Contrasting Surficial Composition of Native Gold from Two Different Types of Gold Ore Deposits
Abstract
:1. Introduction
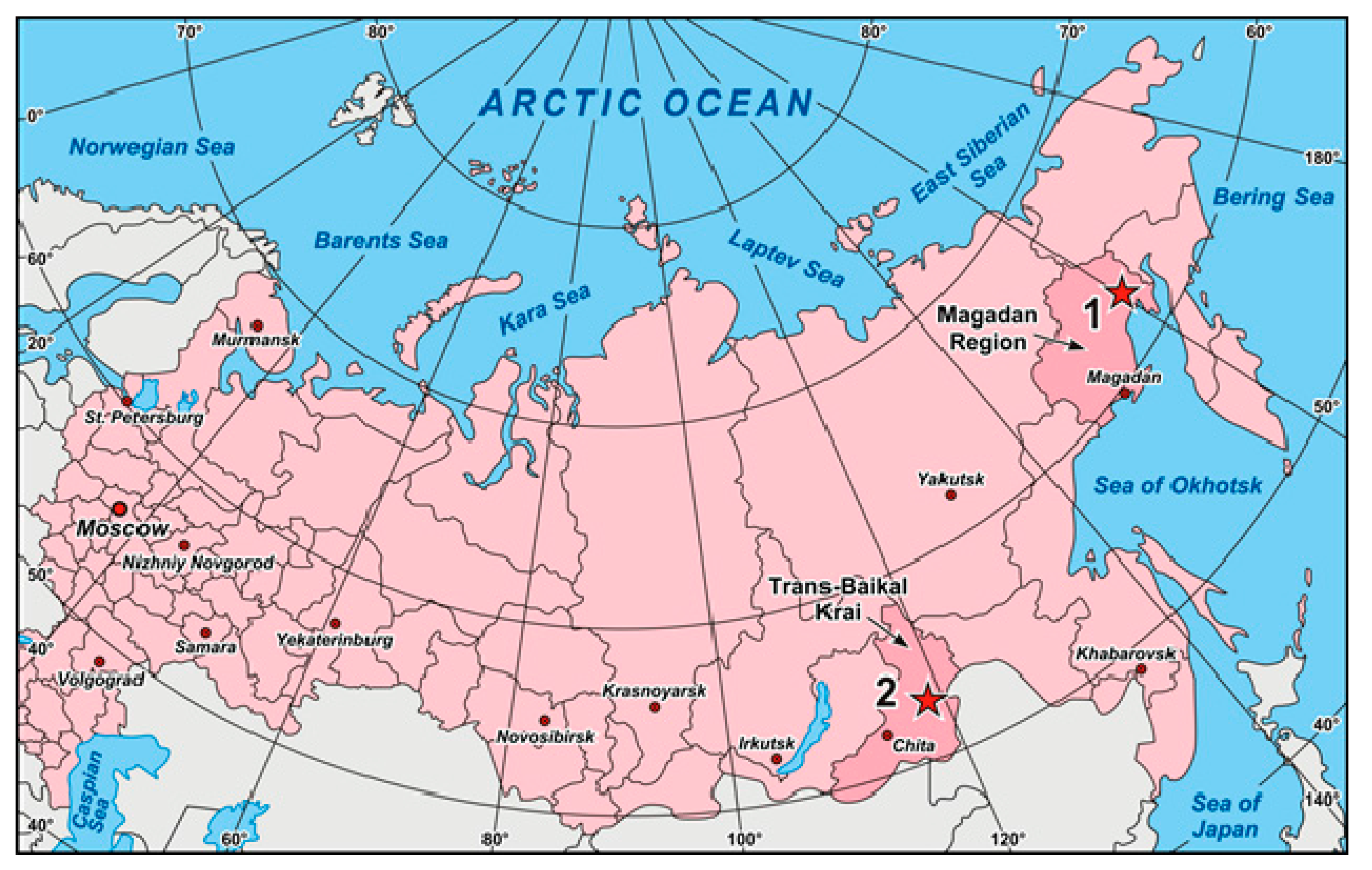
2. Sample Location and Description
2.1. Geological Setting
2.2. Sample Description
3. Methods
3.1. X-ray Photoelectron Spectroscopy and Auger Electron Spectroscopy
3.2. Electron Microprobe Analysis
3.3. Scanning Electron Microscopy with Energy Dispersive X-ray Spectrometry
4. Results
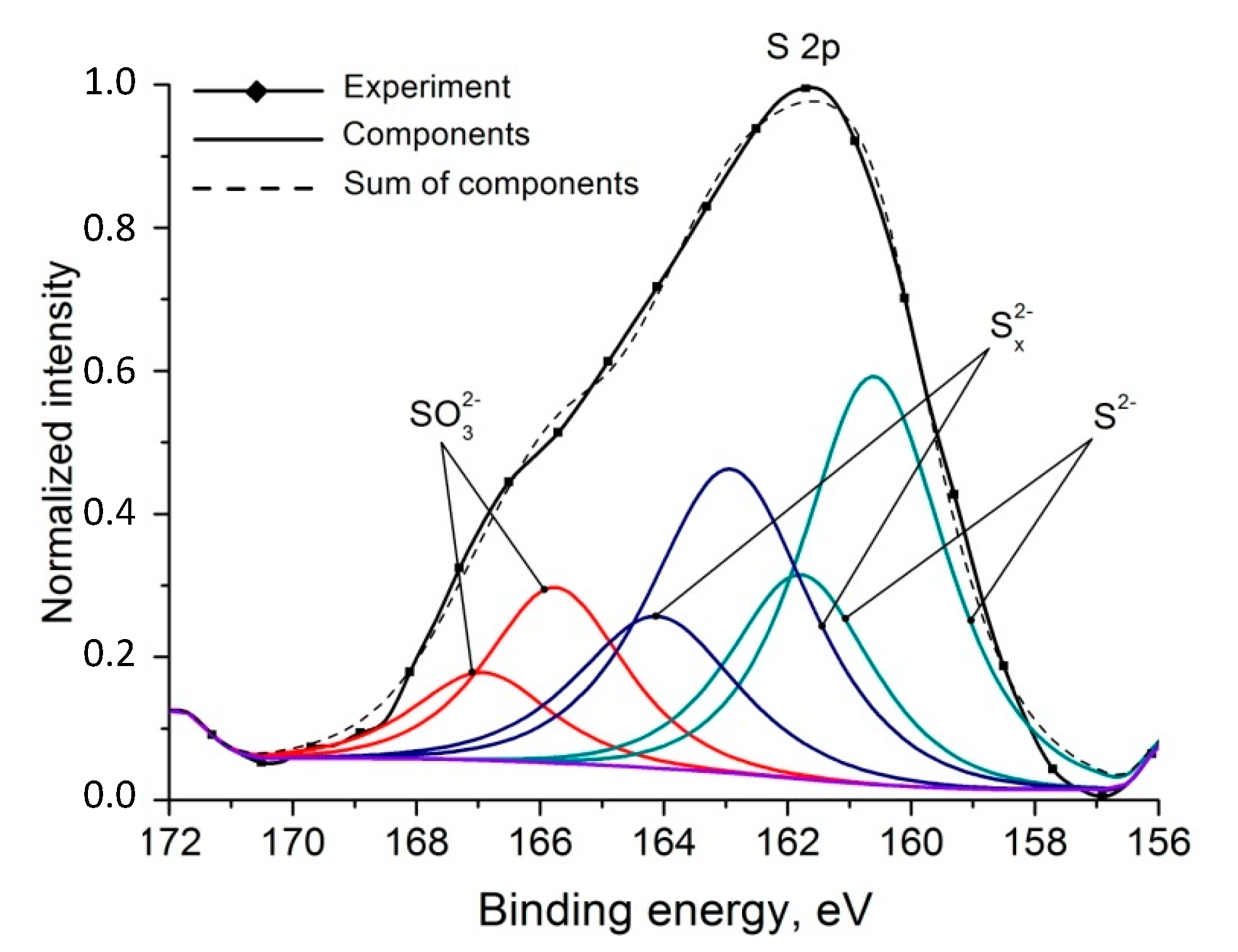
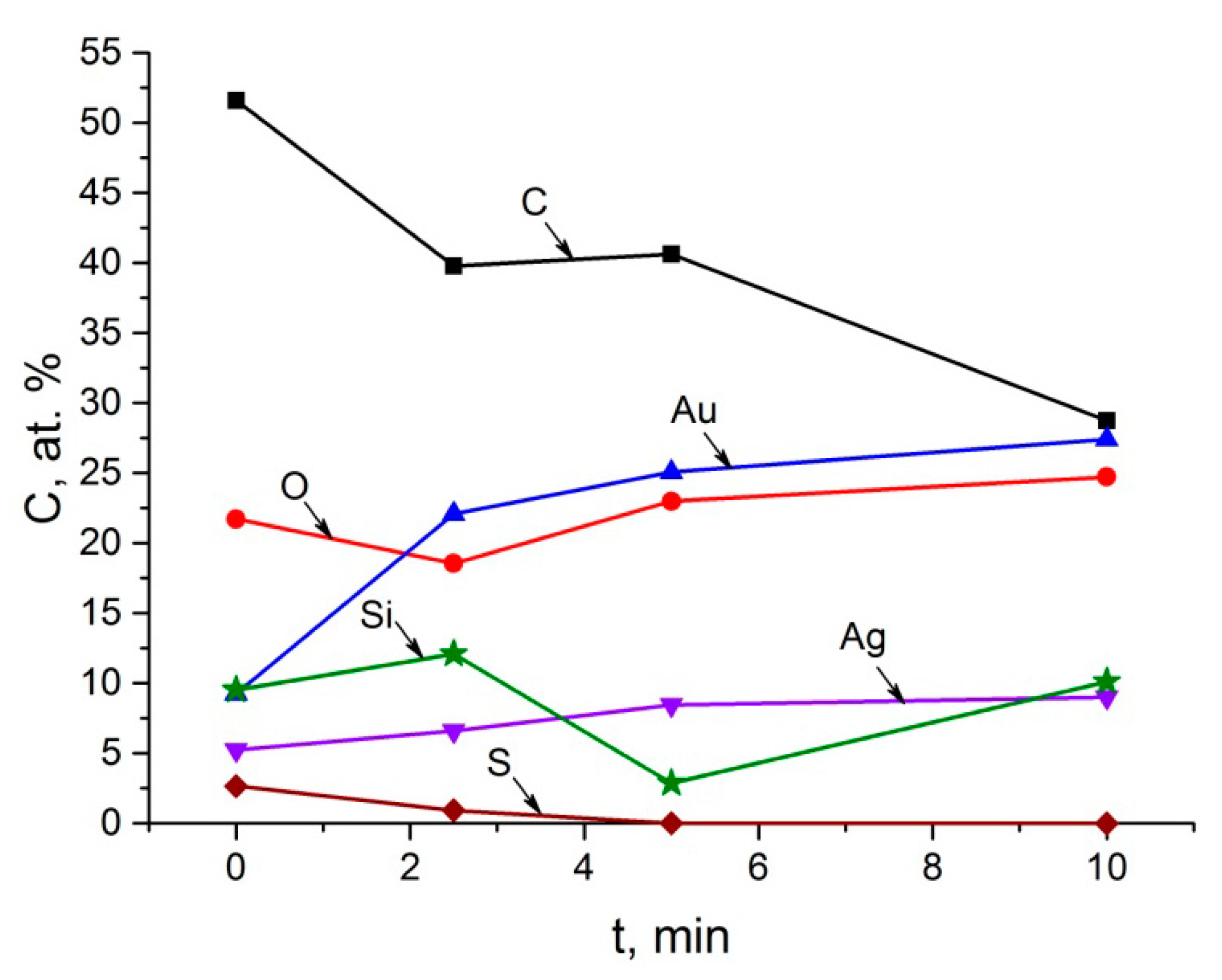
4.1. XPS and AES Data
4.2. EMPA Data
4.3. Chemical Composition of S-Containing Native Gold Samples
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nefedov, V.I.; Zhavoronkov, N.M.; Machavariani, G.V.; Salyn, Ya.V.; Makeev, V.A.; Zelenov, V.I. The variation of silver content in native gold particles as revealed by ESCA. Phys. Chem. Miner. 1982, 8, 193–196. [Google Scholar] [CrossRef]
- Petrovskaya, N.V.; Aleshin, V.G.; Novgorodova, M.I.; Nemoshkalenko, V.V. New data on the composition of surface layers of native gold. Dokl. Earth Sci. 1987, 292, 1241–1244. (In Russian) [Google Scholar]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T.; Ilieva, L.; Iadakiev, V. Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production. Catal. Today 2002, 75, 169–175. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Vericat, C.; Benitez, G.A.; Vela, M.E.; Tognalli, N.; Fainstein, A.; Martiarena, M.L.; Salvarezza, R.C. Spontaneously formed sulfur adlayers on gold in electrolyte solutions: Adsorbed sulfur or gold sulfide? J. Phys. Chem. C 2008, 112, 11394–11402. [Google Scholar] [CrossRef]
- Quek, S.Y.; Biener, M.M.; Biener, J.; Bhattacharjee, J.; Friend, C.M.; Waghmare, U.V.; Kaxiras, E. Rich coordination chemistry of Au adatoms in gold sulfide monolayer on Au (111). J. Phys. Chem. B 2006, 110, 15663–15665. [Google Scholar] [CrossRef] [PubMed]
- Gusmano, G.; Montanari, R.; Kaciulis, S.; Montesperelli, G.; Denk, R. “Gold corrosion”: Red stain on a gold Austrian Ducat. Appl. Phys. A 2004, 79, 205–211. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Ya.V.; Makeev, V.A.; Zelenov, V.I. Surface composition of native gold and Ag/Au alloys. J. Electron. Spectrosc. Relat. Phenom. 1981, 24, 11–17. [Google Scholar] [CrossRef]
