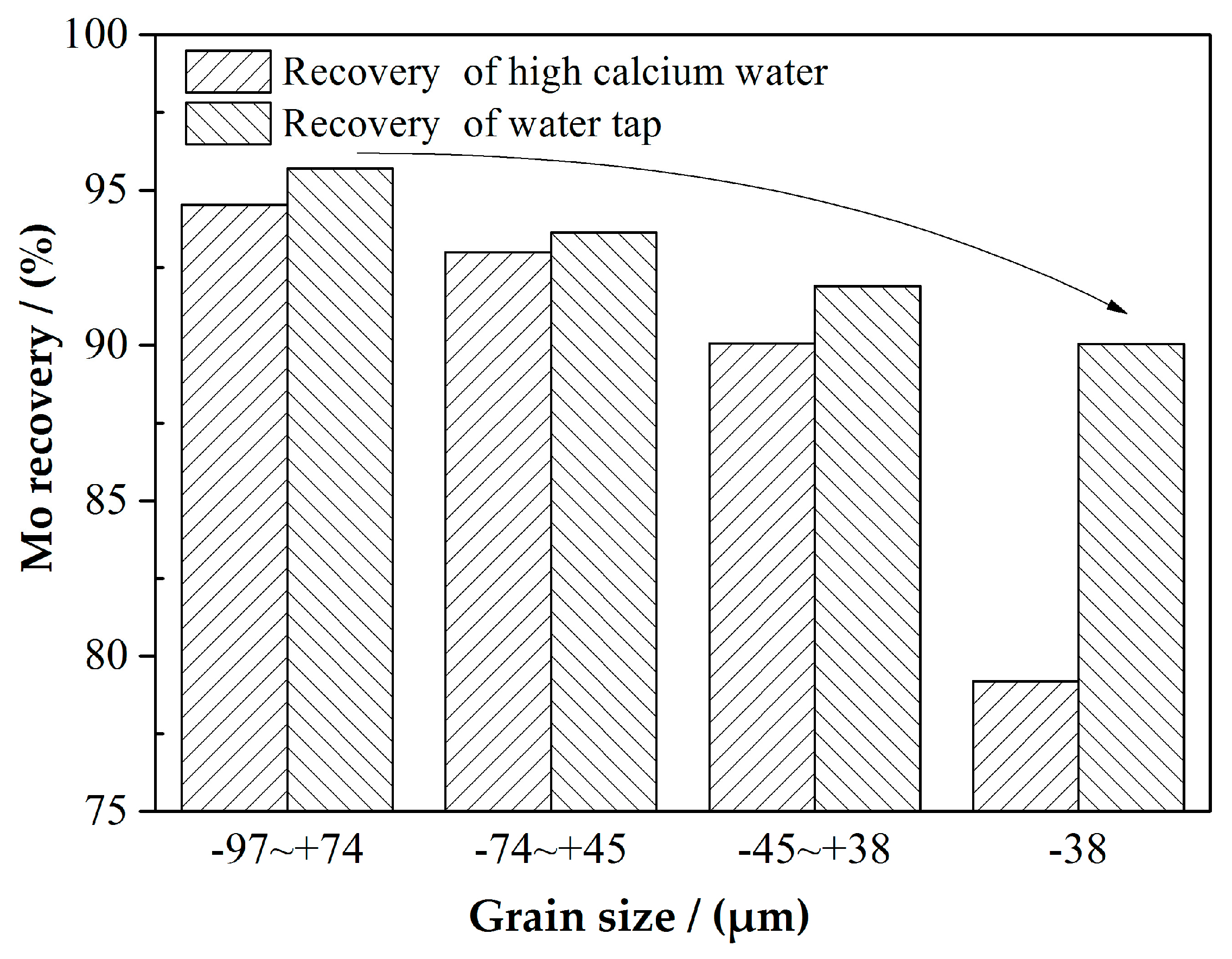
3.1.1. AM Flotation
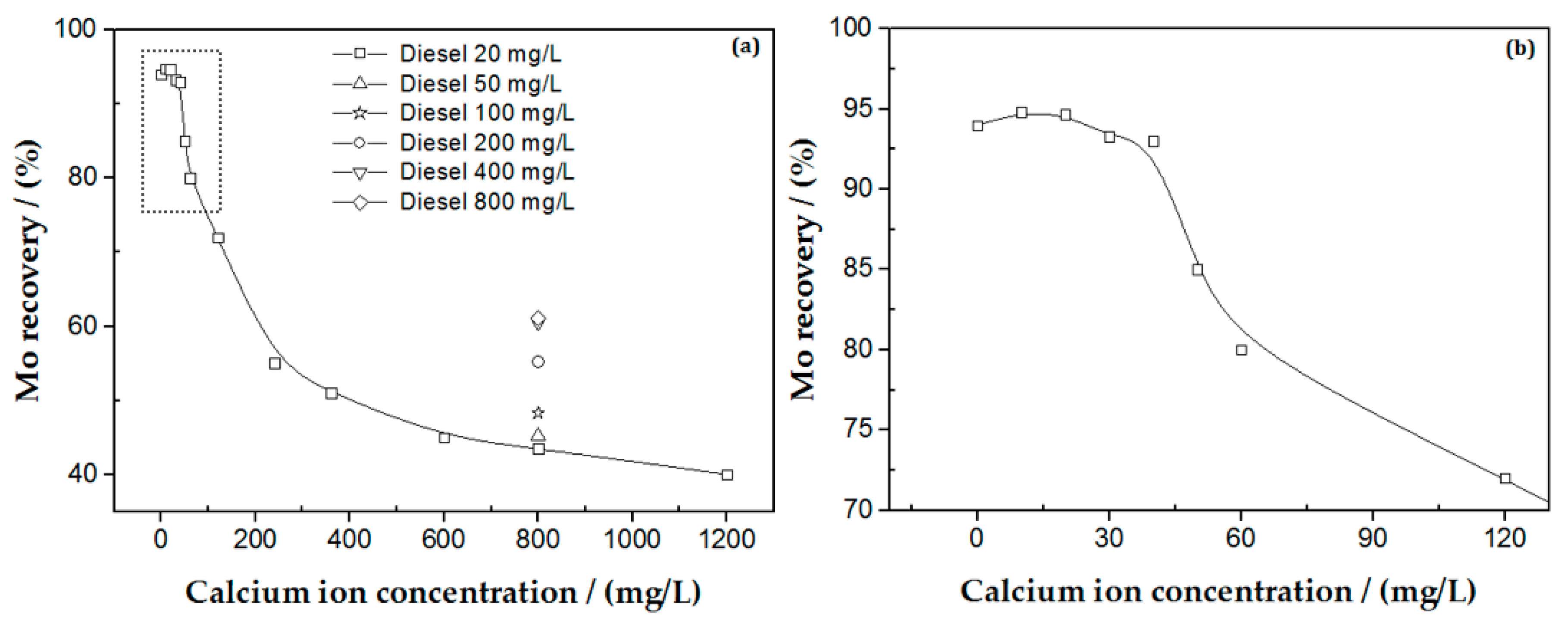
3.1.2. PM Flotation
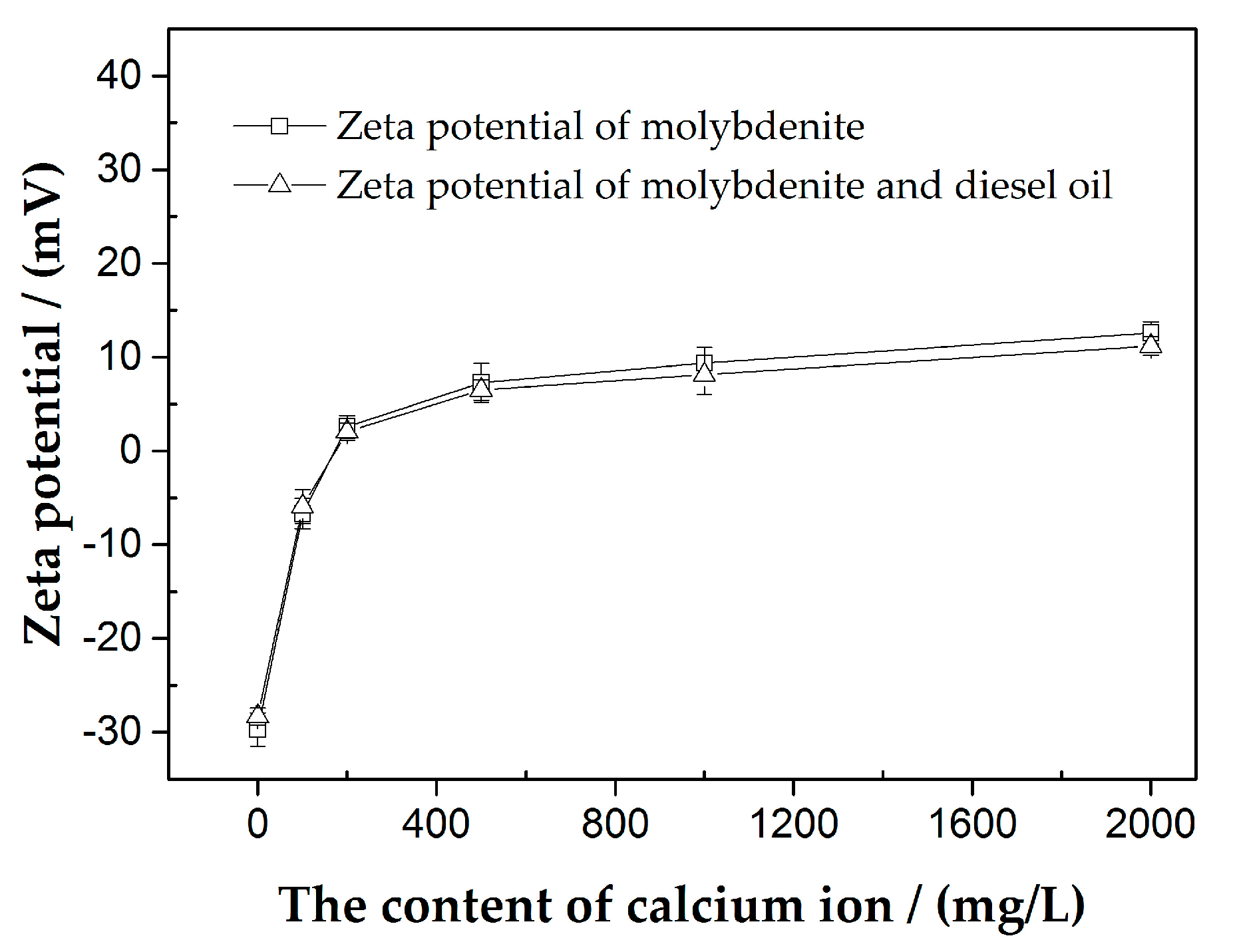
3.2. Zeta Potential Measurements
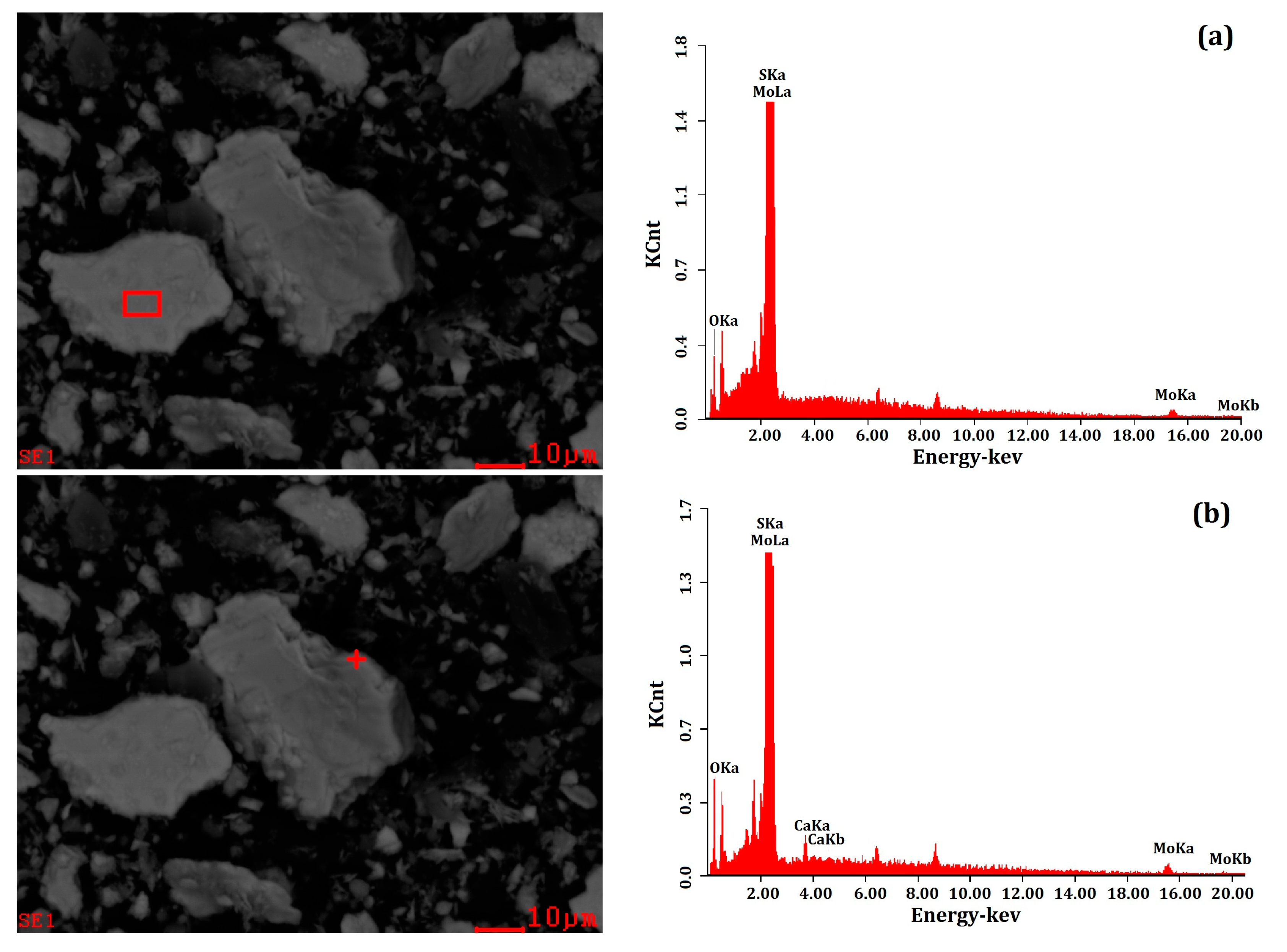
3.3. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometer (SEM-EDS) Analyses
3.4. The Phase Analysis of Molybdenum
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Liu, W.Y.; Moran, C.J.; Vink, S. A review of the effect of water quality on flotation. Miner. Eng. 2013, 53, 90–100. [Google Scholar] [CrossRef]
- Ozkan, S.G.; Acar, A. Investigation of impact of water type on borate ore flotation. Water Res. 2004, 38, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Sinche-Gonzalez, M.; Fornasiero, D.; Zanin, M. Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water. Minerals 2016, 6, 105. [Google Scholar] [CrossRef]
- Muzenda, E. An investigation into the effect of water quality on flotation performance. Proc. World Acad. Sci. Eng. Technol. 2010, 69, 237–241. [Google Scholar]
- Levay, G.; Smart, R.S.C.; Skinner, W.M. The impact of water quality on flotation performance. J. S. Afr. Inst. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Chen, J.M.; Liu, R.Q.; Sun, W.; Qiu, G.Z. Effect of mineral processing wastewater on flotation of sulfide mineral. Trans. Nonferr. Met. Soc. China 2009, 19, 454–457. [Google Scholar] [CrossRef]
