A Low Temperature Detoxification Method for Treatment of Chrysotile-Containing Waste Roofing Slate
Abstract
:1. Introduction
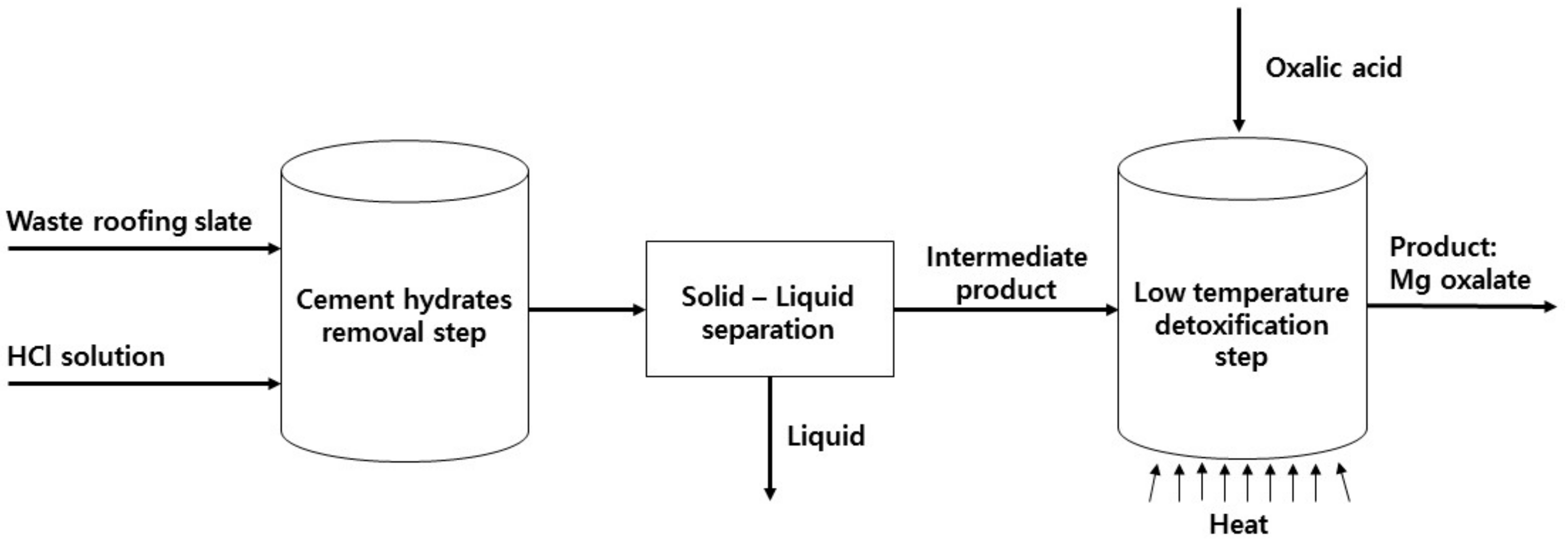
2. Materials and Methods
3. Results and Discussions
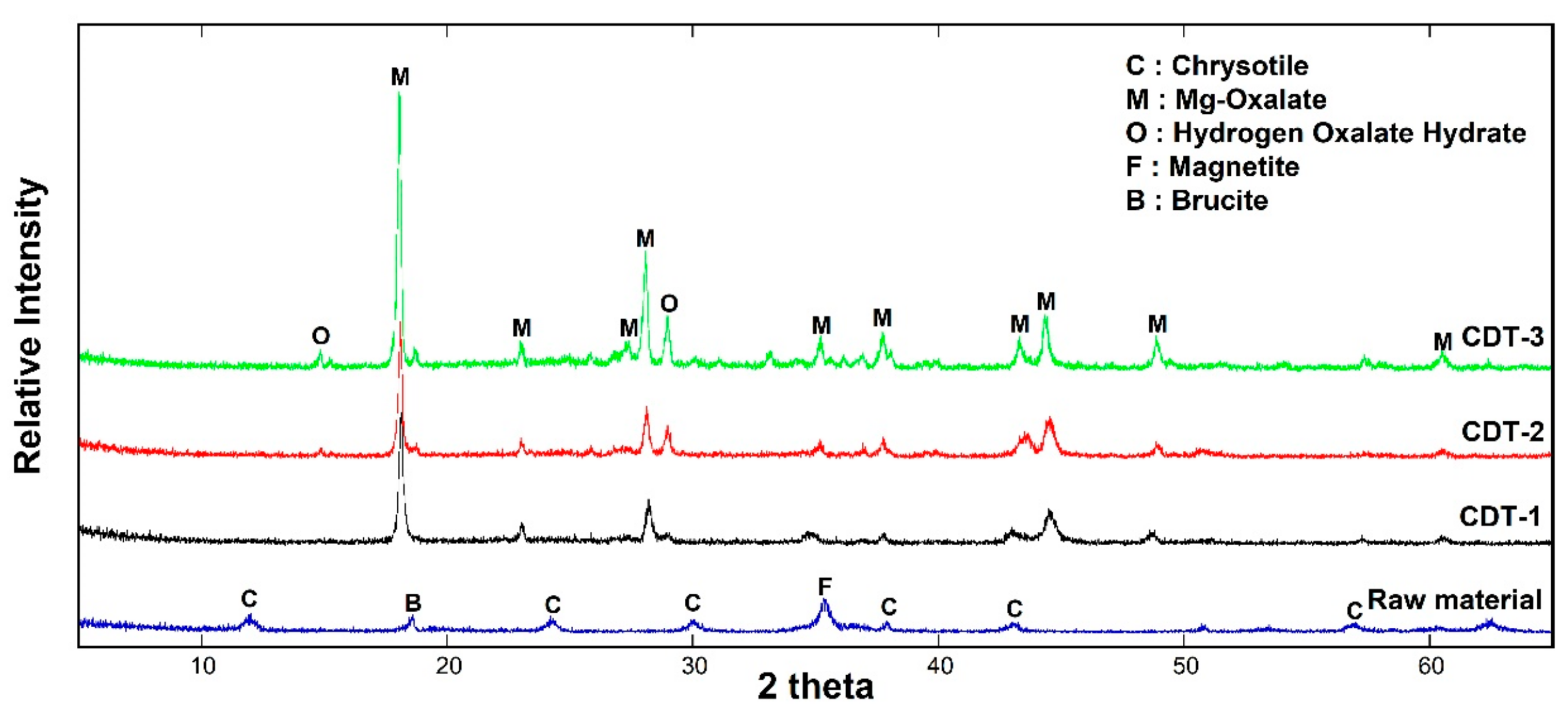
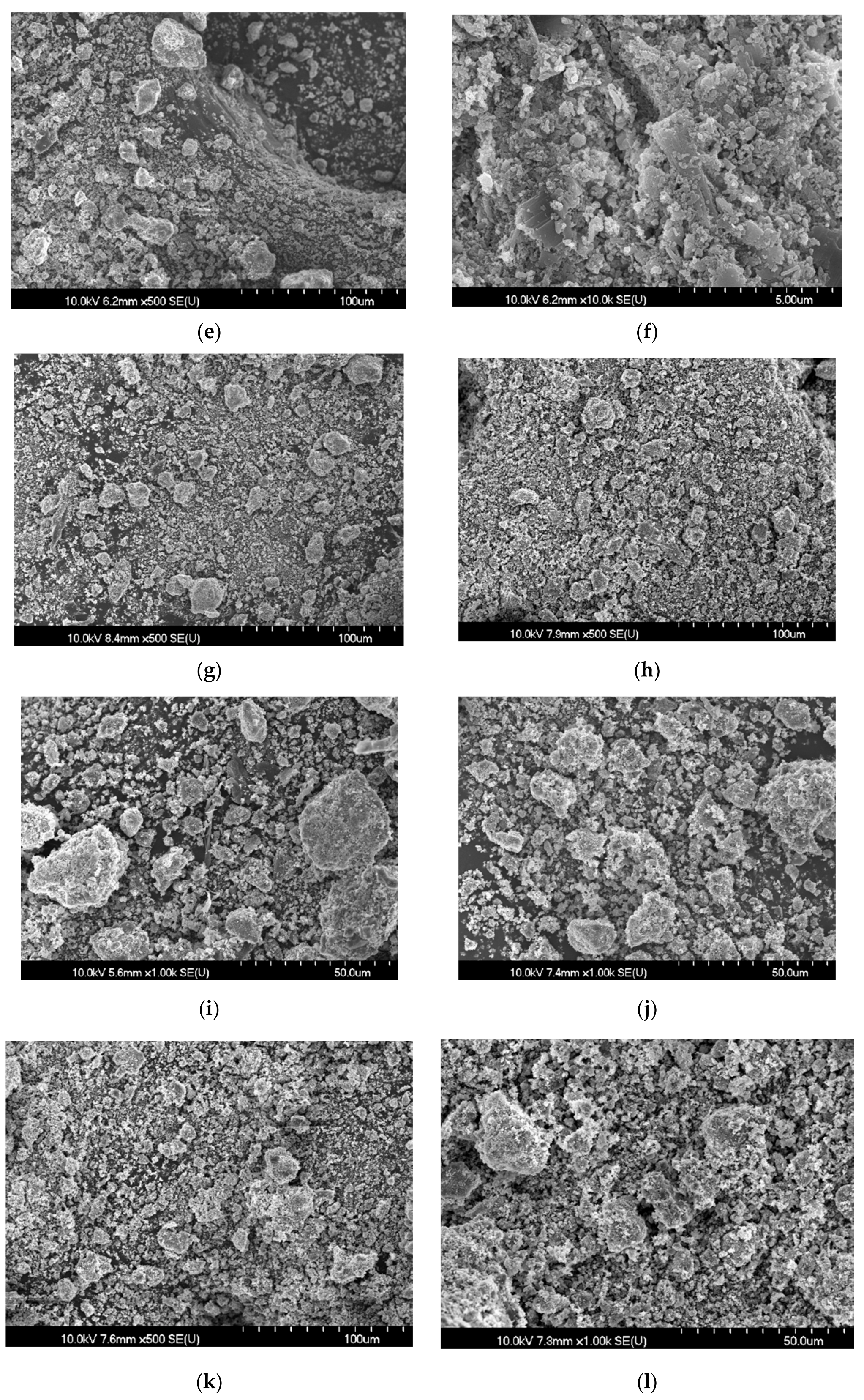
3.1. Low Temperature Detoxification Test Using Chrysotile
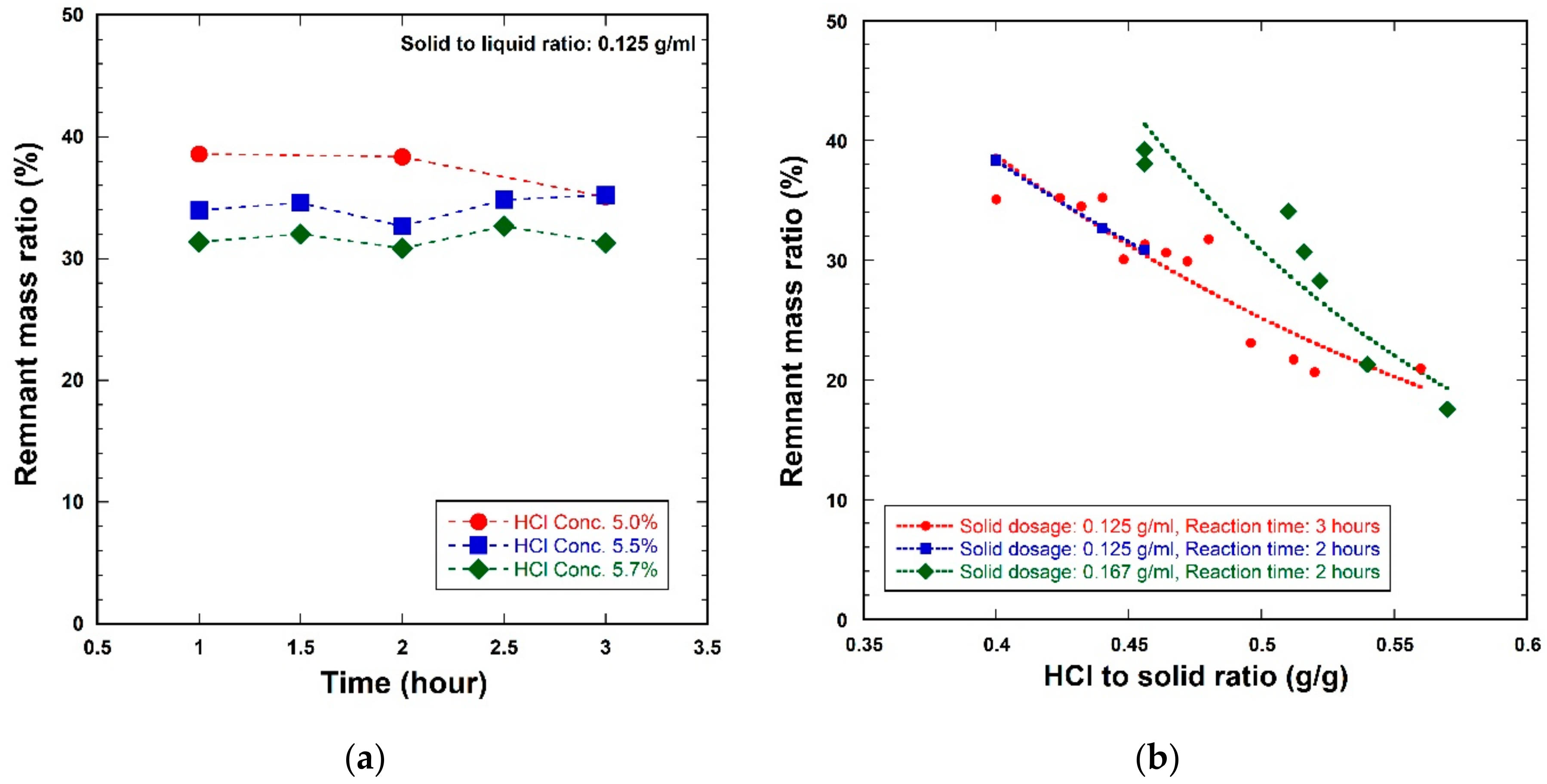
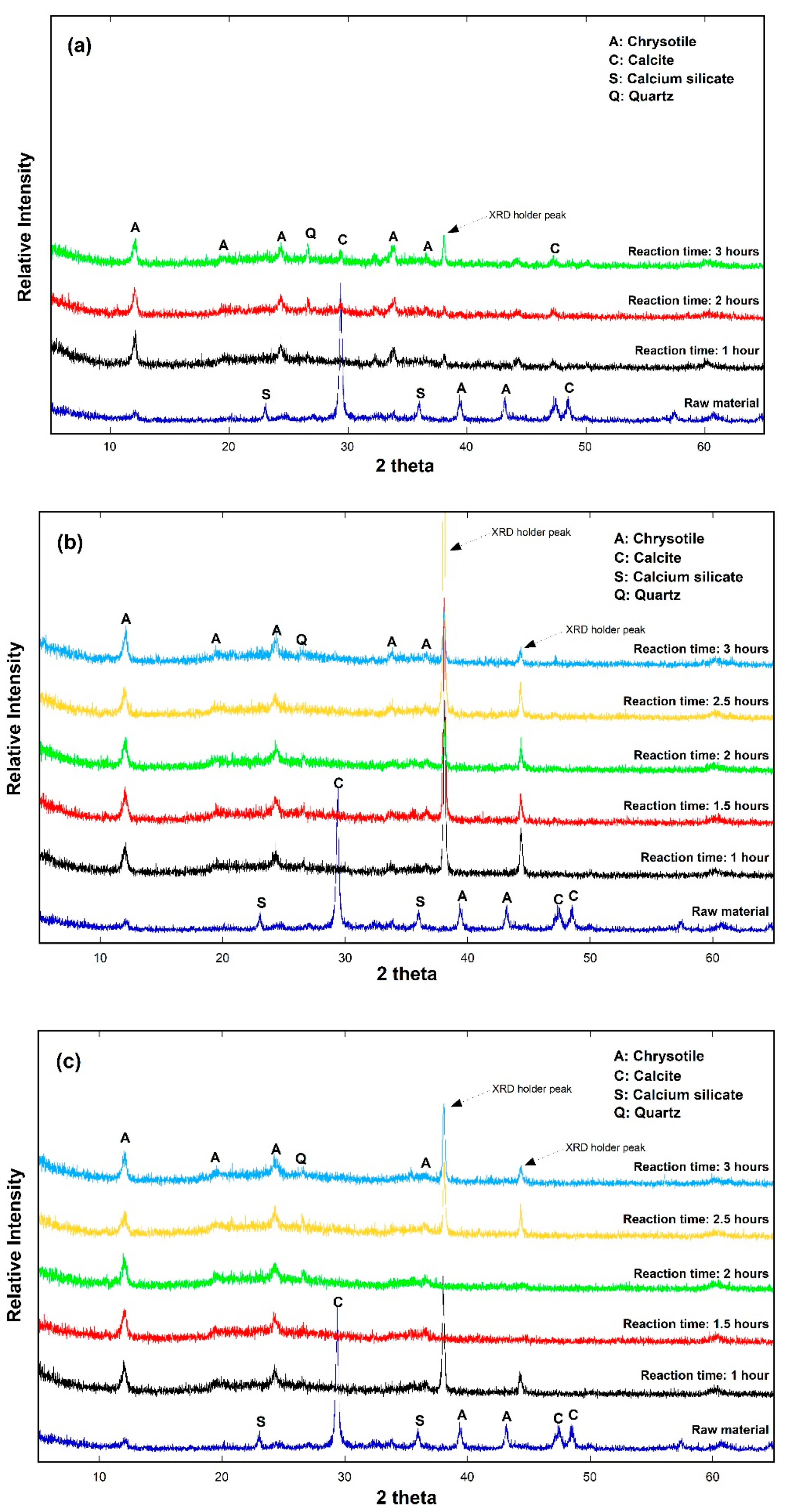
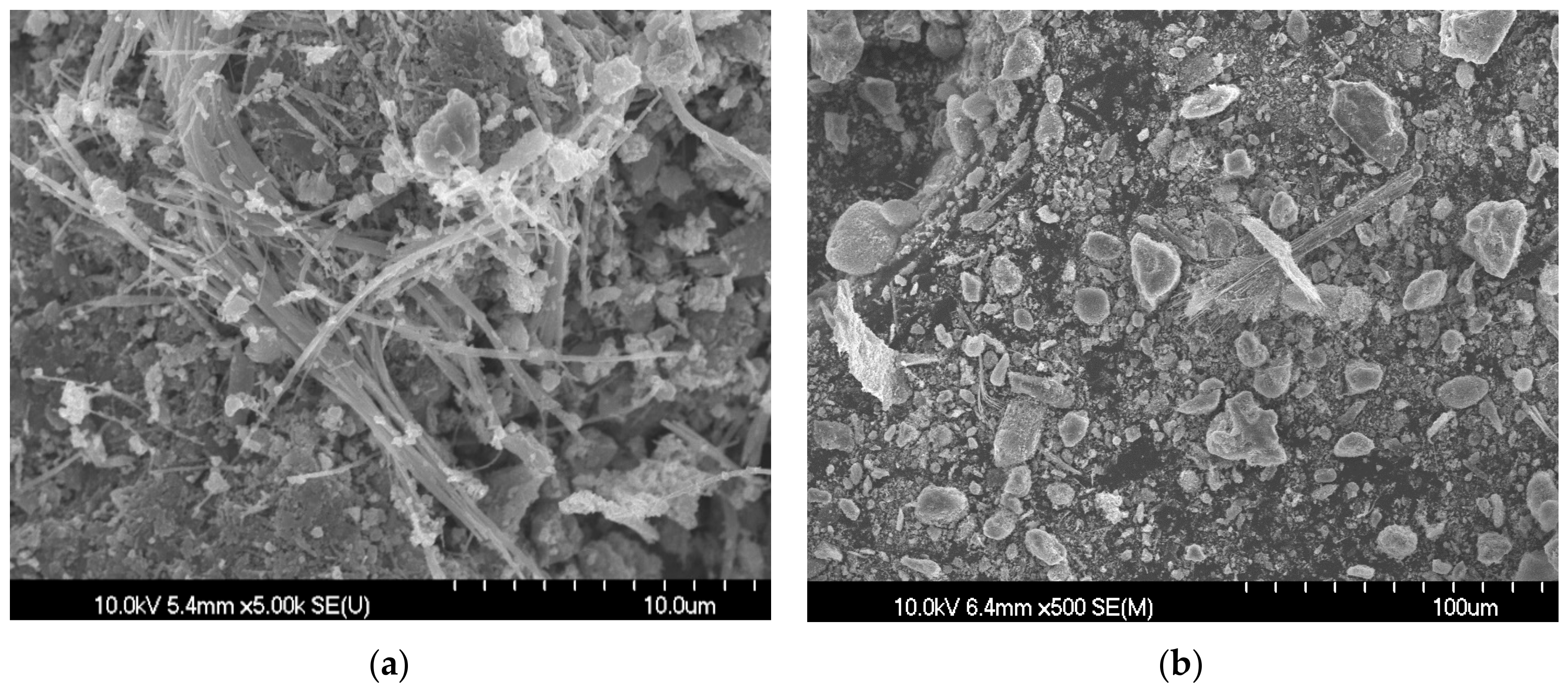
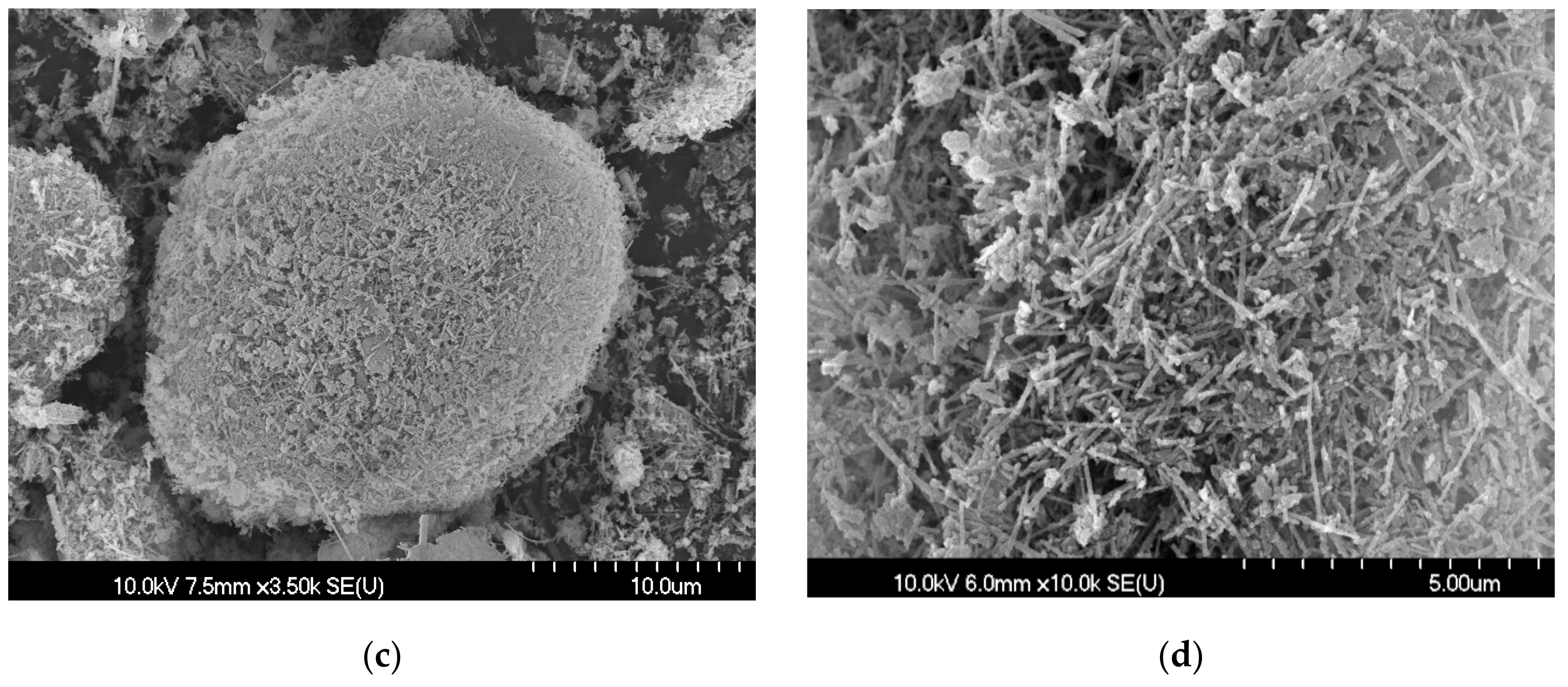
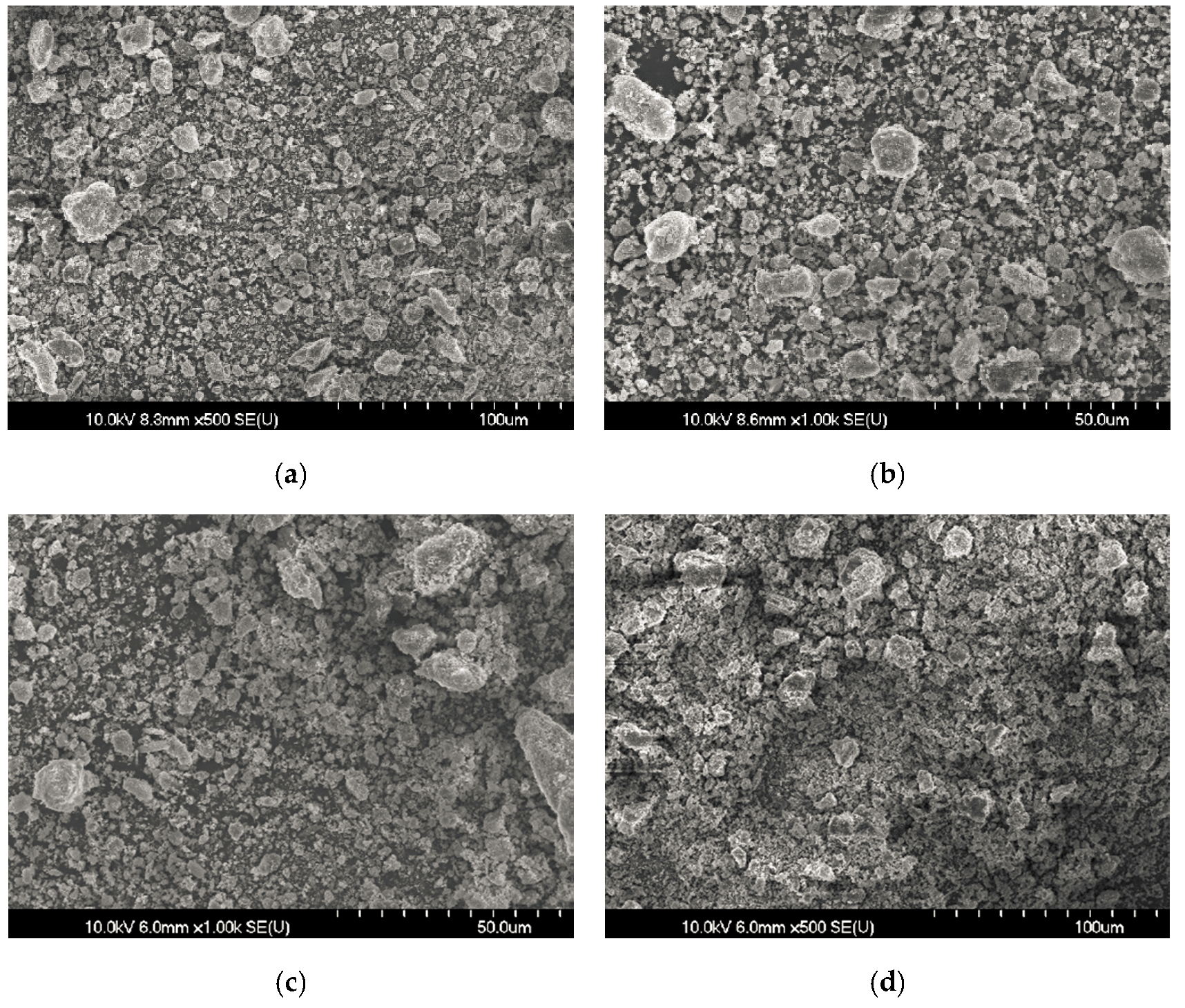
3.2. Cement Hydrate Removal Test Using the Waste Roofing Slate
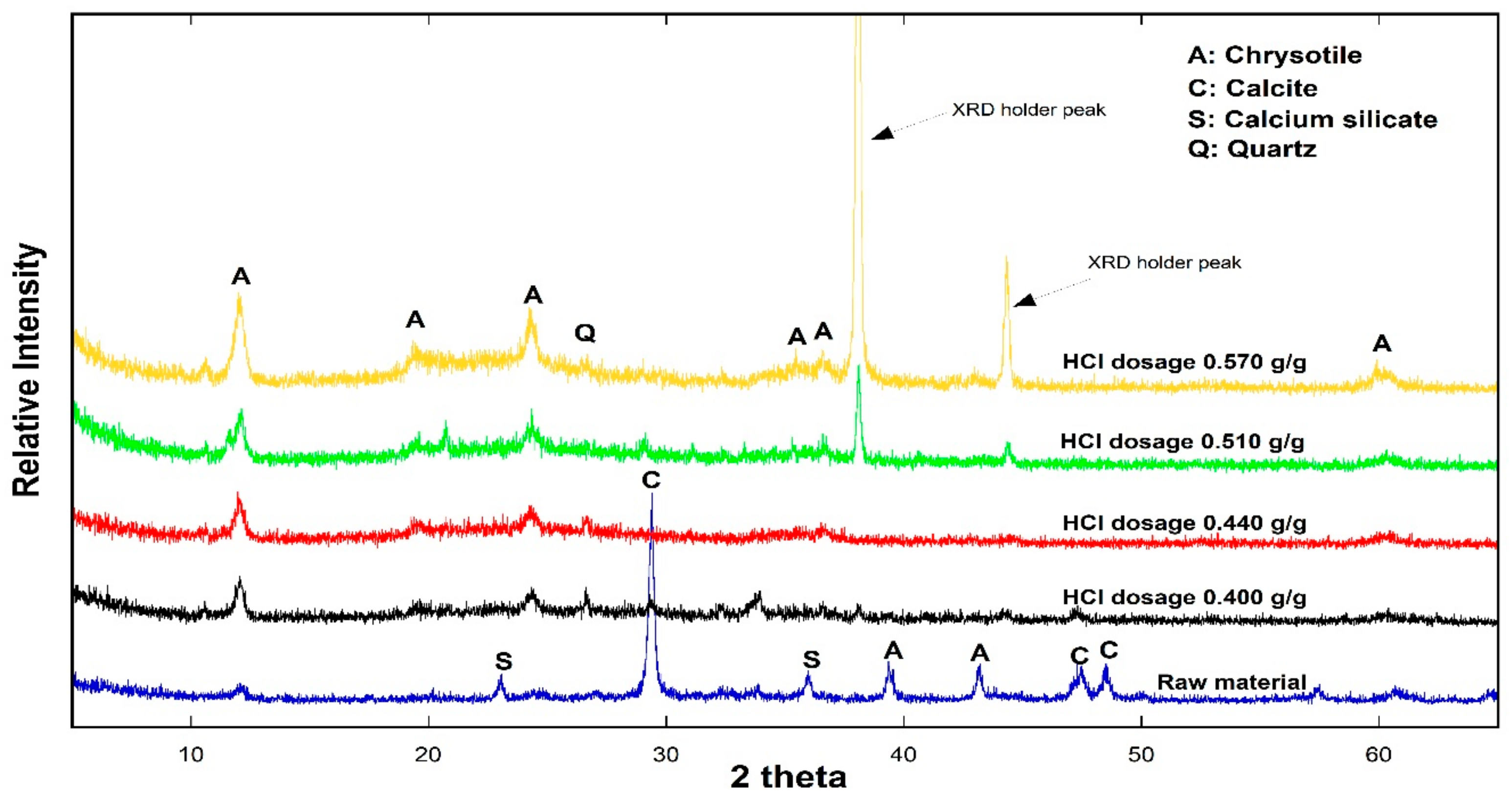
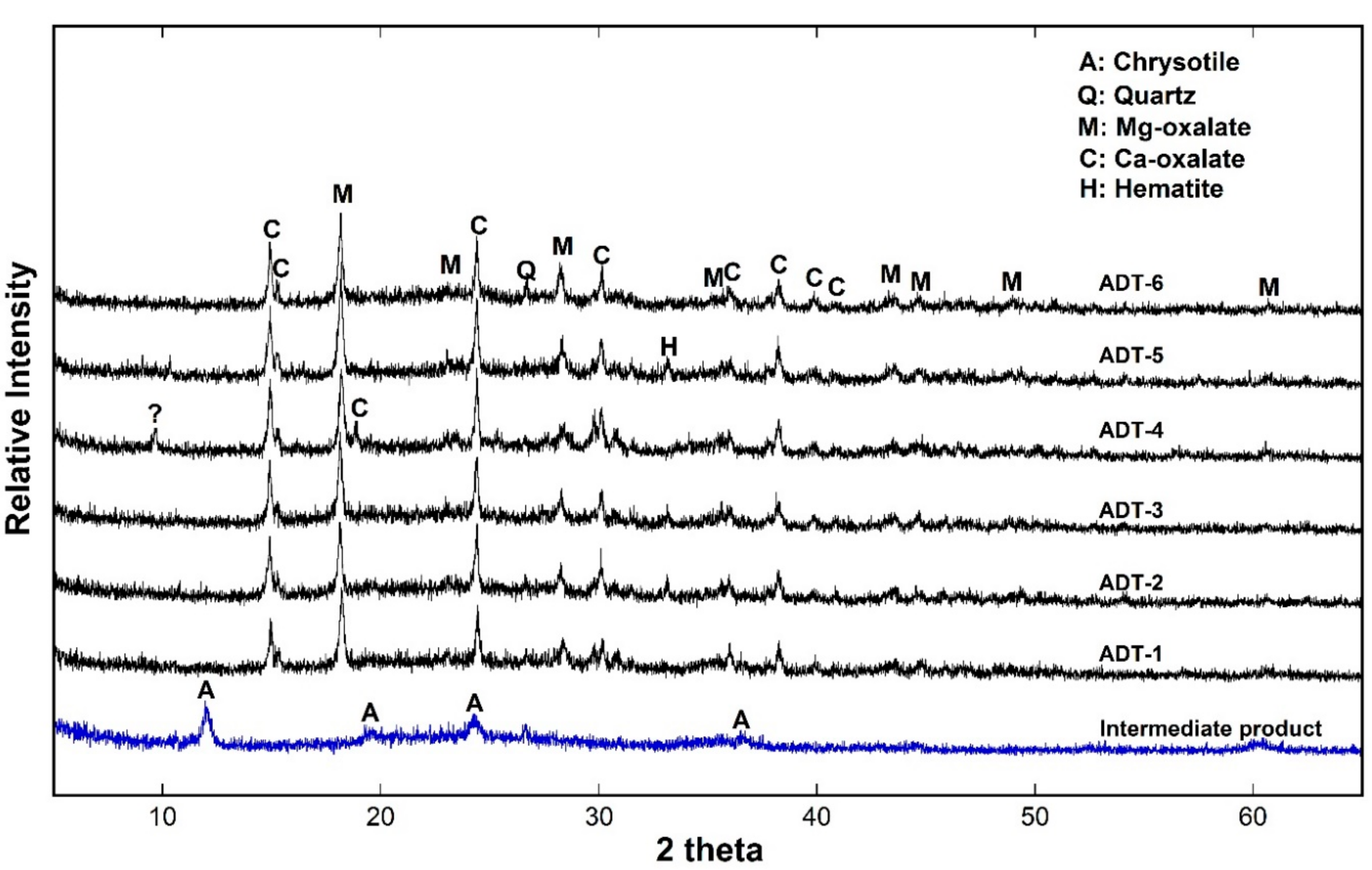
3.3. Low Temperature Detoxification Test Using the Waste Roofing Slate
4. Energy Consumption
