Optical Observations and Geochemical Data in Deep-Sea Hexa- and Octo-Coralla Specimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scleractinian Coral and Octocorallan Coral
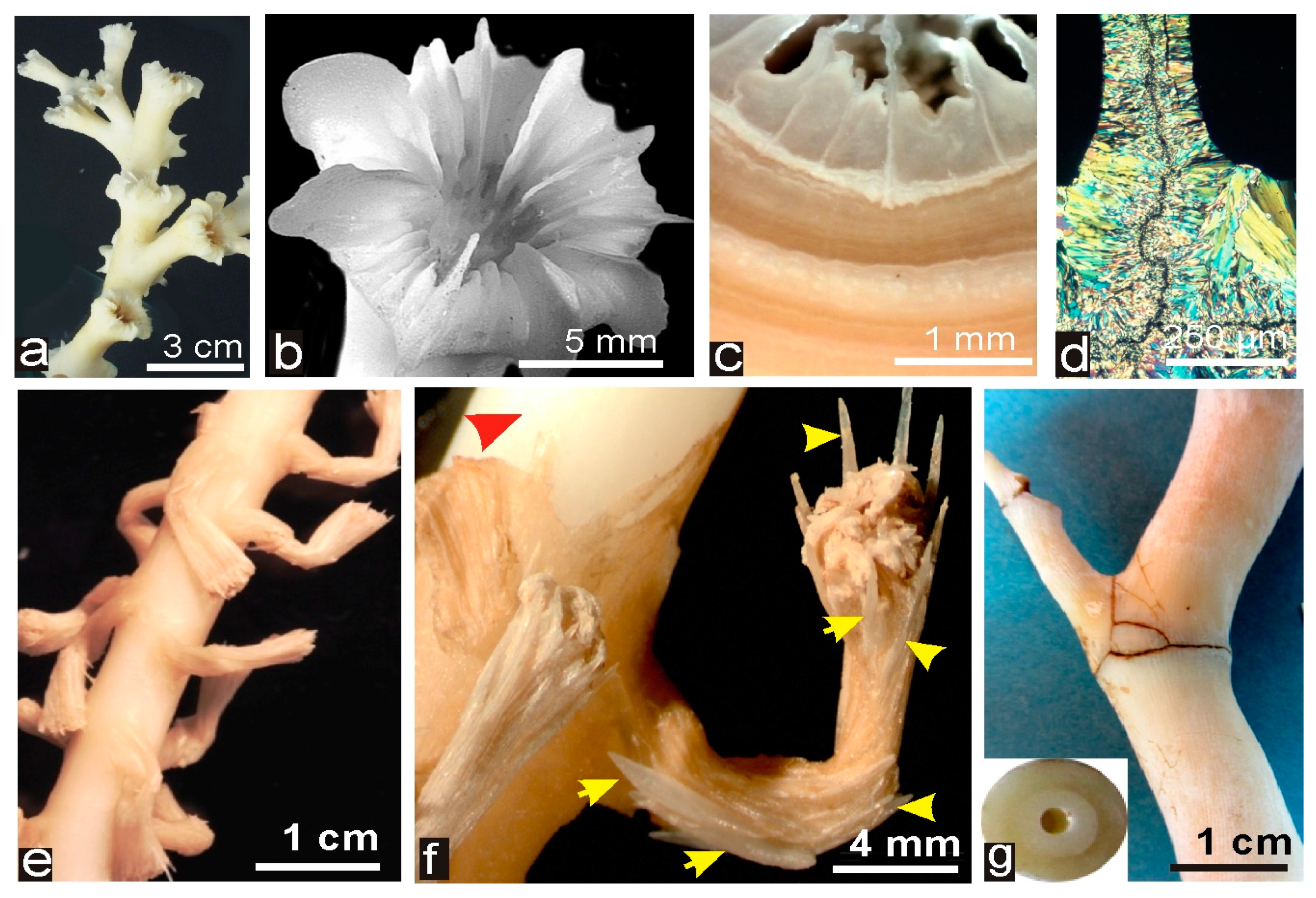
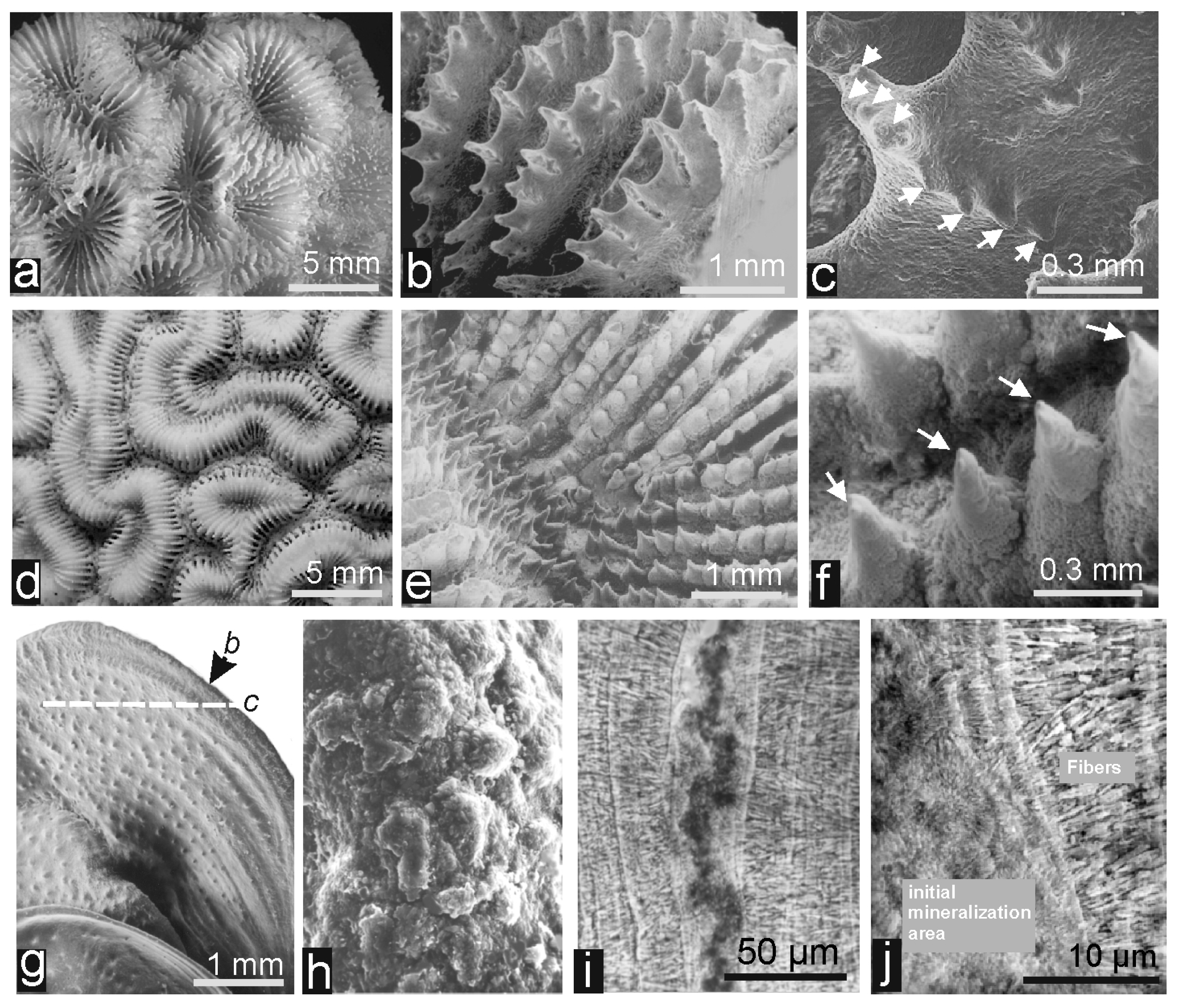
2.2. Description of the Corals Measured in the Study
2.3. Bulk Measurements
2.3.1. Oxygen and Carbon Isotopic Measurements
2.3.2. Trace Element Measurements
2.4. SIMS Measurements
2.4.1. Oxygen and Boron Isotopic Measurements
2.4.2. Trace Elements
3. Results
3.1. Oxygen and Carbon Isotopic Compositions
3.2. Boron Isotopic Compositions
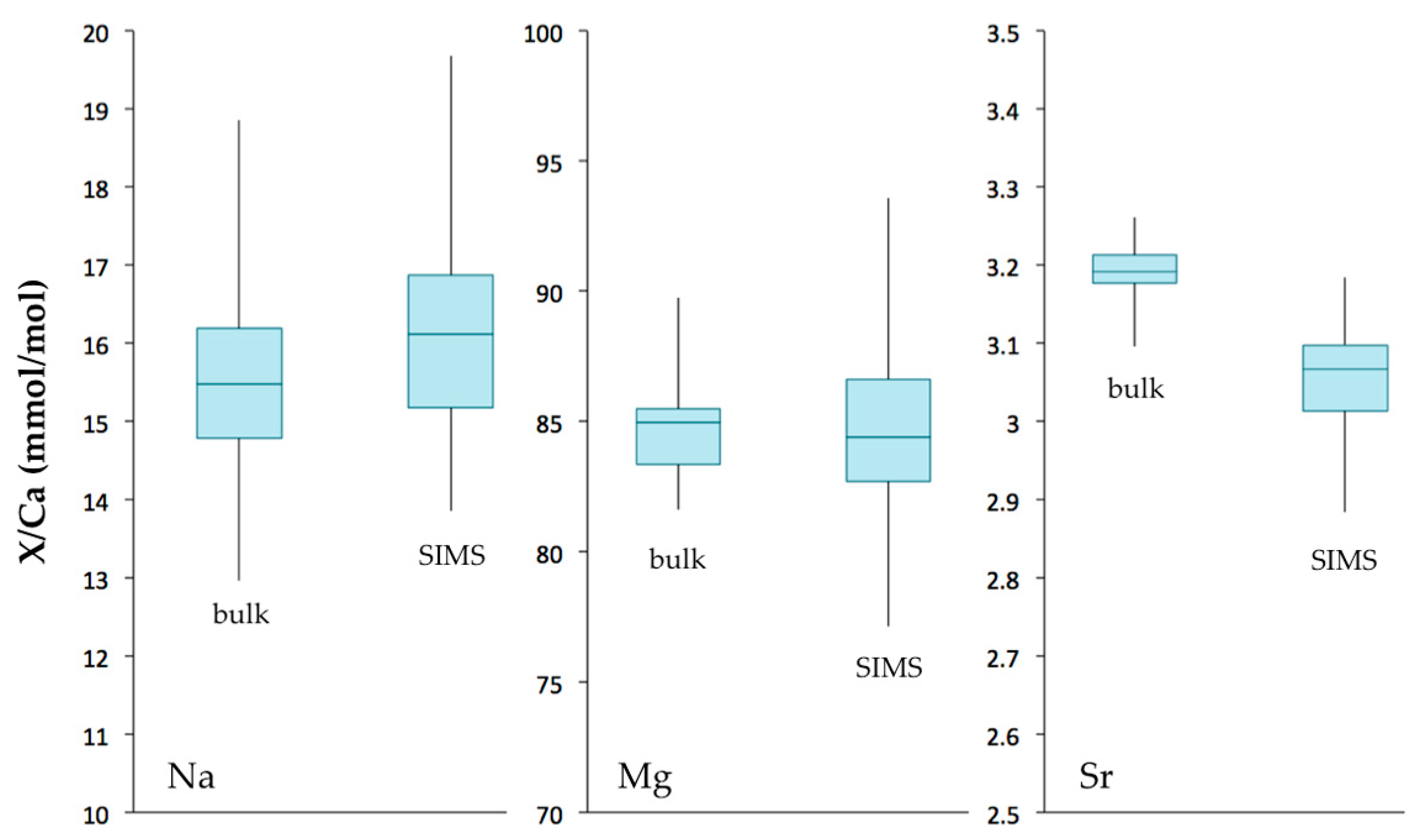
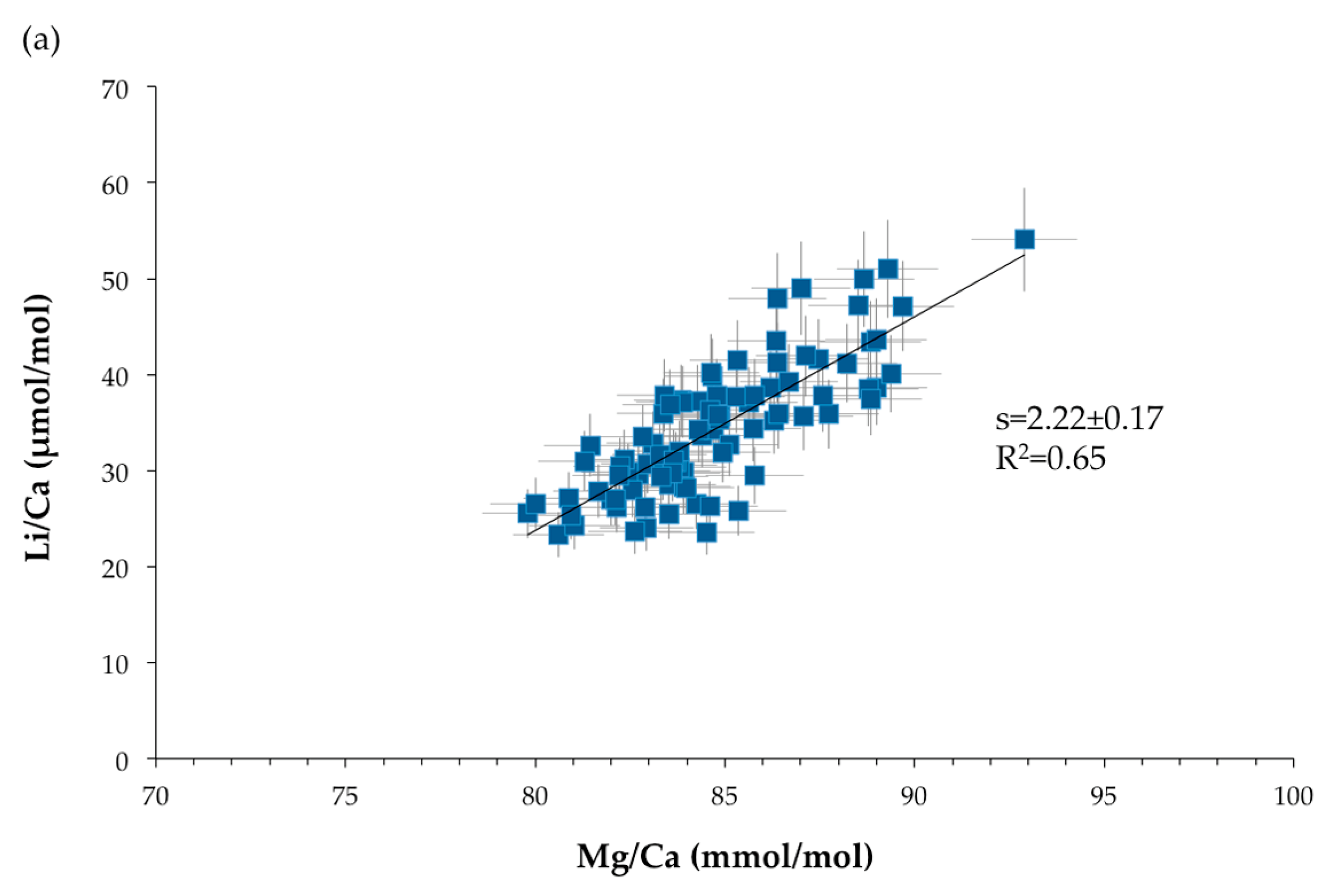
3.3. Trace Element Compositions
4. Discussion
4.1. What Can We Learn from the Geochemical Signatures?
4.1.1. Boron Isotopic Composition as Proxy of Calcification pH
4.1.2. Geochemical Signatures as Temperature Proxies
• The “line method”
• TE/Ca as temperature proxy
4.1.3. Common Mechanism of Biomineralization between Scleractinian and Octocorallian Deep-Sea Corals
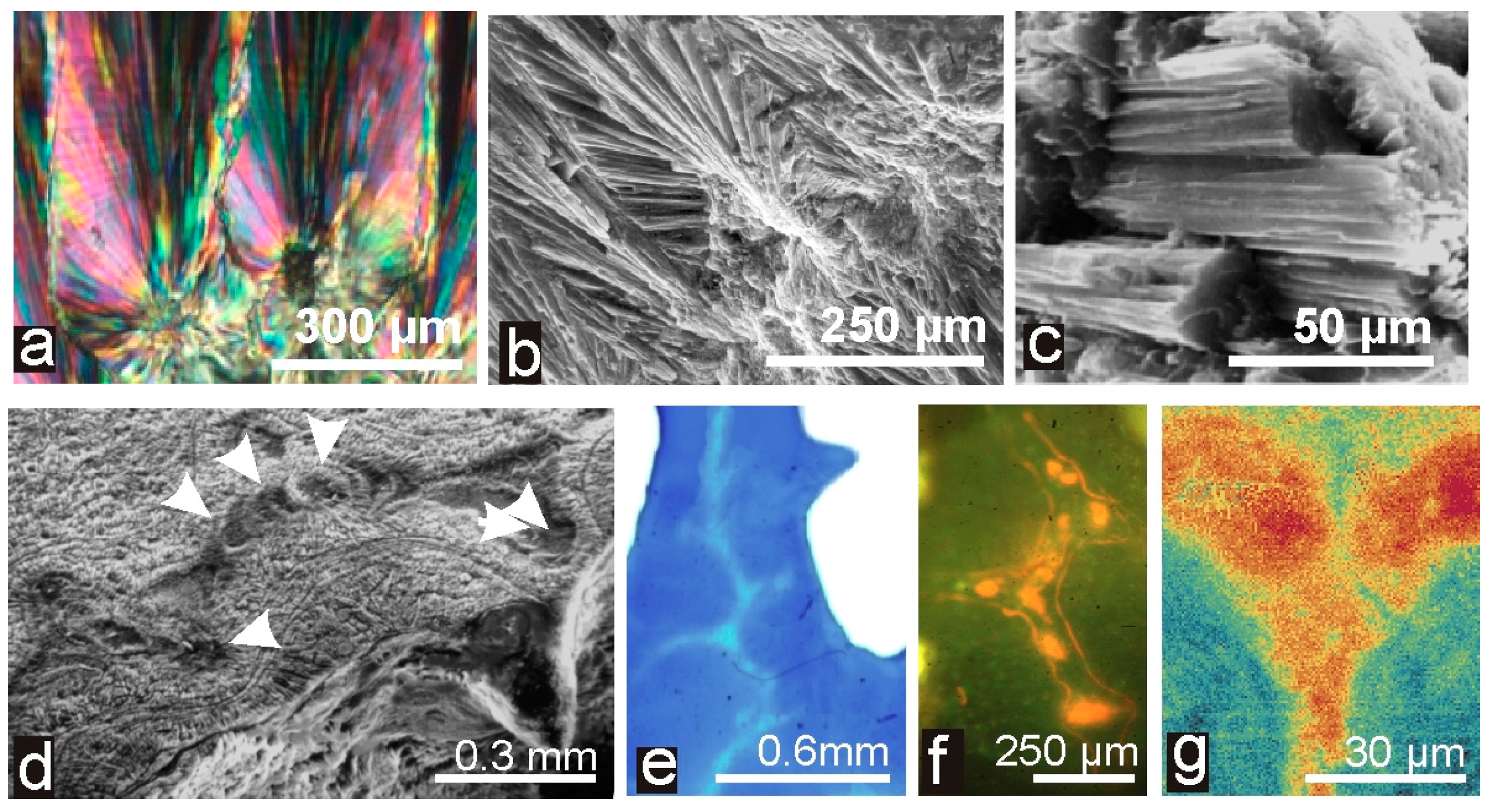
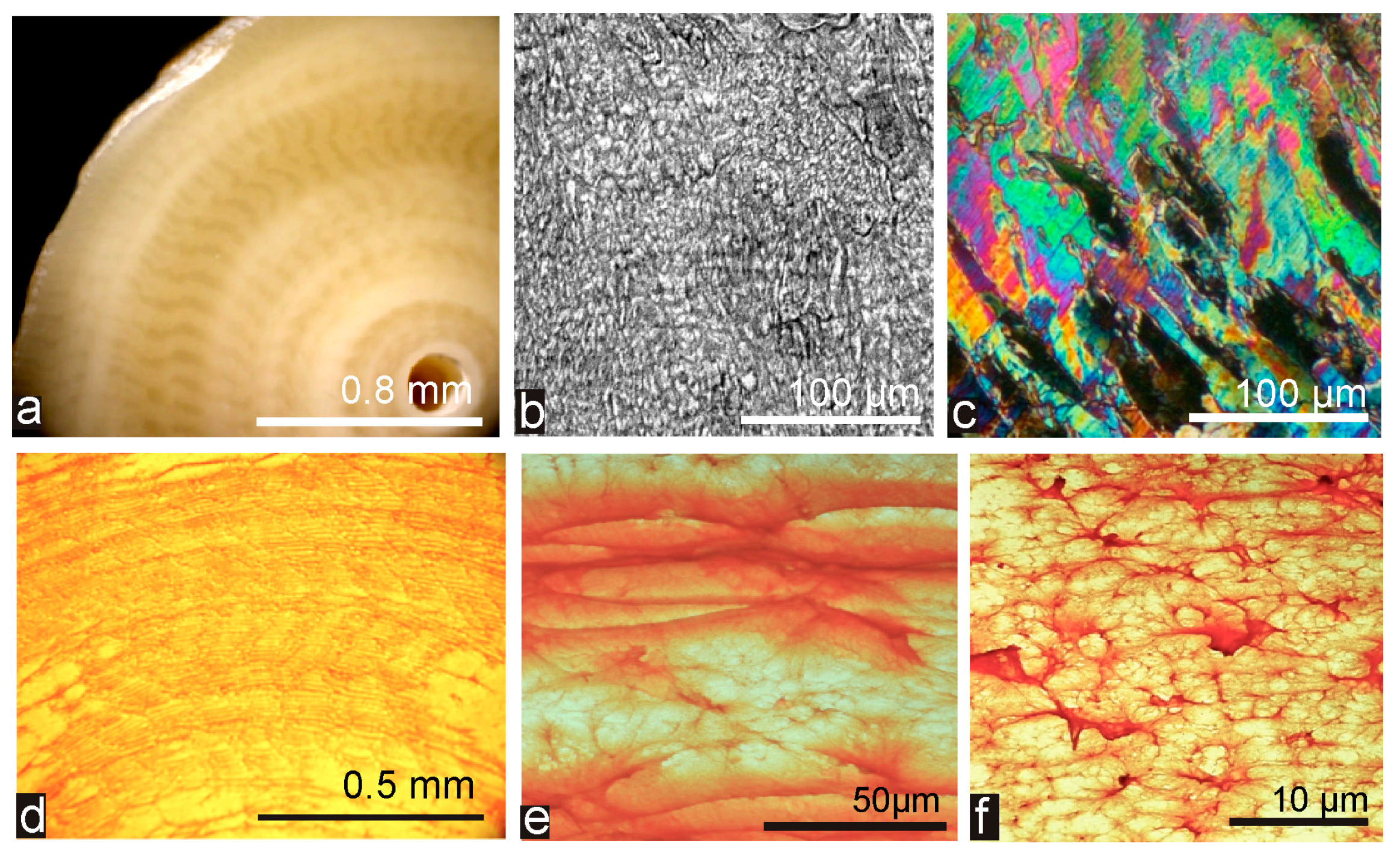
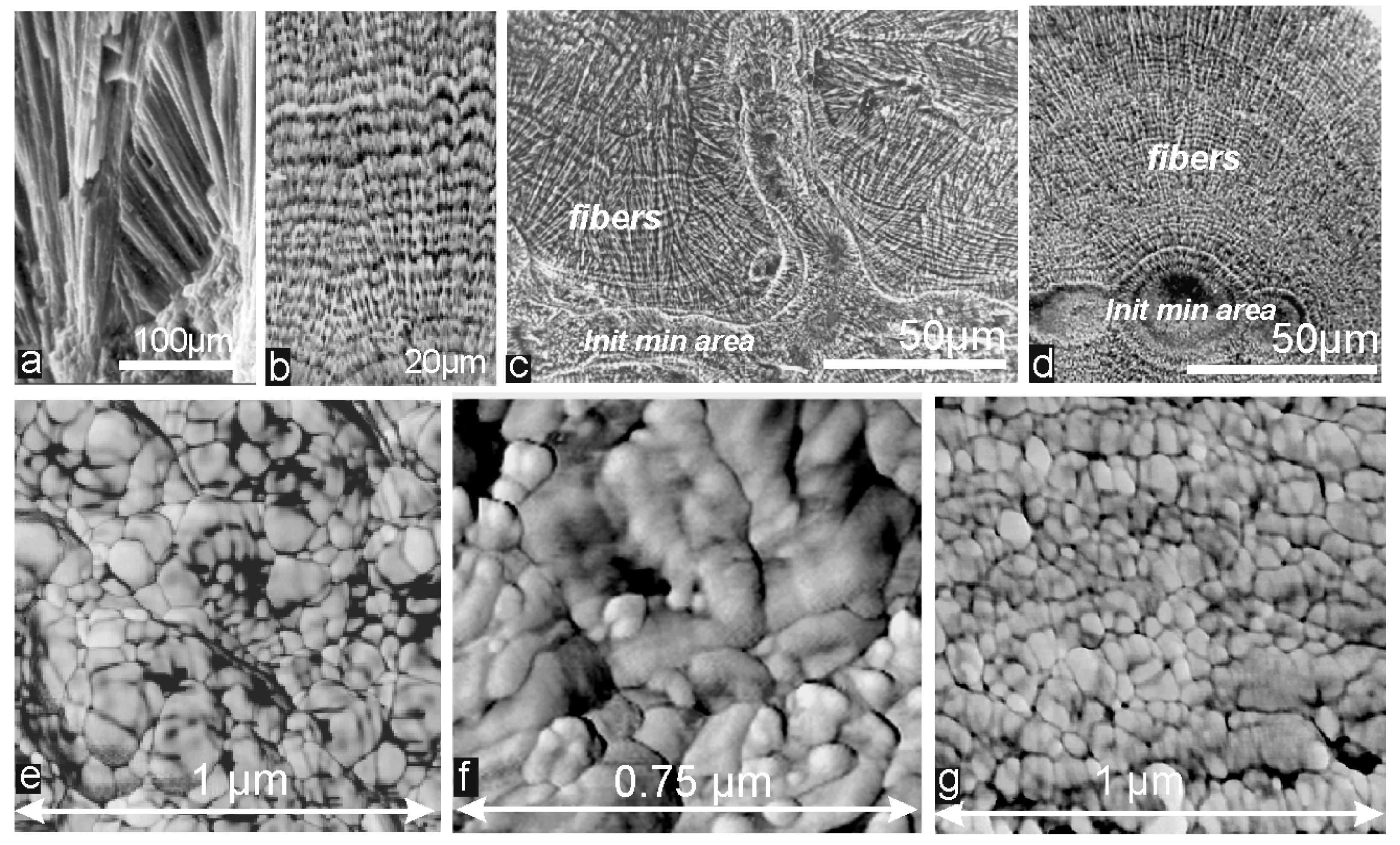
4.2. The Biomineralization Mechanisms Inferred from the Similarity between Calcitic and Aragonitic Ca-Biocarbonates Observed at the Nanometer Scale
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Emiliani, C. Pleistocene temperatures. J. Geol. 1955, 63, 538–578. [Google Scholar] [CrossRef]
- Urey, H.C.; Lowenstam, H.A.; Epstein, S.; McKinney, C.R. Measurement of paleotemperatures and temperatures of the Upper Cretaceous of England, Denmark, and the southeastern United States. Geol. Soc. Am. Bull. 1951, 62, 399–416. [Google Scholar] [CrossRef]
- Epstein, S.; Buchsbaum, R.; Lowenstam, H.A.; Urey, H.C. Revised carbonate-water isotopic temperature scale. Geol. Soc. Am. Bull. 1953, 64, 1315–1326. [Google Scholar] [CrossRef]
- Mikkelsen, N.; Erlenkeuser, H.; Killingley, J.S.; Berger, W.H. Norwegian corals: Radiocarbon and stable isotopes in Lophelia pertusa. Boreas 1982, 11, 163–171. [Google Scholar] [CrossRef]
- Adkins, J.F.; Cheng, H.; Boyle, E.A.; Druffel, E.R.; Edwards, R.L. Deep-sea coral evidence for rapid change in ventilation of the deep North Atlantic 15,400 years ago. Science 1998, 280, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Rapp, H.T.; Båmstedt, U. Oxygen and carbon isotope ratios related to growth line patterns in skeletons of Lophelia pertusa (L)(Anthozoa, Scleractinia): Implications for determination of linear extension rate. Sarsia 1998, 83, 433–446. [Google Scholar] [CrossRef]
- Smith, J.E.; Schwarcz, H.P.; Risk, M.J.; McConnaughey, T.A.; Keller, N. Paleotemperatures from deep-sea corals: Overcoming ‘vital effects’. Palaios 2000, 15, 25–32. [Google Scholar] [CrossRef]
- Robinson, L.F.; Adkins, J.F.; Frank, N.; Gagnon, A.C.; Prouty, N.G.; Brendan Roark, E.; van de Flierdt, T. The geochemistry of deepsea coral skeletons: A review of vital effects and applications for palaeoceanography. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 184–198. [Google Scholar] [CrossRef]