- Shchegol’kov, Yu.V.; Amosov, R.A. Oxidation of native gold in placers. Dokl. Earth Sci. 2000, 370, 520–523. (In Russian) [Google Scholar]
- Shchegol’kov, Yu.V. Formation of thin gold oxide films on the native gold in natural conditions. Zapiski RMO (Proc. Russ. Miner. Soc.) 2013, 142, 126–132. (In Russian) [Google Scholar]
- Palyanova, G.; Karmanov, N.; Savva, N. Sulfidation of native gold. Am. Mineral. 2014, 99, 1095–1103. [Google Scholar] [CrossRef]
- Anisimova, G.S.; Kondrateva, L.A.; Leskova, N.V. Sulfide compounds of gold and silver in gold-ore deposits of East Yakutia. Otechestvennaya Geol. 2008, 5, 24–32. (In Russian) [Google Scholar]
- Barton, M.D. The Ag-Au-S system. Econ. Geol. 1980, 75, 303–316. [Google Scholar] [CrossRef]
- Folmer, J.C.W.; Hofman, P.; Wiegers, G.A. Order-disorder transitions in the system Ag2−xAuxS (0< x <1). J. Less Common Met. 1976, 48, 251–268. [Google Scholar] [CrossRef]
- Savva, N.E.; Pal’yanova, G.A. Genesis of gold and silver sulfides at the Ulakhan deposit (Northeastern Russia). Russ. Geol. Geophys. 2007, 48, 799–810. [Google Scholar] [CrossRef]
- Kucha, H.; Raith, J.G. Gold-oxysulphides in copper deposits of the Greywacke Zone, Austria: A mineral chemical and infrared fluid inclusion study. Ore Geol. Rev. 2009, 35, 87–100. [Google Scholar] [CrossRef]
- Petrovskaya, N.V.; Safonov, Yu.G.; Sher, S.D. Formations of gold deposits. In Ore Formations of Endogenous Deposits; Nauka: Moscow, Russia, 1976; Volume 2, pp. 3–110. (In Russian) [Google Scholar]
- Belyi, V.F. Problems of the geological and isotope age of the Okhotsk-Chukotka volcanogenic belt. Stratigr. Geol. Correl. 2008, 16, 64–75. (In Russian) [Google Scholar] [CrossRef]
- Layer, P.W.; Ivanov, V.V.; Ratkin, V.V.; Bundtsen, T.K. Epithermal gold-silver deposits of North-East Russia: First Ar-40-Ar-39 data of ore ages. Dokl. Earth Sci. 1997, 356, 665–668. (In Russian) [Google Scholar]
- Kravtsova, R.G.; Borovikov, A.A.; Borisenko, A.S.; Prokof’ev, V.Y. Formation conditions of gold-silver deposits in the northern Okhotsk Region, Russia. Geol. Ore Depos. 2003, 45, 395–415. [Google Scholar]
- Spiridonov, A.M.; Zorina, L.D.; Kitaev, N.A. Gold-Bearing Ore-Magmatic Systems of Transbaikalia; Academic Publishing House “Geo”: Novosibirsk, Russia, 2006. (In Russian) [Google Scholar]
