Experimental Investigation of Biotite-Rich Schist Reacting with B-Bearing Fluids at Upper Crustal Conditions and Correlated Tourmaline Formation
Abstract
:1. Introduction
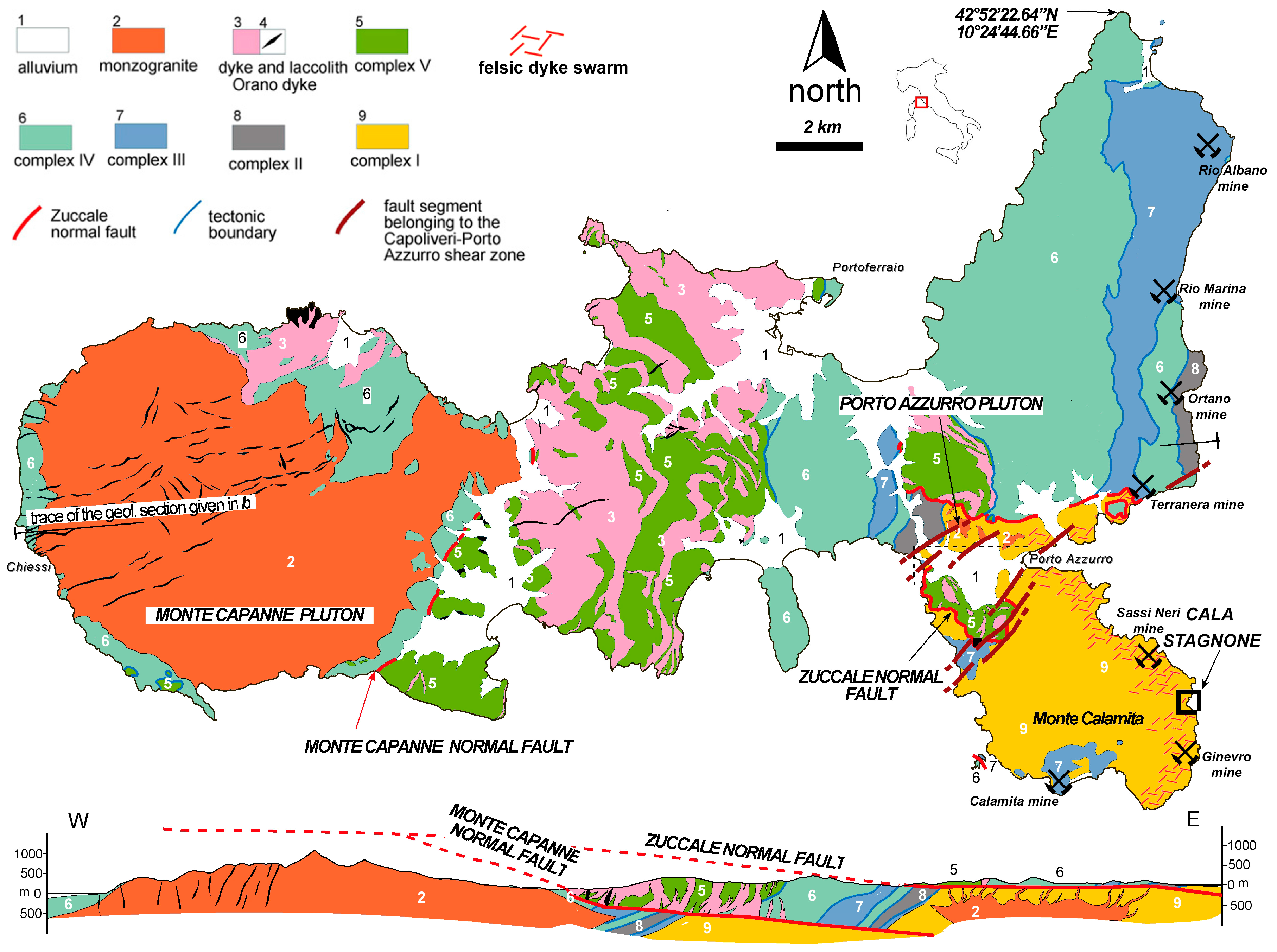
2. Geological Sketch and Iron Deposits of Southeastern Elba Island
3. Tourmaline Veins and Metasomatic Bodies
- (1)
- multi-phase inclusions (liquid + vapour + halite and one or more other solids) characterised by halite final melting temperature between 141 and 415 °C, temperature of bubble disappearance from 192 to 330 °C and a computed salinity range of 29–49 wt % NaCl equivalent;
- (2)
- two-phase inclusions (liquid + vapour) displaying low eutectic temperature (~−55/−50 °C), final ice melting temperature between −31.1 and −7.1 °C, total homogenisation (either to the liquid or the vapour phase) from 242 to 596 °C, and calculated salinity between 11 and 29 wt % NaCl equivalent.