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bianchi, C.; Bianchi, T. Asbestos between science and myth. A 6000-year story. Med. Lav. 2015, 106, 83–90. [Google Scholar] [PubMed]
- Kusiorowski, R.; Zaremba, T.; Gerle, A.; Piotrowski, J.; Simka, W.; Adamek, J. Study on the thermal decomposition of crocidolite asbestos. J. Therm. Anal. Calorim. 2015, 120, 1585–1595. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Asbestos: Selected Health Effects. Asbestos: Selected Cancers; National Academies Press (US): Washington, DC, USA, 2006; pp. 1–327. [Google Scholar]
- World Health Organization. Chrysotile Asbestos; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Choi, J.K.; Paek, D.M.; Paik, N.W. The production, the use, the number of workers and exposure level of asbestos in Korea. J. Korean Soc. Occup. Environ. Hyg. 1998, 8, 242–253. [Google Scholar]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Kozawa, T.; Onda, A.; Yanagisawa, K.; Chiba, O.; Ishiwata, H.; Takanami, T. Thermal decomposition of chrysotile-containing wastes in a water vapor atmosphere. J. Ceram. Soc. Jpn. 2010, 118, 1199–1201. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kashimura, K.; Hashiguchi, M.; Sato, M.; Horikoshi, S.; Mitani, T.; Shinohara, N. Detoxification mechanism of asbestos materials by microwave treatment. J. Hazard. Mater. 2015, 284, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Sumi, T.; Ito, S.; Dillert, R.; Kashimura, K.; Yoshikawa, N.; Sato, M.; Shinohara, N. Microwave-driven asbestos treatment and its scale-up for use after natural disasters. Environ. Sci. Technol. 2014, 48, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Averroes, A.; Sekiguchi, H.; Sakamoto, K. Treatment of airborne asbestos and asbestos-like microfiber particles using atmospheric microwave air plasma. J. Hazard. Mater. 