- Tupyakov, V.E.; Shirokih, I.N.; Rozov, D.N. The model of the ore-metasomatic column of the Kariyskoe ore field (Eastern Transbaikalia). Geol. Geophys. 1982, 23, 33–38. (In Russian) [Google Scholar]
- Prokofiev, V.Yu.; Spiridonov, A.M.; Kuzmina, T.M. Physicochemical conditions of mineralizing processes at the Kariiskoegold deposit, Eastern Transbaikalia. Geokhimiya 1997, 4, 423–434. (In Russian) [Google Scholar]
- Tauson, V.L.; Goettlicher, J.; Sapozhnikov, A.N.; Mangold, S.; Lustenberg, E.E. Sulphur speciation in lazurite-type minerals (Na,Ca)8[Al6Si6O24](SO4,S)2 and their annealing products: A comparative XPS and XAS study. Eur. J. Mineral. 2012, 24, 133–152. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lustenberg, E.E. Quantitative determination of modes of gold occurrence in minerals by the statistical analysis of analytical data sampling. Geochem. Int. 2008, 46, 423–428. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Koshcheyev, S.V.; Boronin, A.I. Oxidation of the polycrystalline gold foil surfaces and X-ray photoelectronic spectroscopy study of oxygen states in oxide layers. Vestn. Mosc. Univ. Khimia 2007, 48, 418–426. (In Russian) [Google Scholar]
- Kuo, C.-L.; Huang, M.H. Hydrothermal synthesis of free-floating Au2S nanoparticle superstructures. J. Phys. Chem. C 2008, 112, 11661–11666. [Google Scholar] [CrossRef]
- Osadchii, E.G.; Rappo, O.A. Determination of standard thermodynamic properties of sulfides in the Ag-Au-S system by means of a solid-state galvanic cell. Am. Mineral. 2004, 89, 1405–1410. [Google Scholar] [CrossRef]
- Cocker, H.A.; Mauk, J.L.; Rabone, S.D.C. The origin of Ag-Au-S-Se minerals in adularia-sericite epithermal deposits: Constrains from the Broken Hills deposit, Hauraki Goldfield, New Zealand. Miner. Depos. 2013, 48, 249–266. [Google Scholar] [CrossRef]
- Tauson, V.L.; Kravtsova, R.G. Chemical typomorphism of mineral surfaces: Surface composition specifics (by the example of gold-bearing pyrite from epithermal deposit). Russ. Geol. Geophys. 2004, 45, 222–227. [Google Scholar]
- Wu, Z.; Jiang, D.; Lanni, E.; Bier, M.E.; Jin, R.C. Sequential observation of AgnS4− (1 ≤ n ≤ 7) gas phase clusters in MS/MS and prediction of their structures. J. Phys. Chem. Lett. 2010, 1, 1423–1427. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, Y.L.; Lu, W.; Wang, J.; Yin, W.P. Density functional study of AgnAum and AgnAum+ (n + m ≤ 5) clusters interaction with a single S atom. Comput. Theor. Chem. 2013, 1017, 188–193. [Google Scholar] [CrossRef]