4. Materials and Methods
4.1. Starting Material
4.1.1. Solids
4.1.2. Fluids
4.2. Experimental Conditions and Apparatus
4.3. Analyses
4.3.1. Solids
- XRD: Philips PW 1050/37 diffractometer (Philips, Amsterdam, The Netherlands), Cu Kα source, 2° 2Θ/min step size. Rietveld refinements of powder diffraction patterns were performed on some selected spectra using the MAUD software package [39];
- SEM: Zeiss EVO MA15 instrument (Zeiss, Oberkochen, Germany), equipped with an Oxford Inca 250 EDS detector (Oxford Instruments, Abingdon-on-Thames, UK), 20 kV accelerating voltage. EDS spectra and semi-quantitative analyses were acquired for the whole set of experimental products;
- EMPA: JEOL JXA-8600 (Jeol Ltd., Tokyo, Japan) electron microprobe using 15 kV accelerating voltage, 10 nA beam current, spot size ~5 µm, and 15 s counting times for each element (Si, Ti, Al, Cr, Fe, Mn, Mg, Ca, K) but10 s for Na. Data were collected considering the PAP model [40]. Mineral analyses by this technique were performed only on some selected experimental products.
4.3.2. Fluids
- the capsule was immersed in milli-Q water into a test tube with a porous stopper;
- the capsule was crimped (and opened) using pliers, thus allowing the fluid into the capsule to dilute into the milli-Q water. Knowing the amount of the fluid inserted into the capsule the dilution was then calculated;
- the immersion of the test tube in ultrasonic bath for 3 h favoured the homogenisation of the aqueous solution and the powder removal from the capsule;
- after 48 h, the powder fully deposited to the bottom of the test tube and the solution was thus recovered through a pipette, and subsequently analysed.