- Smith, J.E.; Risk, M.J.; Schwarcz, H.P.; McConnaughey, T.A. Rapid climate change in the North Atlantic during the Younger Dryas recorded by deep-sea corals. Nature 1997, 386, 818–820. [Google Scholar] [CrossRef]
- Smith, J.E.; Brand, U.; Risk, M.J.; Schwarcz, H.P. Mid-Atlantic Ridge hydrothermal events recorded by deep-sea corals. Can. J. Earth Sci. 1999, 36, 511–517. [Google Scholar] [CrossRef]
- Frank, N.; Paterne, M.; Ayliffe, L.; van Weering, T.; Henriet, J.P.; Blamart, D. Eastern North Atlantic deep-sea corals: Tracing upper intermediate water Δ14C during the Holocene. Earth Planet. Sci. Lett. 2004, 219, 297–309. [Google Scholar] [CrossRef]
- Marali, S.; Wisshak, M.; Correa, M.L.; Freiwald, A. Skeletal microstructure and stable isotope signature of three bathyal solitary cold-water corals from the Azores. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 373, 25–38. [Google Scholar] [CrossRef]
- Raddatz, J.; Rüggeberg, A.; Flögel, S.; Hathorne, E.C.; Liebetrau, V.; Eisenhauer, A.; Dullo, W.C. The influence of seawater pH on U/Ca ratios in the scleractinian cold-water coral Lophelia pertusa. Biogeosciences 2014, 11, 1863–1871. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Kusakabe, M.; Nakai, S.; Ishii, T.; Watanabe, T.; Hiyagon, H.; Sano, Y. Deep-sea coral geochemistry: Implication for the vital effect. Chem. Geol. 2005, 224, 212–222. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D. SIMS method and examples of applications in coral biomineralization. In Biomineralization Sourcebook: Characterization of Biominerals and Biomimetic Materials; DiMasi, E., Gower, L.B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 249–261. [Google Scholar]
- Spooner, P.T.; Guo, W.; Robinson, L.F.; Thiagarajan, N.; Hendry, K.R.; Rosenheim, B.E.; Leng, M.J. Clumped isotope composition of cold-water corals: A role for vital effects? Geochim. Cosmochim. Acta 2016, 179, 123–141. [Google Scholar] [CrossRef] [Green Version]
- Noé, S.U.; Dullo, W.C. Skeletal morphogenesis and growth mode of modern and fossil deep-water isidid gorgonians (Octocorallia) in the West Pacific (New Zealand and Sea of Okhotsk). Coral Reefs 2006, 25, 303–320. [Google Scholar] [CrossRef]
- Thresher, R.; Rintoul, S.R.; Koslow, J.A.; Weidman, C.; Adkins, J.; Proctor, C. Oceanic evidence of climate change in southern Australia over the last three centuries. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Sherwood, O.A.; Thresher, R.E.; Fallon, S.J.; Davies, D.M.; Trull, T.W. Multi-century time-series of 15N and 14C in bamboo corals from deep Tasmanian seamounts: Evidence for stable oceanographic conditions. Mar. Ecol. Prog. Ser. 2009, 397, 209–218. [Google Scholar] [CrossRef]
- LaVigne, M.; Hill, T.M.; Spero, H.J.; Guilderson, T.P. Bamboo coral Ba/Ca: Calibration of a new deep ocean refractory nutrient proxy. Earth Planet. Sci. Lett. 2011, 312, 506–515. [Google Scholar] [CrossRef]