- Banfield, J.F.; Welch, S.A.; Zhang, H.Z.; Ebert, T.T.; Penn, R.L. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Fedorov, P.P.; Baranchikov, A.Y.; Osiko, V.V. Oriented attachment of particles: 100 years of investigations of non-classical crystal growth. Russ. Chem. Rev. 2014, 83, 1204–1222. [Google Scholar] [CrossRef]
- Abramovich, M.G.; Shmakin, B.M.; Tauson, V.L.; Akimov, V.V. Mineral typochemistry: Anomalous trace-element concentrations in solid solutions with defect structures. Int. Geol. Rev. 1990, 32, 608–615. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Yanakieva, D.Y. First results of structural study of macroscopic native silver economic deposits on the nanolevel. Dokl. Earth Sci. 2012, 444, 639–641. [Google Scholar] [CrossRef]
- Ostapenko, L.A.; Ryzhov, O.B.; Shchegol’kov, Yu.V.; Aristov, V.V. Typical features of placer gold from the Adycha river basin, East Yakutia: Grain morphology, chemistry and origin of coatings. Otechestvennaya Geol. 2011, 1, 29–42. (In Russian) [Google Scholar]

| Deposit | Sample No. | Sampling Point | Sample Characteristics | |
|---|---|---|---|---|
| Rock Sample | Native Gold | |||
| Kvartsevaya Sopka | P;-5706 | Natural outcrop, Trench 203 | Quartz-adularia vein with sulfides (acanthite, silver sulfosalts, pyrite), native silver and native gold (electrum) | Euhedral crystals and their aggregates 0.2–0.5 mm in size. Impurities, wt % (Table 4): 28.4–32.5 (av. 30.5) Ag, bdl-0.17 Te, bdl-0.54 S. |
| KK-1 | Adit No. 2, rise 1 | Quartz-adularia vein with sulfides (acanthite, silver sulfosalts, galena, sphalerite, pyrite) and native gold (electrum) | Euhedral crystals and their aggregates 0.5–1 mm in size. Impurities, wt % (Table 4): 27.8–31.7 (av. 29.2) Ag, bdl-0.29 Pb, bdl-0.16 Zn, bdl-0.39 S. | |
| P-5707 | Adit No. 4, inset 24 | Quartz-adularia vein with sulfides (acanthite, silver sulfosalts, minerals of the tetrahedrite group, pyrite, galena, sphalerite, chalcopyrite), naumannite and native gold (electrum) | Euhedral crystals and their aggregates 0.3–1 mm in size. Impurities, wt % (Table 4): 27.4–36.1 (av. 31.5) Ag, bdl-0.31 Cu, bdl-0.41 Pb, bdl-0.25 Zn, bdl-1.19 Bi, bdl-0.54 Fe, bdl-0.27 S, bdl-0.75 Se. | |