5. Results
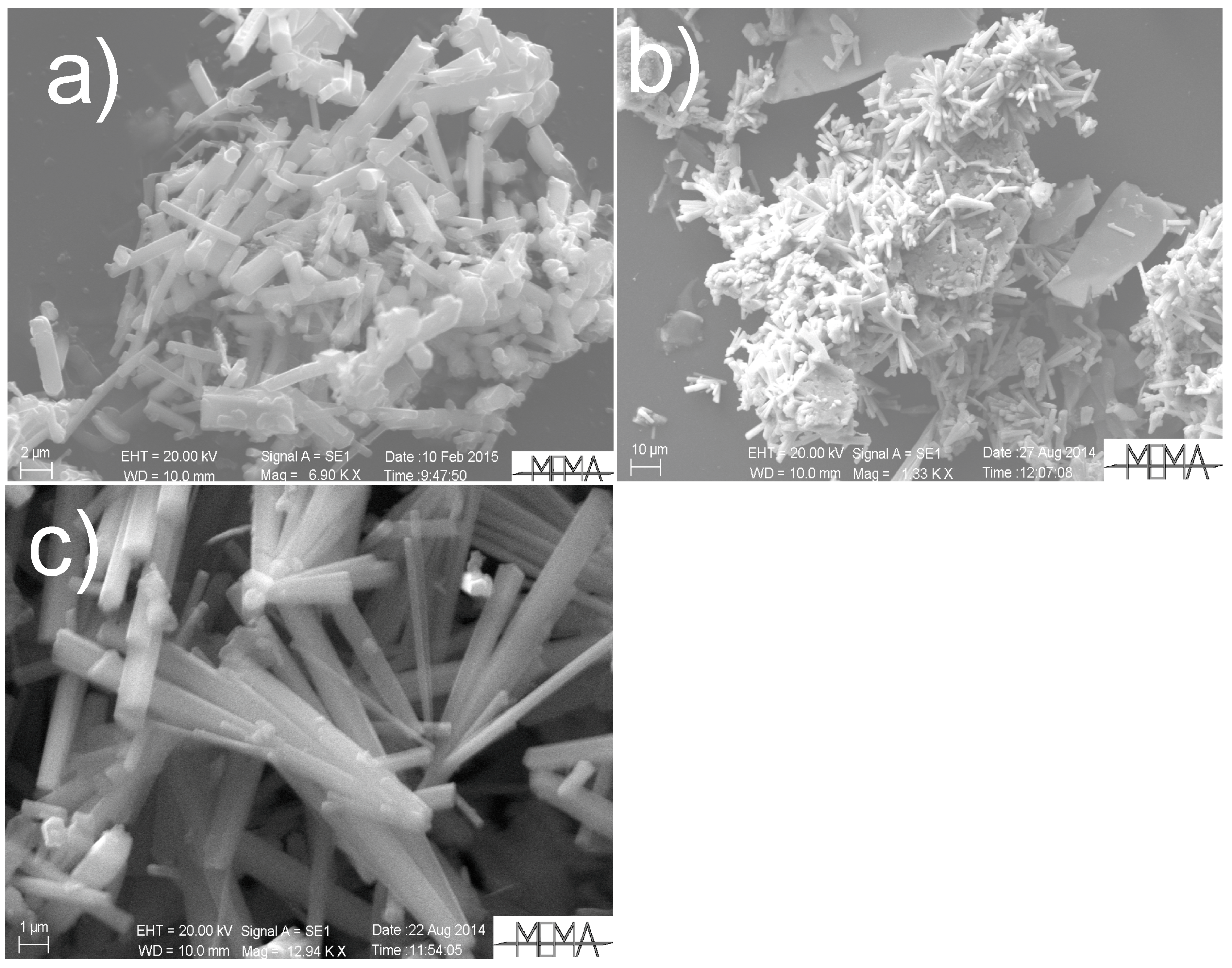
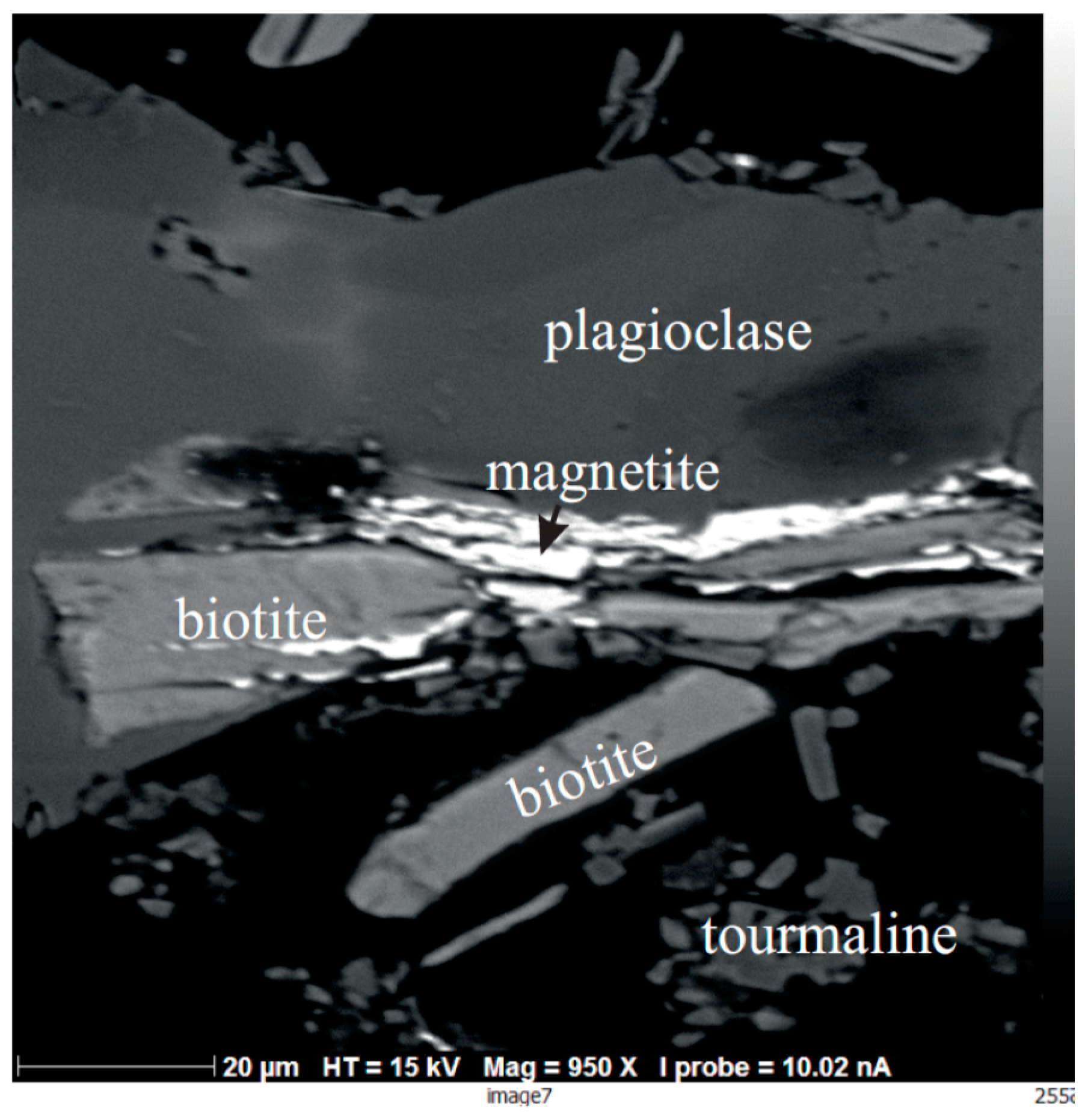
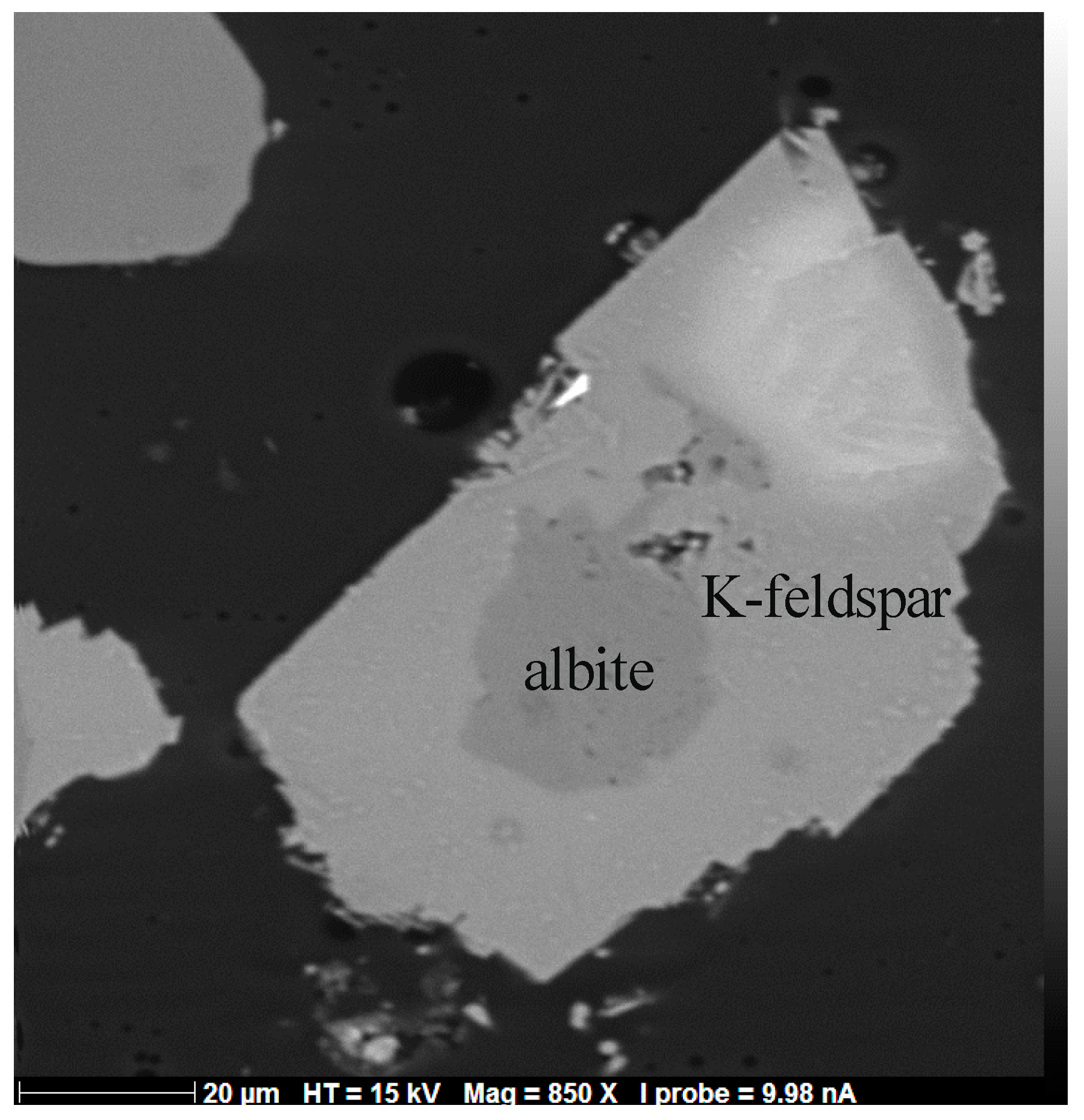
5.1. Solid Phases
5.1.1. General Remarks
5.1.2. Experiments Performed Using Saline Aqueous Solutions
5.1.3. Experiments Performed Using Pure Water
5.2. Fluids
6. Discussion
6.1. Tourmaline Formation
6.2. Synthetic and Natural Tourmalines
6.3. Implications of Tourmalinisation for the Metallogeny of Eastern Elba Iron Deposits and the Exploration of the Larderello-Travale Geothermal Field
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- London, D.; Manning, D.A.C. Chemical variation and significance of tourmaline from Southwest England. Econ. Geol. 1995, 90, 495–519. [Google Scholar] [CrossRef]
- London, D. Granitic Pegmatites. Geol. Soc. Am. Spec. Pap. 1996, 315, 305–319. [Google Scholar]
- London, D. Experimental synthesis and stability of tourmaline: A historical overview. Can. Mineral. 2011, 49, 117–136. [Google Scholar] [CrossRef]
- Buriánek, D.; Novák, M. Compositional evolution and substitutions in disseminated and nodular tourmaline from leucocratic granites: Examples from the Bohemian Massif, Czech Republic. Lithos 2007, 95, 148–164. [Google Scholar] [CrossRef]
- Slack, J.F.; Trumbull, R.B. Tourmaline as a recorder of ore-forming processes. Elements 2011, 7, 321–326. [Google Scholar] [CrossRef]
- Gawęda, A.; Müller, A.; Stein, H.; Kądziołko-Gaweł, M.; Mikulski, S. Age and origin of the tourmaline-rich hydraulic breccias in the Tatra Granite, Western Carpathians. J. Geosci. 2013, 58, 133–148. [Google Scholar] [CrossRef]