2011, 195, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-N.; Jeong, S.; Lim, H. Thermochemical destruction of asbestos-containing roofing slate and the feasibility of using recycled waste sulfuric acid. J. Hazard. Mater. 2014, 265, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sugama, T.; Sabatini, R.; Petrakis, L. Decomposition of chrysotile asbestos by fluorosulfonic acid. Ind. Eng. Chem. Res. 1998, 37, 79–88. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Kozawa, T.; Onda, A.; Kanazawa, M.; Shinohara, J.; Takanami, T.; Shiraishi, M. A novel decomposition technique of friable asbestos by CHClF2-decomposed acidic gas. J. Hazard. Mater. 2009, 163, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Shono, A.; Ghaffar, A. Decomposition of asbestos by a supernatant used for immobilization of heavy metals in fly ash. J. Mater. Cycles Waste Manag. 2016, 18, 483–492. [Google Scholar] [CrossRef]
- Turci, F.; Tomatis, M.; Mantegna, S.; Cravotto, G.; Fubini, B. The combination of oxalic acid with power ultrasound fully degrades chrysotile asbestos fibres. J. Environ. Monit. 2007, 9, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Rozalen, M.; Huertas, F.J. Comparative effect of chrysotile leaching in nitric, sulfuric and oxalic acids at room temperature. Chem. Geol. 2013, 352, 134–142. [Google Scholar] [CrossRef]
- Valouma, A.; Verganelaki, A.; Maravelaki-Kalaitzaki, P.; Gidarakos, E. Chrysotile asbestos detoxification with a combined treatment of oxalic acid and silicates producing amorphous silica and biomaterial. J. Hazard. Mater. 