| P-4581 | Adit No. 4, inset 2 | Quartz-adularia vein with sulfides (acanthite, silver sulfosalts, minerals of the tetrahedrite group, pyrite, galena, sphalerite, chalcopyrite), naumannite and native gold (electrum) | Euhedral crystals and their aggregates 0.1–0.5 mm in size. Impurities, wt % (Table 4): 23.9–28.6 (av. 27.6) Ag, bdl-0.22 As, bdl-0.22 Cu, 0.5–1.5 (av. 1.2) Bi, bdl-0.3 Fe, bdl-0.36 S. | |
| Amurskie Daiki | V-62/3 | Natural outcrop (North-East) | Veinlets of quartz and actinolite-quartz composition with native gold in propylitized and pyritized hybrid porphyries | Aggregates of euhedral crystals 0.1–0.4 mm in size. Impurities, wt % (Table 5): 1.0–2.7 (av. 1.8) Ag, bdl-0.29 Sb, 0.1–0.5 (av. 0.2) Cu, bdl-0.1 S. |
| KP-52 | Natural outcrop (South-West) | K-feldspar-quartz veinlets with native gold in pyritized metasomatites of chlorite-carbonate-albite compositions, developed along cataclastic diorite | Aggregates of euhedral crystals 0.5–3 mm in size. Impurities, wt % (Table 5): 1.8–5.0 (av. 2.6) Ag, 0.1–0.5 (av. 0.3) Cu, bdl-0.1 Fe, bdl-0.1 S. | |
| Sample No. | Photo-Electron Peak | Binding Energy (eV) | FWHM (eV) a | MPE b | MF c |
|---|---|---|---|---|---|
| KP-52 | Au 4f7/2 | 83.9 | 2.5 | Au(0) | 1 |
| Au 4f5/2 | 87.6 | 2.5 | |||
| V-62/3 | Au 4f7/2 | 83.7 | 3.1 | Au(0) | 1 |
| Au 4f5/2 | 87.4 | 3.1 | |||
| P-5706 | Au 4f7/2 | 84 | 3.5 | Au(0) | 1 |
| Au 4f5/2 | 87.7 | 3.5 | |||
| KK-1 | Au 4f7/2 | 84 | 3.1 | Au(0) | 1 |
| Au 4f5/2 | 87.7 | 3.1 | |||
| P-4581 | Au 4f7/2 | 83.5 | 2.4 | Au(0) | 0.63 |
| Au 4f5/2 | 87.2 | 2.4 | |||
| Au 4f7/2 | 85 | 2.5 | Au(ox) | 0.37 | |
| Au 4f5/2 | 88.6 | 2.5 | |||
| P-5707 | Au 4f7/2 | 84.3 | 2.3 | Au(0) | 0.43 |
| Au 4f5/2 | 88 | 2.3 | |||
| Au 4f7/2 | 85.8 | 2.6 | Au(ox) | 0.57 | |
| Au 4f5/2 | 89.5 | 2.6 | |||
| S 2p3/2 | 160.6 | 2.7 | S2− | 0.44 | |
| S 2p1/2 | 161.8 | 2.7 | |||
| S 2p3/2 | 162.9 | 3 | Sx2− | 0.37 | |
| S 2p1/2 | 164.1 | 3 | |||
| S 2p3/2 | 165.8 | 2.6 | SO32− | 0.19 | |
| S 2p1/2 | 166.9 | 2.6 |
| Sample No. | Atomic Percent * | Ag/Au | (Ag + Au)/S | |||||
|---|---|---|---|---|---|---|---|---|
| Ag | Au | S | O | Cl | Sb | |||
| KK-1 | 12.5 | 3.4 | 12.0 | 4.5 | 1.3 | 2.4 | 3.68 | 1.32 |
| P-4581 | 12.1 | 2.5 | 11.0 | 3.1 | 0.8 | - | 4.84 | 1.33 |
| P-5706 | 4.9 | 2.2 | 8.0 | 3.5 | - | - | 2.23 | 0.89 |
| P-5707 | 12.1 | 3.0 | 21.1 | 3.4 | 0.9 | - | 4.03 | 0.72 |