- London, D. Stability of tourmaline in peraluminous granite systems: The boron cycle from anatexis to hydrothermal aureoles. Eur. J. Mineral. 1999, 11, 253–262. [Google Scholar] [CrossRef]
- Dini, A.; Mazzarini, F.; Musumeci, G.; Rocchi, S. Multiple hydro-fracturing by boron-rich fluids in the Late Miocene contact aureole of eastern Elba Island (Tuscany, Italy). Terra Nova 2008, 20, 318–332. [Google Scholar] [CrossRef]
- Liotta, D.; Brogi, A.; Meccheri, M.; Dini, A.; Bianco, C.; Ruggieri, G. Coexistence od low-angle normal and high-angle strike- to oblique-slip faults during Late Miocene mineralization in eastern Elba Island (Italy). Tectonophysics 2015, 660, 17–34. [Google Scholar] [CrossRef]
- Trevisan, L. L’Elba orientale e la sua tettonica di scivolamento per gravità. Mem. Istituto Geol. Univ. Padova 1950, 16, 1–30. (In Italian) [Google Scholar]
- Zucchi, M.; Brogi, M.; Liotta, D.; Rimondi, V.; Ruggieri, G.; Montegrossi, G.; Caggianelli, A.; Dini, A. Permeability and hydraulic conductivity of faulted micaschist in the eastern Elba Island exhumed geo-thermal system (Tyrrhenian Sea, Italy): Insights from Cala Stagnone. Geothermics 2017, 70, 125–145. [Google Scholar] [CrossRef]
- Liotta, D.; Brogi, A.; Zucchi, M.; Bianco, C.; Ciacci, M.; Fregola, R.A.; Caggianelli, A.; Friðleifsson, G.Ó.; Hersir, G.P.; Helgadóttir, H.M.; et al. IMAGE Project, Public Deliverables IMAGE-D3.01, 2015. Final Report on: Understanding from Deep Drilling and Exhumed Systems. Available online: http://www.image-fp7.eu/reference-documents/deliverables/IMAGE-D3.01-v2015.10.31-deep%20drilling%20and%20exhumed%20systems-incl-annex%20-%20public.pdf (accessed on 23 August 2017).