- Chander, S.; Fuerstenau, D.W. On the natural floatability of molybdenite. Trans. Am. Inst. Min. Metall. Eng. 1972, 252, 62–69. [Google Scholar]
- Raghavan, S.; Hsu, L.L. Factors affecting the flotation recovery of molybdenite from porphyry copper ores. Int. J. Miner. Process. 1984, 12, 145–162. [Google Scholar] [CrossRef]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
- Zhou, L. Molybdenite Flotation. Master’s Thesis, Ian Wark Research Institute and University of South Australia, Mawson Lakes, Australia, May 2010; p. 14. [Google Scholar]
- Nagaraj, D.R.; Farinato, R. Chemical factor effects in saline and hypersaline waters in the flotation of Cu and Cu-Mo ores. In Proceedings of the XXVII International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Triffett, B.; Bradshaw, D. The role of morphology and host rock lithology on the flotation behaviour of molybdenite at Kennecott Utah Copper. In Proceedings of the 9th International Congress for Applied Mineralogy: ICAM 2008, Brisbane, Australia, 8–10 September 2008; pp. 465–473. [Google Scholar]
- Triffett, B.; Veloo, C.; Adair, B.J.I.; Bradshaw, D. An investigation of the factors affecting the recovery of molybdenite in the Kennecott Utah copper bulk flotation circuit. Miner. Eng. 2008, 21, 832–840. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Lucay, F.; Cisternas, L.A.; Gálvez, E.D.; López-Valdivieso, A. Study of the natural floatability of molybdenite fines in saline solutions and effect of gypsum precipitation. Metall. Process. 2015, 32, 203–208. [Google Scholar]