| n | Au | Ag | As | Te | Se | Cu | Pb | Zn | Bi | Fe | S | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural Outcrop, Trench 203. Sample P-5706 | ||||||||||||
| Grain 1 | ||||||||||||
| 1 | 67.76 | 30.86 | < | < | < | < | < | < | < | < | 0.19 | 98.81 |
| 2 | 69.96 | 28.40 | < | < | < | < | < | < | < | < | < | 98.36 |
| 3 | 70.01 | 28.47 | < | < | < | < | < | < | < | < | 0.13 | 98.61 |
| 4 | 70.02 | 29.08 | < | 0.15 | < | < | < | < | < | < | < | 99.25 |
| Grain 2 | ||||||||||||
| 5 | 67.46 | 31.55 | < | < | < | 0.13 | < | < | < | < | 0.34 | 99.48 |
| 6 | 68.47 | 31.30 | < | 0.17 | < | < | < | < | < | < | 0.54 | 100.48 |
| 7 | 68.53 | 31.75 | < | 0.12 | < | < | < | < | < | < | 0.35 | 100.75 |
| 8 | 66.84 | 32.55 | < | < | < | < | < | < | < | < | 0.50 | 99.90 |
| Adit 2, rise 1. Sample KK-1 (depth from the surface 50 m) | ||||||||||||
| Grain 1 | ||||||||||||
| 9 | 67.07 | 31.71 | < | < | < | < | < | < | < | < | 0.39 | 99.17 |
| 10 | 68.97 | 29.25 | < | < | < | < | < | < | < | < | 0.14 | 98.36 |
| 11 | 69.45 | 30.65 | < | < | < | < | 0.29 | < | < | < | < | 100.39 |
| Grain 2 | ||||||||||||
| 12 | 70.47 | 27.83 | < | 0.12 | < | < | < | < | < | < | 0.21 | 98.63 |
| 13 | 70.79 | 28.53 | < | 0.15 | < | < | 0.19 | < | < | < | 0.14 | 99.80 |
| 14 | 70.85 | 28.07 | < | 0.13 | < | < | < | < | < | < | 0.15 | 99.20 |
| 15 | 70.54 | 28.13 | < | < | < | < | 0.15 | 0.16 | < | < | < | 98.98 |
| Adit 4, inset 24. Sample P-5707 (depth from the surface 150 m) | ||||||||||||
| Grain 1 | ||||||||||||
| 16 | 70.66 | 28.45 | < | < | < | < | < | < | < | 0.10 | 0.10 | 99.31 |
| 17 | 70.72 | 27.99 | < | < | < | < | < | < | < | 0.12 | 0.12 | 98.95 |
| 18 | 70.91 | 27.69 | < | < | < | < | < | < | < | < | 0.16 | 98.76 |
| Grain 2 | ||||||||||||
| 19 | 70.16 | 28.85 | < | < | < | < | < | < | < | < | 0.19 | 99.20 |
| 20 | 70.19 | 28.25 | < | < | < | < | 0.10 | < | < | 0.10 | 0.17 | 98.81 |
| 21 | 71.97 | 27.35 | < | < | < | < | 0.18 | < | < | < | < | 99.50 |
| Grain 3 | ||||||||||||
| 22 | 63.83 | 34.91 | < | < | < | 0.28 | < | 0.25 | < | < | 0.27 | 99.54 |
| 23 | 62.11 | 36.11 | < | < | < | 0.31 | < | < | < | 0.54 | < | 99.07 |
| 24 | 63.66 | 35.50 | < | < | < | < | < | 0.24 | < | < | 0.27 | 99.67 |
| 25 | 63.76 | 33.85 | < | < | < | < | 0.41 | < | 1.19 | < | 0.17 | 99.38 |
| 26 | 64.28 | 34.62 | < | < | 0.75 | < | < | < | < | < | < | 99.65 |