- Hill, T.M.; LaVigne, M.; Spero, H.J.; Guilderson, T.; Gaylord, B.; Clague, D. Variations in seawater Sr/Ca recorded in deep-sea bamboo corals. Paleoceanography 2012, 27. [Google Scholar] [CrossRef]
- Farmer, J.R.; Hoenisch, B.; Robinson, L.F.; Hill, T.M. Effects of seawater-pH and biomineralization on the boron isotopic composition of deep-sea bamboo corals. Geochim. Cosmochim. Acta 2015, 155, 86–106. [Google Scholar] [CrossRef]
- Thresher, R.E.; Fallon, S.J.; Townsend, A.T. A “core-top” screen for trace element proxies of environmental conditions and growth rates in the calcite skeletons of bamboo corals (Isididae). Geochim. Cosmochim. Acta 2016, 193, 75–99. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A.; Cairns, S.D. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats; Roberts, J.M., Wheeler, A.J., Freiwald, A., Cairns, S.D., Eds.; Cambridge University Press: Cambridge, UK, 2009; 333p. [Google Scholar]
- Adkins, J.F.; Boyle, E.A.; Curry, W.B.; Lutringer, A. Stable isotopes in deep-sea corals and a new mechanism for “vital effects”. Geochim. Cosmochim. Acta 2003, 67, 1129–1143. [Google Scholar] [CrossRef]
- Sinclair, D.J.; Risk, M.J. A numerical model of trace-element coprecipitation in a physicochemical calcification system: Application to coral biomineralization and trace-element ‘vital effects’. Geochim. Cosmochim. Acta 2006, 70, 3855–3868. [Google Scholar] [CrossRef]
- Blamart, D.; Rollion-Bard, C.; Meibom, A.; Cuif, J.P.; Juillet-Leclerc, A.; Dauphin, Y. Correlation of boron isotopic composition with ultrastructure in the deep-sea coral Lophelia pertusa: Implications for biomineralization and paleo-pH. Geochem. Geophys. Geosyst. 2007, 8. [Google Scholar] [CrossRef]
- McConnaughey, T. 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochim. Cosmochim. Acta 1989, 53, 151–162. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D.; Cuif, J.P.; Dauphin, Y. In situ measurements of oxygen isotopic composition in deep-sea coral, Lophelia pertusa: Re-examination of the current geochemical models of biomineralization. Geochim. Cosmochim. Acta 2010, 74, 1338–1349. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D. Possible controls on Li, Na, and Mg incorporation into aragonite coral skeletons. Chem. Geol. 2015, 396, 98–111. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y. Microstructural and physico-chemical characterizations of the“centers of calcification” in the septa of some recent Scleractinian corals. Paläont. Zeit. 1998, 72, 257–270. [Google Scholar] [CrossRef]
- Ogilvie, M.M. Microscopic and systematic study of Madreporarian types of corals. Philos. Trans. R. Soc. B 1896, 187, 83–345. [Google Scholar] [CrossRef]
- Milne-Edwards, H. Histoire Naturelle des Coralliaires ou Polypes Proprement Dits; Roret: Paris, France, 1860; Volume 1, 326p; Volume 2, 633p.; Volume 3, 560p. [Google Scholar]
- Wells, J.W. Scleractinia. In Treatise on Invertebrate Paleontology; Coelenterata, P.F., Moore, R.C., Eds.; The University of Kansas Press: Lawrence, KS, USA, 1956; pp. F328–F344. [Google Scholar]