- Hirajim, T.; Suyantar, G.P.W.; Ichikaw, O.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner. Eng. 2016, 96–97, 83–93. [Google Scholar] [CrossRef]
- Li, C.Q.; Xue, J. Phase analysis of molybdenum ores and determination of molybdenum by catalytic polarography. Part B Chem. Anal. 2012, 48, 420–422. (In Chinese) [Google Scholar]
- Zheng, M.Q.; Yu, S.X.; Cheng, X.H. A rapid phase analysis method for molybdenum ores. Rock Miner. Anal. 2011, 30, 40–42. (In Chinese) [Google Scholar]
- Song, S.; Zhang, X.; Yang, B.; Lopez-Mendoza, A. Flotation of molybdenite fines as hydrophobic agglomerates. Sep. Purif. Technol. 2012, 98, 451–455. [Google Scholar] [CrossRef]
- Ornelas Tabares, J.; Madrid Ortega, I.; Reyes Bahena, J.L.; Sánchez López, A.A.; Valdez Pérez, D.; LópezValdivieso, A. Surface properties and floatability of molybdenite. In Proceedings of the 2006 China-Mexico Workshop on Minerals Particle Technology, San Luis Potosí, Mexico, 5–7 December 2006; pp. 115–124. [Google Scholar]
- Hoover, R.; Malhotra, D. Emulsion flotation of molybdenite. In Flotation; Gaudin Memorial, A.M., Ed.; American Institute of Mining, Metallurgical, and Petroleum Engineers: New York, NY, USA, 1976; Volume 1, pp. 485–503. [Google Scholar]
- Smit, F.J.; Bhasin, A.K. Relationship of petroleum hydrocarbon characteristics and molybdenite flotation. Int. J. Miner. Process. 1985, 15, 19–40. [Google Scholar] [CrossRef]