| 27 | 63.87 | 34.62 | < | < | 0.67 | < | < | < | < | < | < | 99.16 |
| Adit 4, inset 2. Sample P-4581 (depth from the surface 150 m) | ||||||||||||
| Grain 1 | ||||||||||||
| 28 | 70.08 | 28.08 | < | < | < | 0.09 | < | < | 1.16 | 0.18 | 0.36 | 99.95 |
| 29 | 69.53 | 28.57 | < | < | < | < | < | < | 1.44 | < | 0.18 | 99.72 |
| 30 | 70.19 | 28.24 | 0.12 | < | < | 0.12 | < | < | 1.01 | 0.14 | 0.21 | 100.03 |
| 31 | 70.05 | 28.35 | < | < | < | 0.11 | < | < | 1.12 | 0.16 | 0.12 | 99.91 |
| Grain 2 | ||||||||||||
| 32 | 70.07 | 28.50 | < | < | < | 0.22 | < | < | 1.12 | < | 0.22 | 100.13 |
| 33 | 71.49 | 26.33 | 0.22 | < | < | 0.08 | < | < | 1.46 | 0.19 | 0.28 | 100.05 |
| 34 | 71.09 | 23.95 | 0.14 | < | < | < | < | < | 1.05 | 0.15 | 0.24 | 96.62 |
| Grain 3 | ||||||||||||
| 35 | 71.92 | 26.21 | < | < | < | 0.06 | < | < | 1.31 | 0.30 | < | 99.80 |
| 36 | 70.08 | 28.21 | 0.08 | < | < | 0.06 | < | < | 1.41 | < | 0.16 | 100.00 |
| 37 | 70.36 | 27.78 | 0.13 | < | < | 0.05 | < | < | 1.28 | 0.12 | 0.08 | 99.80 |
| 38 | 70.22 | 29.23 | 0.06 | < | < | 0.06 | < | < | 0.50 | < | < | 100.07 |
| n | Au | Ag | Sb | Cu | Fe | S | Total |
|---|---|---|---|---|---|---|---|
| Natural Outcrop (North-East). Profile V. Sample V-62/3 | |||||||
| Grain 1 | |||||||
| 1 | 98.92 | 1.39 | 0.22 | 0.09 | < | 0.11 | 100.73 |
| 2 | 98.42 | 1.23 | < | 0.52 | < | < | 100.17 |
| 3 | 98.11 | 2.25 | < | 0.33 | < | < | 100.69 |
| 4 | 98.24 | 2.54 | < | 0.32 | < | < | 101.10 |
| 5 | 98.42 | 1.24 | < | 0.23 | < | < | 99.89 |
| Grain 2 | |||||||
| 6 | 98.21 | 2.26 | 0.22 | 0.28 | < | 0.10 | 101.07 |
| 7 | 98.21 | 2.36 | 0.23 | 0.23 | < | < | 101.03 |
| 8 | 98.30 | 2.47 | < | 0.21 | < | < | 100.98 |
| 9 | 98.04 | 2.70 | < | 0.29 | < | 0.10 | 101.13 |
| 10 | 99.71 | 1.23 | 0.29 | 0.09 | < | < | 101.32 |
| 11 | 98.30 | 2.50 | < | 0.20 | < | < | 101.00 |
| Grain 3 | |||||||
| 12 | 98.76 | 0.99 | < | 0.17 | < | < | 99.92 |
| 13 | 99.01 | 1.06 | < | 0.12 | < | 0.10 | 100.29 |
| 14 | 99.18 | 1.06 | < | 0.16 | < | < | 100.40 |
| 15 | 98.42 | 1.77 | < | 0.18 | < | < | 100.37 |
| 16 | 98.11 | 2.20 | < | 0.11 | < | < | 100.42 |
| 17 | 98.52 | 1.62 | < | 0.17 | < | < | 100.31 |
| Natural outcrop (South-West). Profile III. Sample KP-52 | |||||||
| Grain 1 | |||||||
| 18 | 97.55 | 2.06 | < | 0.27 | < | < | 99.88 |