- Bakker, R.J.; Schilli, S.E. Formation conditions of leucogranite dykes and aplite-pegmatite dykes in the eastern Mt. Capanne plutonic complex (Elba, Italy): Fluid inclusion studies in quartz, tourmaline, andalusite and plagioclase. Mineral. Petrol. 2016, 110, 43–63. [Google Scholar] [CrossRef]
- Fuchs, Y.; Lagache, M.; Linares, J. Fe-tourmaline synthesis under different T and fO2 conditions. Am. Mineral. 1998, 83, 525–534. [Google Scholar] [CrossRef]
- Van Hinsberg, V.J.; Henry, D.J.; Marschall, H.R. Tourmaline: An ideal indicator of its host environment. Can. Mineral. 2011, 49, 1–16. [Google Scholar] [CrossRef]
- Dutrow, B.L.; Henry, D.J. Tourmaline. Elements 2011, 7, 301–306. [Google Scholar] [CrossRef]
- Cavarretta, G.; Puxeddu, M. Schorl-dravite-ferridravite tourmalines deposited by hydrothermal magmatic fluids during early evolution of the Larderello geothermal field, Italy. Econ. Geol. 1990, 85, 1236–1251. [Google Scholar] [CrossRef]
- Garfagnoli, F.; Menna, F.; Pandeli, E.; Principi, G. The Porto Azzurro Unit (Mt. Calamita Promontory, southeastern Elba Island, Tuscany): Stratigraphic, tectonic and metamorphic evolution. Boll. Soc. Geol. Ital. 2005, 3, 119–138. [Google Scholar]
- Mazzarini, F.; Musumeci, G.; Cruden, A.R. Vein development during folding in the upper brittle crust: The case of tourmaline-rich veins of eastern Elba Island, northern Tyrrhenian Sea, Italy. J. Struct. Geol. 2011, 33, 1509–1522. [Google Scholar] [CrossRef]