- Bryan, W.H.; Hill, D. Spherulitic crystallization as a mechanism of skeletal growth in the hexacorals. Proc. R. Soc. Qld. 1941, 52, 78–91. [Google Scholar]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. A window on biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.N.; Woodhead, P.M. Temperature dependence of oxygen-18 concentration in reef coral carbonates. J. Geophys. Res. 1972, 77, 463–473. [Google Scholar] [CrossRef]
- Volz, W. Die Korallen der Schichten von St. Cassian in Süd-Tirol (Frech, Fritz; Volz, Wilhelm: Die Korallenfauna der Trias. Monographisch bearbeitet. II). Palaeontographica 1896, 2, 1–124. [Google Scholar]
- Balan, E.; Aufort, J.; Pouillé, S.; Dabos, M.; Blanchard, M.; Lazzeri, M.; Rollion-Bard, C.; Blamart, D. Infrared spectroscopic study of sulfate-bearing calcite from deep-sea bamboo coral. Eur. J. Mineral. 2017, 29, 1–12. [Google Scholar] [CrossRef]
- Tisnérat-Laborde, N.; Paterne, M.; Métivier, B.; Arnold, M.; Yiou, P.; Blamart, D.; Raynaud, S. Variability of the northeast Atlantic sea surface Δ14C and marine reservoir age and the North Atlantic Oscillation (NAO). Quat. Sci. Rev. 2010, 29, 2633–2646. [Google Scholar] [CrossRef]
- Mook, W.G.; Van Der Plicht, J. Reporting 14C activities and concentrations. Radiocarbon 1999, 41, 227–239. [Google Scholar] [CrossRef]
- Roark, E.B.; Guilderson, T.P.; Dunbar, R.B.; Ingram, B.L. Radiocarbon-based ages and growth rates of Hawaiian deep-sea corals. Mar. Ecol. Prog. Ser. 2006, 327, 1–14. [Google Scholar] [CrossRef]
- Thresher, R.E. Environmental and compositional correlates of growth rate in deep-water bamboo corals (Gorgonacea; Isididae). Mar. Ecol. Prog. Ser. 2009, 397, 187–196. [Google Scholar] [CrossRef]
- Farmer, J.R.; Robinson, L.F.; Hönisch, B. Growth rate determinations from radiocarbon in bamboo corals (genus Keratoisis). Deep Sea Res. Part I 2015, 105, 26–40. [Google Scholar] [CrossRef]
- Lazier, A.V.; Smith, J.E.; Risk, M.J.; Schwarcz, H.P. The skeletal structure of Desmophyllum cristagalli: The use of deep-water corals in sclerochronology. Lethaia 1999, 32, 119–130. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y.; Doucet, J.; Salome, M.; Susini, J. XANES mapping of organic sulfate in three scleractinian coral skeletons. Geochim. Cosmochim. Acta 2003, 67, 75–83. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D.; Cuif, J.P.; Juillet-Leclerc, A. Microanalysis of C and O isotopes of azooxanthellate and zooxanthellate corals by ion microprobe. Coral Reefs 2003, 22, 405–415. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Chaussidon, M.; France-Lanord, C. pH control on oxygen isotopic composition of symbiotic corals. Earth Planet. Sc. Lett. 2003, 215, 275–288. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Mangin, D.; Champenois, M. Development and application of oxygen and carbon isotopic measurements of biogenic carbonates by ion microprobe. Geostand. Geoanal. Res. 2007, 31, 39–50. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Marin-Carbonne, J. Determination of SIMS matrix effects on oxygen isotopic compositions in carbonates. J. Anal. At. Spectrom. 2011, 26, 1285–1289. [Google Scholar] [CrossRef]