2016, 305, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Owada, S.; Sugiyama, N. The effect of hcl treatment on zeta-potential, flocculation dispersion characteristics and floatability of silicate minerals. Shigen-to-Sozai 1989, 105, 1059–1065. [Google Scholar] [CrossRef]
- Mohandes, F.; Davar, F.; Salavati-Niasari, M. Magnesium oxide nanocrystals via thermal decomposition of magnesium oxalate. J. Phys. Chem. Solids 2010, 71, 1623–1628. [Google Scholar] [CrossRef]

| Material | Chemical Composition (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | MnO | |
| Chrysotile | 26.9 | 0.34 | 31.7 | n.d. 1 | 24.9 | n.d. | 1.90 | 0.44 |
| Waste Roofing Slate | 18.7 | 4.44 | 3.60 | 35.1 | 6.77 | 0.28 | 0.11 | 0.07 |
| No. | Chrysotile (g) | Oxalic Acid (g) | Water (mL) | Solid to Oxalic Acid Ratio (g/g) | Hot Platetemperature (°C) | Reaction Time (min) | Stirring Rate (rpm) |
|---|---|---|---|---|---|---|---|
| CDT-1 | 3 | 3.0 | 30 | 1.0 | 120 | 180 | 150 |
| CDT-2 | 3 | 4.5 | 30 | 1.5 | 120 | 180 | 150 |
| CDT-3 | 3 | 4.5 | 30 | 1.5 | 180 | 60 | 150 |
| Experiment Variables | Experiment Conditions |
|---|---|
| Waste roofing slate (g) | 50, 200, 400, 2000 |
| HCl to solid ratio (g/g) | 0.400~0.520 |
| Amount of HCl solution (mL) | 400~8000 |
| Reaction time (h) | 1~3 |
| Solid to liquid ratio (g/mL) | 0.125~0.250 |
| No. | Intermediate Product (g) | Oxalic Acid (g) | Water (mL) | Oxalic Acid to Solid Ratio (g/g) | Reaction Temperature (°C) | Reaction Time (min) | Mass of Product (g) |
|---|---|---|---|---|---|---|---|
| ADT-1 | 60 | 40 | 120 | 0.67 | 100.5 | 75 | 92.6 |
| ADT-2 | 60 | 43 | 140 | 0.72 | 100.7 | 90 | 90.2 |
| ADT-3 | 60 | 45 | 140 | 0.75 | 100.7 | 100 | 90.2 |
| ADT-4 | 60 | 47 | 140 | 0.78 | 101.3 | 90 | 94.2 |
| ADT-5 | 60 | 50 | 140 | 0.83 | 101.4 | 90 | 97.3 |
| ADT-6 | 90 | 68 | 210 | 0.75 | 101.4 | 135 | 147.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Jang, Y.N.; Jo, J.H. A Low Temperature Detoxification Method for Treatment of Chrysotile-Containing Waste Roofing Slate. Minerals 2017, 7, 144. https://doi.org/10.3390/min7080144
Jo H, Jang YN, Jo JH. A Low Temperature Detoxification Method for Treatment of Chrysotile-Containing Waste Roofing Slate. Minerals. 2017; 7(8):144. https://doi.org/10.3390/min7080144
Chicago/Turabian StyleJo, Hwanju, Young Nam Jang, and Jung Hyun Jo. 2017. "A Low Temperature Detoxification Method for Treatment of Chrysotile-Containing Waste Roofing Slate" Minerals 7, no. 8: 144. https://doi.org/10.3390/min7080144