- Musumeci, G.; Mazzarini, F.; Tiepolo, M.; Di Vincenzo, G. U-Pb and 40Ar-39Ar geochronology of Palaeozoic units in the northern Apennines: Determining protolith age and alpine evolution using the Calamita Schist and Ortano Porphyroid. Geol. J. 2011, 46, 288–310. [Google Scholar] [CrossRef]
- Maineri, C.; Benvenuti, M.; Costagliola, P.; Dini, A.; Lattanzi, P.; Ruggieri, G.; Villa, I.M. Sericitic alteration at the La Crocetta mine (Elba Island, Italy): Interplay between magmatism, tectonics, and hydrothermal activity. Miner. Depos. 2003, 38, 67–86. [Google Scholar] [CrossRef]
- Innocenti, F.; Serri, G.; Manetti, P.; Ferrara, G.; Tonarini, S. Genesis and classification of the rocks of the Tuscan Magmatic Province: Thirty years after Marinelli’s model. Acta Vulcanol. 1992, 2, 247–265. [Google Scholar]
- Peccerillo, A. The Tuscany Province. In Cenozoic Volcanism in the Tyrrhenian Sea Region; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mazzarini, F.; Musumeci, G. Hydrofracturing related sill and dyke emplacement at shallow crustal level: The eastern Elba Dyke Complex, Italy. In Structure and Emplacement of High-Level Magmatic Systems; Thomson, K., Petford, N., Eds.; Special Publications; Geological Society: London, UK, 2008; Volume 302, pp. 121–129. [Google Scholar]
- Duranti, S.; Palmeri, R.; Pertusati, P.C.; Ricci, C.A. Geological evolution and metamorphic petrology of the basal sequences of Eastern Elba (Complex II). Acta Vulcanol. 1992, 2, 213–229. [Google Scholar]
- Principi, G.; Bortolotti, V.; Pandeli, E.; Benvenuti, M.; Fanucci, F.; Chiari, M.; Dini, A.; Fazzuoli, M.; Menna, F.; Moretti, S.; et al. Note Illustrative della Carta Geologica d’Italia alla Scala 1:50.000—2015, Foglio 316, 317, 328, 329 Isola d’Elba; Selca: Firenze, Italy, 2011. [Google Scholar]