- Bice, K.L.; Layne, G.D.; Dahl, K. Application of secondary ion mass spectrometry to the determination of Mg/Ca in rare, delicate, or altered planktonic foraminifera: Examples from the Holocene, Paleogene, and Cretaceous. Geochem. Geophys. Geosyst. 2005, 6. [Google Scholar] [CrossRef]
- Vigier, N.; Rollion-Bard, C.; Spezzaferri, S.; Brunet, F. In situ measurements of Li isotopes in foraminifera. Geochem. Geophys. Geosyst. 2007, 8. [Google Scholar] [CrossRef]
- Kimball, J.B.; Dunbar, R.B.; Guilderson, T.P. Oxygen and carbon isotope fractionation in calcitic deep-sea corals: Implications for paleotemperature reconstruction. Chem. Geol. 2014, 381, 223–233. [Google Scholar] [CrossRef]
- Hill, T.M.; Spero, H.J.; Guilderson, T.; LaVigne, M.; Clague, D.; Macalello, S.; Jang, N. Temperature and vital effect controls on bamboo coral (Isididae) isotope geochemistry: A test of the “lines method”. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Thresher, R.E.; Neil, H. Scale dependence of environmental and physiological correlates of δ18O and δ13C in the magnesium calcite skeletons of bamboo corals (Gorgonacea; Isididae). Geochim. Cosmochim. Acta 2016, 187, 260–278. [Google Scholar] [CrossRef]
- Chaabane, S.; Correa, M.L.; Montagna, P.; Kallel, N.; Taviani, M.; Linares, C.; Ziveri, P. Exploring the oxygen and carbon isotopic composition of the Mediterranean red coral (Corallium rubrum) for seawater temperature reconstruction. Mar. Chem. 2016, 186, 11–23. [Google Scholar] [CrossRef]
- Saenger, C.; Gabitov, R.I.; Farmer, J.; Watkins, J.M.; Stone, R. Linear correlations in bamboo coral δ13C and δ18O sampled by SIMS and micromill: Evaluating paleoceanographic potential and biomineralization mechanisms using δ11B and ∆47 composition. Chem. Geol. 2017, 454, 1–14. [Google Scholar] [CrossRef]
- Risk, M.J.; Hall-Spencer, J.; Williams, B. Climate records from the Faroe-Shetland Channel using Lophelia pertusa (Linnaeus, 1758). In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Germany, 2005; pp. 1097–1108. [Google Scholar]
- Lutringer, A.; Blamart, D.; Frank, N.; Labeyrie, L. Paleotemperatures from deep-sea corals: Scale effects. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Germany, 2005; pp. 1081–1096. [Google Scholar]
- Gabitov, R.I.; Rollion-Bard, C.; Tripati, A.; Sadekov, A. In situ study of boron partitioning between calcite and fluid at different crystal growth rates. Geochim. Cosmochim. Acta 2014, 137, 81–92. [Google Scholar] [CrossRef]
- Noireaux, J.; Mavromatis, V.; Gaillardet, J.; Schott, J.; Montouillout, V.; Louvat, P.; Rollion-Bard, C.; Neuville, D.R. Crystallographic control on the boron isotope paleo-pH proxy. Earth Planet. Sci. Lett. 2015, 430, 398–407. [Google Scholar] [CrossRef]