- Lv, J.Y.; Shen, Y.P.; Zhang, H.E. Surface properties of molybdenite and its floatability. Nonferr. Met. Miner. Process. Sect. 1992, 41, 4–8. (In Chinese) [Google Scholar]
- Lin, C.Y. Molybdenum Ore Dressing and Further Processing, 1st ed.; Metallurgy Industry Press: Beijing, China, 1996; pp. 52–53. (In Chinese) [Google Scholar]
- Pu, C.Y.; Liu, T.Y.; Zhang, Q.R. Electronic structure of CaMoO4 crystal with point defects. J. Univ. Shanghai Sci. Technol. 2008, 30, 112–115. (In Chinese) [Google Scholar]
- Gontijo, C.D.F.; Fornasiero, D.; Ralston, J. The Limits of Fine and Coarse Particle Flotation. Can. J. Chem. Eng. 2007, 85, 739–747. [Google Scholar] [CrossRef]
- Krishnaswamy, P. Kinetics of the Aqueous Oxidation of Molybdenite and the Role of Crystal Anisotropy on the Electrochemical Mechanisms of the Process. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1981; p. 101. [Google Scholar]
- Essington, M.E. Formation of calcium and magnesium molybdate complexes in dilute aqueous solutions. Soil Sci. Soc. Am. 1992, 56, 1124–1127. [Google Scholar] [CrossRef]
- Qiu, Z.H.; Liu, G.Y.; Liu, Q.X.; Zhong, H. Understanding the roles of high salinity in inhibiting the molybdenite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 123–129. [Google Scholar] [CrossRef]
| Compositions | Mo | WO3 | Cu | Pb | Zn | S | CaO |