| 19 | 96.34 | 2.15 | < | 0.08 | < | < | 98.57 |
| 20 | 96.96 | 1.83 | < | 0.19 | < | < | 98.98 |
| 21 | 96.13 | 2.74 | < | 0.08 | < | 0.09 | 99.04 |
| 22 | 97.23 | 1.80 | < | 0.21 | < | < | 99.24 |
| Grain 2 | |||||||
| 23 | 97.46 | 2.28 | < | 0.11 | < | 0.10 | 99.95 |
| 24 | 97.73 | 2.33 | < | 0.44 | < | < | 100.50 |
| 25 | 97.58 | 2.23 | < | 0.33 | < | < | 100.14 |
| 26 | 97.49 | 2.28 | < | 0.53 | 0.11 | 0.10 | 100.51 |
| 27 | 95.71 | 4.25 | < | 0.31 | 0.07 | 0.08 | 100.42 |
| 28 | 95.48 | 4.95 | < | 0.26 | 0.12 | 0.11 | 100.92 |
| Sample No. | S | Se | Ag | Au | Ag/Au | (Ag + Au)/(S + Se) |
|---|---|---|---|---|---|---|
| KK-1 | 22.69 | 3.27 | 39.29 | 1.91 | 20.57 | 1.59 |
| 23.76 | 0.87 | 43.47 | 0.2 | 217.35 | 1.77 | |
| 23.57 | 1.61 | 28.53 | 4.08 | 6.99 | 2.23 | |
| 23.57 | 2.63 | 40.81 | 3.09 | 13.21 | 1.68 | |
| P-4581 | 29.55 | 3.19 | 45.86 | 2.46 | 18.64 | 1.48 |
| 29.4 | 3.3 | 46.55 | 2.43 | 19.16 | 1.5 | |
| 27.23 | 3.95 | 46.04 | 4.62 | 9.97 | 1.62 | |
| 26.57 | 0.8 | 37.45 | 12.52 | 2.99 | 1.83 | |
| 21.8 | 3.09 | 33.08 | 9.51 | 3.48 | 1.71 | |
| 20.56 | - | 29.51 | 11.42 | 2.58 | 1.99 | |
| 24.32 | - | 34.33 | 13.11 | 2.62 | 1.95 | |
| 26.3 | - | 40.4 | 14.21 | 2.84 | 2.08 | |
| P-5706 | 30.47 | 1.97 | 44.73 | 3.04 | 14.71 | 1.47 |
| 30.72 | 1.71 | 46.37 | 2.84 | 16.33 | 1.52 | |
| P-5707 | 18.12 | 2.37 | 45.49 | 10.24 | 4.44 | 2.72 |
| 15.93 | 2.25 | 46.32 | 7.86 | 5.89 | 2.98 | |
| 26.75 | - | 42.73 | 12.58 | 3.4 | 2.07 | |
| 35.13 | - | 35.76 | 15.69 | 2.28 | 1.46 | |
| 31.89 | 2.02 | 43.69 | 1.25 | 34.95 | 1.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauson, V.L.; Kravtsova, R.G.; Makshakov, A.S.; Lipko, S.V.; Arsent’ev, K.Y. Contrasting Surficial Composition of Native Gold from Two Different Types of Gold Ore Deposits. Minerals 2017, 7, 142. https://doi.org/10.3390/min7080142
Tauson VL, Kravtsova RG, Makshakov AS, Lipko SV, Arsent’ev KY. Contrasting Surficial Composition of Native Gold from Two Different Types of Gold Ore Deposits. Minerals. 2017; 7(8):142. https://doi.org/10.3390/min7080142
Chicago/Turabian StyleTauson, Vladimir L., Raisa G. Kravtsova, Artem S. Makshakov, Sergey V. Lipko, and Kirill Yu. Arsent’ev. 2017. "Contrasting Surficial Composition of Native Gold from Two Different Types of Gold Ore Deposits" Minerals 7, no. 8: 142. https://doi.org/10.3390/min7080142