- Thresher, R.E.; Wilson, N.C.; MacRae, C.M.; Neil, H. Temperature effects on the calcite skeletal composition of deep-water gorgonians (Isididae). Geochim. Cosmochim. Acta 2010, 74, 4655–4670. [Google Scholar] [CrossRef]
- Sherwood, O.A.; Heikoop, J.M.; Scott, D.B.; Risk, M.J.; Guilderson, T.P.; McKinney, R.A. Stable isotopic composition of deep-sea gorgonian corals Primnoa spp.: A new archive of surface processes. Mar. Ecol. Prog. Ser. 2005, 301, 135–148. [Google Scholar] [CrossRef]
- Sinclair, D.; Sherwood, O.; Risk, M.; Hillaire-Marcel, C.; Tubrett, M.; Sylvester, P.; McCulloch, M.; Kinsley, L. Testing the reproducibility of Mg/Ca profiles in the deep-water coral Primnoa resedaeformis: Putting the proxy through its paces. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Germany, 2005; pp. 1039–1060. [Google Scholar]
- Sinclair, D.J.; Williams, B.; Allard, G.; Ghaleb, B.; Fallon, S.; Ross, S.W.; Risk, M. Reproducibility of trace element profiles in a specimen of the deep-water bamboo coral Keratoisis sp. Geochim. Cosmochim. Acta 2011, 75, 5101–5121. [Google Scholar] [CrossRef]
- Pilkey, O.H.; Harris, R.C. The effect of intertidal environment on the composition of calcareous skeletal material. Limnol. Oceanogr. 1966, 11, 381–385. [Google Scholar] [CrossRef]
- Bond, Z.A.; Cohen, A.L.; Smith, S.R.; Jenkins, W.J. Growth and composition of high-Mg calcite in the skeleton of a Bermudian gorgonian (Plexaurella dichotoma): Potential for paleothermometry. Geochem. Geophys. Geosyst. 2005, 6. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Gagnon, A.C.; Guan, Y.; Eiler, J.M.; Adkins, J.F. Accurate Mg/Ca, Sr/Ca, and Ba/Ca ratio measurements in carbonates by SIMS and NanoSIMS and an assessment of heterogeneity in common calcium carbonate standards. Chem. Geol. 2013, 356, 94–108. [Google Scholar] [CrossRef]
- Case, D.H.; Robinson, L.F.; Auro, M.E.; Gagnon, A.C. Environmental and biological controls on Mg and Li in deep-sea scleractinian corals. Earth Planet. Sci. Lett. 2010, 300, 215–225. [Google Scholar] [CrossRef]
- Hathorne, E.C.; Felis, T.; Suzuki, A.; Kawahata, H.; Cabioch, G. Lithium in the aragonite skeletons of massive Porites corals: A new tool to reconstruct tropical sea surface temperatures. Paleoceanography 2013, 28, 143–152. [Google Scholar] [CrossRef]
- Raddatz, J.; Liebetrau, V.; Rüggeberg, A.; Hathorne, E.; Krabbenhöft, A.; Eisenhauer, A.; Böhm, F.; Vollstaedt, H.; Fietzke, J.; Lopez-Correa, M.; et al. Stable Sr-isotope, Sr/Ca, Mg/Ca, Li/Ca and Mg/Li ratios in the scleractinian cold-water coral Lophelia pertusa. Chem. Geol. 2013, 352, 143–152. [Google Scholar] [CrossRef]
- Montagna, P.; McCulloch, M.; Douville, E.; Correa, M.L.; Trotter, J.; Rodolfo-Metalpa, R.; Dissard, D.; Taviani, M.; Goldstein, S.; Mazzoli, C.; et al. Li/Mg systematics in scleractinian corals: Calibration of the thermometer. Geochim. Cosmochim. Acta 2014, 132, 288–310. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; De Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419–426. [Google Scholar] [CrossRef]