| Content | 0.12 | 0.07 | 0.007 | 0.005 | 0.016 | 1.81 | 21.98 |
| Compositions | Al2O3 | SiO2 | MgO | Na2O | K2O | TFe 1 | |
| Content | 7.89 | 48.50 | 2.86 | 1.08 | 0.82 | 10.12 |
| Compositions | Molybdenite | Powethite | Scheelite | Chalcopyrite |
| Content | 0.19 | trace | 0.09 | 0.02 |
| Compositions | Sphalerite | Galena | Pyrite | Magnetite |
| Content | 0.24 | trace | 1.10 | 1.00 |
| Compositions | Garnet | Diopside | Wollastonite | Quartz |
| Content | 25.70 | 18.70 | 16.30 | 14.60 |
| Compositions | Biotite | Feldspar | Calcite | Loss |
| Content | 10.10 | 9.10 | 2.00 | 0.85 |
| Title | Before Reacting | After Reacting | |
|---|---|---|---|
| Concentration of calcium in pulp (mg/L) | - | 0 | 800 |
| CaMoO4/MoS2(mg/g) | 0.00 | 0.00 | 1.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Yang, W.; Cao, W.; He, T.; Liu, Y.; Yang, J.; Guo, L.; Peng, Y. The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation. Minerals 2017, 7, 141. https://doi.org/10.3390/min7080141
Wan H, Yang W, Cao W, He T, Liu Y, Yang J, Guo L, Peng Y. The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation. Minerals. 2017; 7(8):141. https://doi.org/10.3390/min7080141
Chicago/Turabian StyleWan, He, Wei Yang, Weicheng Cao, Tingshu He, Yanying Liu, Jianbo Yang, Lin Guo, and Yongjun Peng. 2017. "The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation" Minerals 7, no. 8: 141. https://doi.org/10.3390/min7080141