- Venn, A.A.; Tambutté, E.; Lotto, S.; Zoccola, D.; Allemand, D.; Tambutté, S. Imaging intracellular pH in a reef coral and symbiotic anemone. Proc. Natl. Acad. Sci. USA 2009, 106, 16574–16579. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.A.; Tambutté, E.; Holcomb, M.; Allemand, D.; Tambutté, S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 2011, 6, e20013. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.A.; Tambutté, E.; Holcomb, M.; Laurent, J.; Allemand, D.; Tambutté, S. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA 2013, 110, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Klochko, K.; Kaufman, A.J.; Yao, W.; Byrne, R.H.; Tossell, J.A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 2006, 248, 276–285. [Google Scholar] [CrossRef]
- Hemming, N.G.; Hanson, G.N. Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta 1992, 56, 537–543. [Google Scholar] [CrossRef]
- Klochko, K.; Cody, G.D.; Tossell, J.A.; Dera, P.; Kaufman, A.J. Re-evaluating boron speciation in biogenic calcite and aragonite using 11 B MAS NMR. Geochim. Cosmochim. Acta 2009, 73, 1890–1900. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D.; Trebosc, J.; Tricot, G.; Mussi, A.; Cuif, J.P. Boron isotopes as pH proxy: A new look at boron speciation in deep-sea corals using 11B MAS NMR and EELS. Geochim. Cosmochim. Acta 2011, 75, 1003–1012. [Google Scholar] [CrossRef]
- Cusack, M.; Kamenos, N.A.; Rollion-Bard, C.; Tricot, G. Red coralline algae assessed as marine pH proxies using 11B MAS NMR. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.L.; Pogge von Strandmann, P.A.E.; Rae, J.W.B. Boron and magnesium isotopic composition of seawater. Geochem. Geophys. Geosyst. 2010, 11. [Google Scholar] [CrossRef]
- Dickson, A.G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A 1990, 37, 755–766. [Google Scholar] [CrossRef]
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2012, 2, 623–627. [Google Scholar] [CrossRef]
- Ip, Y.K.; Lim, A.L.L.; Lim, R.W.L. Some properties of calcium-activated adenosine triphosphatase from the hermatypic coral Galaxea fascicularis. Mar. Biol. 1991, 111, 191–197. [Google Scholar] [CrossRef]
- Kingsley, R.J.; Watabe, N. An autoradiographic study of calcium transport in spicule formation in the gorgonian Leptogorgia virgulata (Lamarck) (Coelenterata: Gorgonacea). Cell Tissue Res. 1985, 239, 305–310. [Google Scholar] [CrossRef]
- Anagnostou, E.; Huang, K.F.; You, C.F.; Sikes, E.L.; Sherrell, R.M. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment. Earth Planet Sci. Lett. 2012, 349, 251–260. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Brandstätter, F.; Velimirov, B. On the potential use of magnesium and strontium concentrations as ecological indicators in the calcite skeleton of the red coral (Corallium rubrum). Mar. Biol. 2000, 137, 801–809. [Google Scholar] [CrossRef]
- Oomori, T.; Kaneshima, H.; Maezato, Y.; Kitano, Y. Distribution coefficient of Mg2+ ions between calcite and solution at 10–50 °C. Mar. Chem. 1987, 20, 327–336. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y.; Sorauf, J.E. Biominerals and Fossils through Time; Cambridge University Press: Cambridge, UK, 2010; 512p. [Google Scholar]
- Cuif, J.P. Calcification in the Cnidaria through Time: An Overview of Their Skeletal Patterns from Individual to Evolutionary Viewpoints. In The Cnidaria, Past, Present and Future; Springer: Berlin, Germany, 2016; pp. 163–179. [Google Scholar]
- Cuif, J.P. The Rugosa-Scleractinia gap re-examined through microstructural and biochemical evidence: A tribute to HC Wang. Palaeoworld 2014, 23, 1–14. [Google Scholar] [CrossRef]
- Beniash, E.; Aizenberg, J.; Addadi, L.; Weiner, S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B Biol. Sci. 1997, 264, 461–465. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y.; Farre, B.; Nehrke, G. Distribution of sulphated polysaccharides within calcareous biominerals indicates a widely shared layered growth-mode for the Invertebrate skeletons and suggests a two-step crystallization process for the mineral growth units. Mineral. Mag. 2008, 72, 233–237. [Google Scholar] [CrossRef]
- Meibom, A.; Cuif, J.P.; Hillion, F.; Constantz, B.R.; Juillet-Leclerc, A.; Dauphin, Y.; Watanabe, T.; Dunbar, R.B. Distribution of magnesium in coral skeleton. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y. The environmental recording unit in coral skeleton—A synthesis of structural and chemical evidences for a biochemically driven, stepping-growth process in fibres. Biogeosciences 2005, 2, 61–73. [Google Scholar] [CrossRef]
- Sutton, J.N.; Liu, Y.W.; Ries, J.B.; Guillermic, M.; Ponzevera, E.; Eagle, R.A. δ11B as monitor of calcification site pH in marine calcifying organisms. Biogeosci. Dis. 2017. [Google Scholar] [CrossRef]
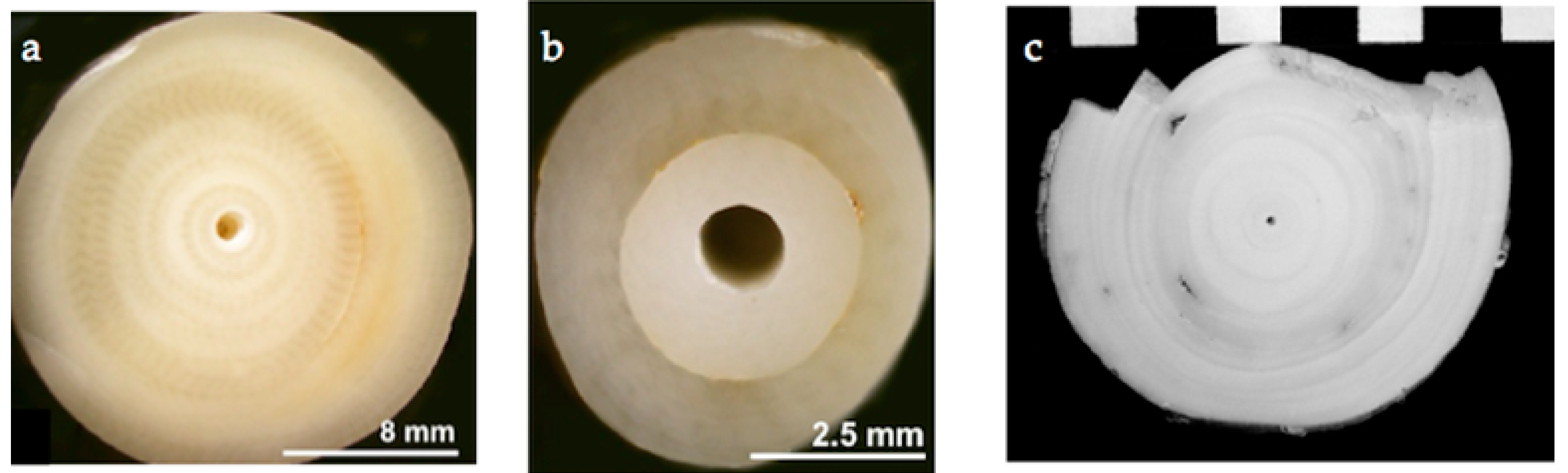

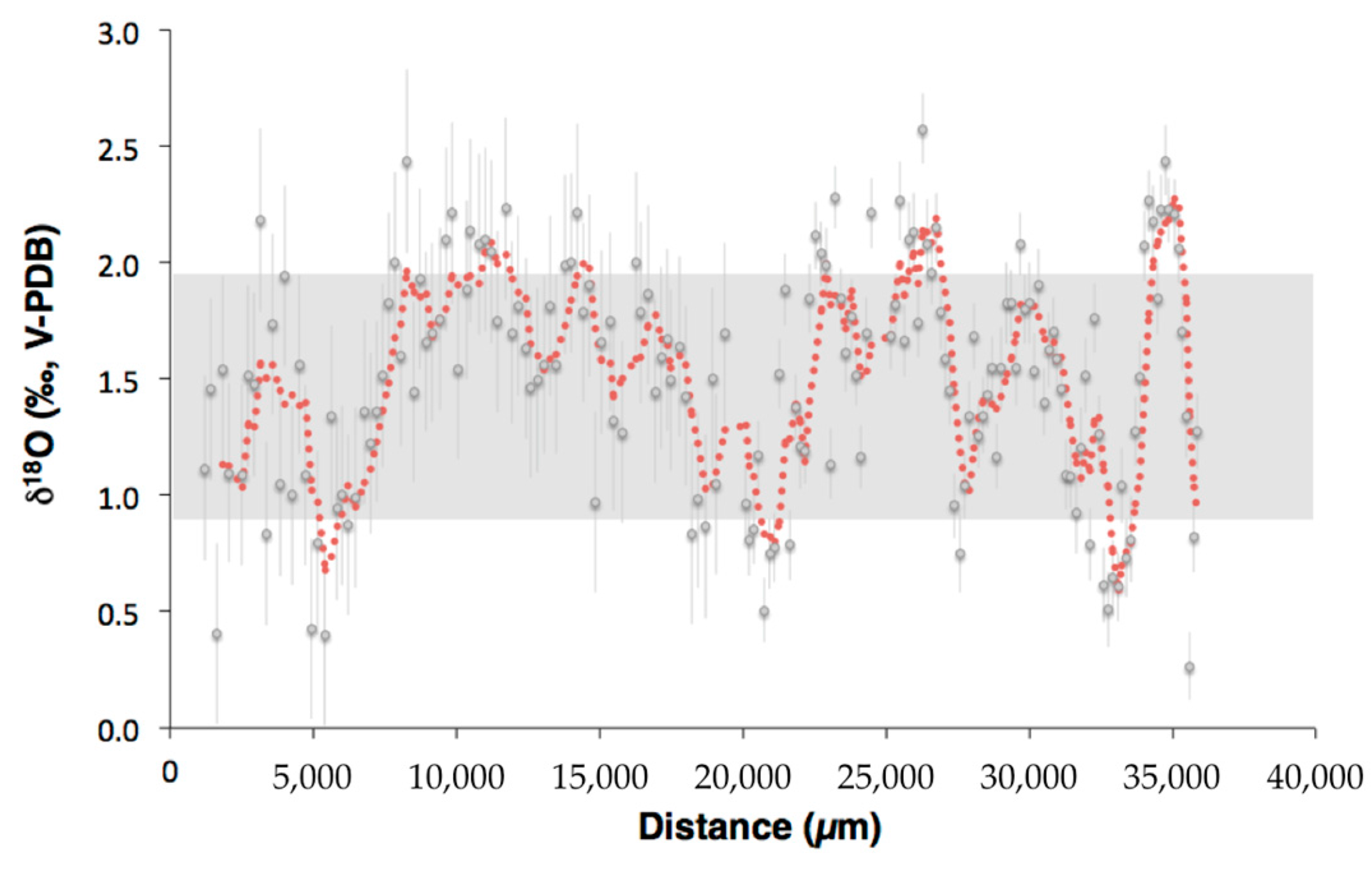
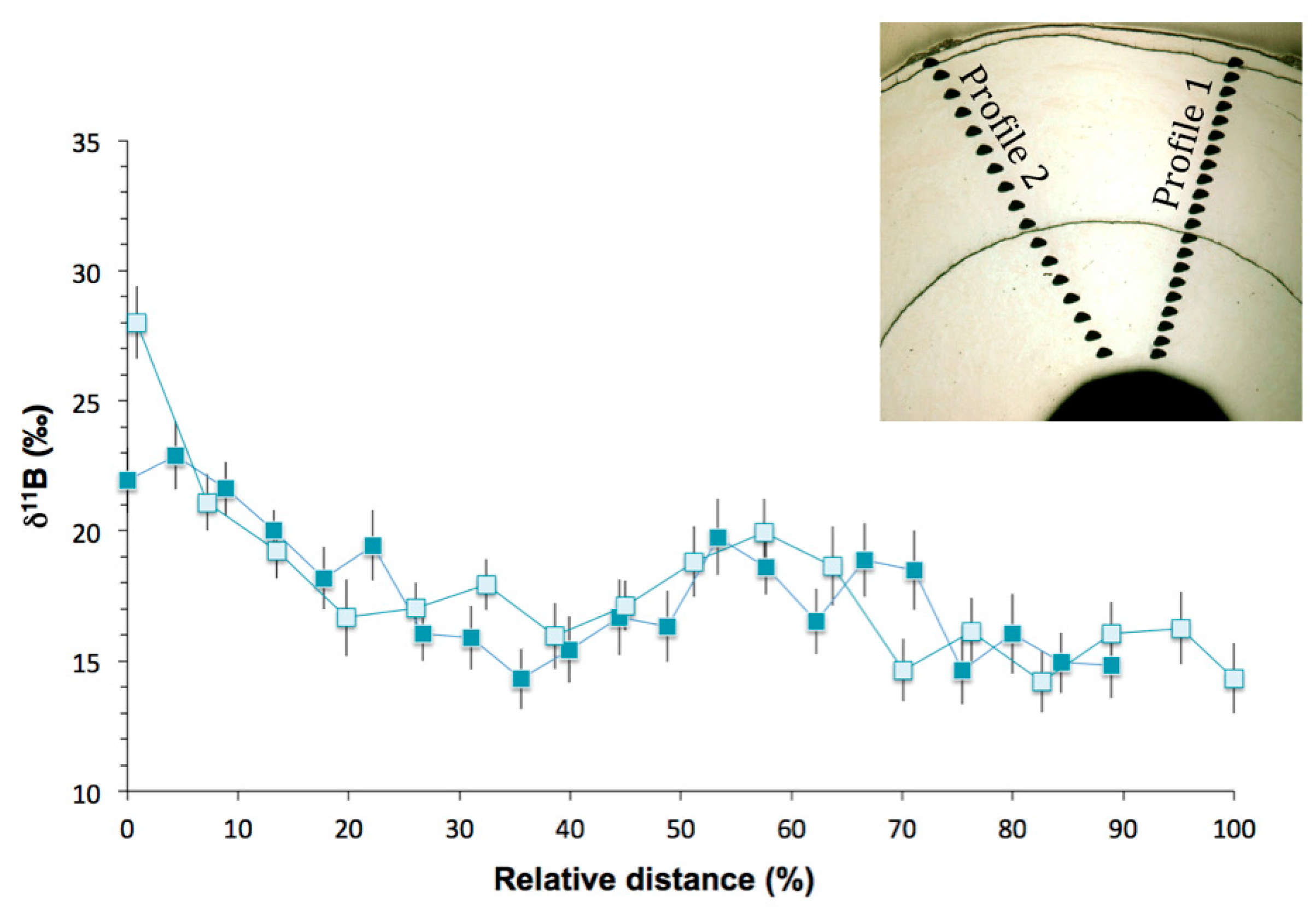
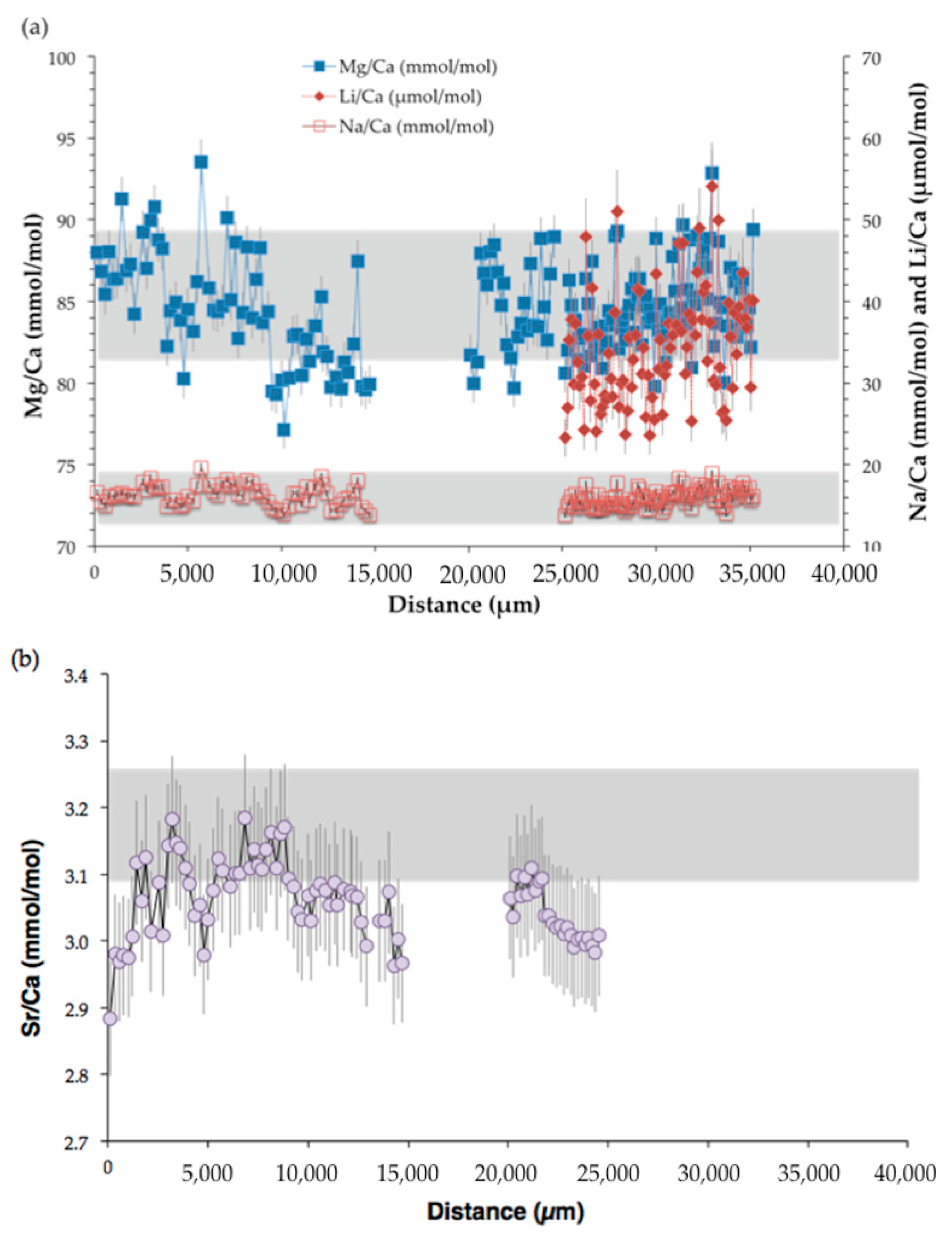


| Sample Ref | Sample Name | mg C | Age BP (1σ) | Age AD Calibrated (2σ) |
|---|---|---|---|---|
| Sac40169 | B1 2.50 | 1.26 | 1505 (±30) | 530–637 |
| Sac40170 | B2 9.00 | 1.24 | 1470 (±30) | 545–645 |
| Sac40171 | B3 15.50 | 1.41 | 1300 (±30) | 661–773 |
| Sac40172 | B4 21.50 | 1.36 | 1015 (±30) | 972–1047 |
| Sac40173 | B5 28.80 | 1.41 | 910 (±30) | 1034–1189 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollion-Bard, C.; Cuif, J.-P.; Blamart, D. Optical Observations and Geochemical Data in Deep-Sea Hexa- and Octo-Coralla Specimens. Minerals 2017, 7, 154. https://doi.org/10.3390/min7090154
Rollion-Bard C, Cuif J-P, Blamart D. Optical Observations and Geochemical Data in Deep-Sea Hexa- and Octo-Coralla Specimens. Minerals. 2017; 7(9):154. https://doi.org/10.3390/min7090154
Chicago/Turabian StyleRollion-Bard, Claire, Jean-Pierre Cuif, and Dominique Blamart. 2017. "Optical Observations and Geochemical Data in Deep-Sea Hexa- and Octo-Coralla Specimens" Minerals 7, no. 9: 154. https://doi.org/10.3390/min7090154