- Tanelli, G.; Benvenuti, M.; Costagliola, P.; Dini, A.; Lattanzi, P.; Maineri, C.; Mascaro, I.; Ruggieri, G. The iron mineral deposits of Elba Island: State of the art. Ofioliti 2001, 26, 239–248. [Google Scholar]
- Savi, P. Sulla miniera di ferro dell’Isola d’Elba. In Nuovo Giornale Letteratura; Nistri: Pisa, Italy, 1836; Volume 31, 45p. [Google Scholar]
- Lotti, B. Carta Geologica Dell’isola d’Elba alla Scala 1:25.000; Regio Ufficio Geologico d’Italia: Roma, Italy, 1884. [Google Scholar]
- Marinelli, G. II magmatismo recente in Toscana e le sue implicazioni minerogenetiche. Mem. Soc. Geol. Ital. 1983, 25, 111–124. [Google Scholar]
- Deschamps, Y.; Dagallier, G.; Macaudière, J.; Marignac, C.; Moine, B.; Saupé, F. Le gisement de pyrite-hématite de ValleGiove (Rio Marina, Ile d’Elbe, Italie). Schweiz. Mineralog. Petrogr. Mitt. 1983, 63, 149–165. [Google Scholar]
- Lattanzi, P.; Benvenuti, M.; Costagliola, P.; Tanelli, G. Anoverview on recent research on the metallogeny of Tuscany, with special reference to the Apuane Alps. Mem. Soc. Geol. Ital. 1994, 48, 613–625. [Google Scholar]
- Tanelli, G. Mineralizzazioni metallifere e minerogenesi in Toscana. Mem. Soc. Geol. Ital. 1983, 25, 91–109. [Google Scholar]
- Rossetti, F.; Tecce, F.; Billi, A.; Brilli, M. Patterns of fluid flow in the contact aureole of the Late Miocene Monte Capanne pluton (Elba Island, Italy): The role of structures and rheology. Contrib. Mineral. Petrol. 2007, 153, 743–760. [Google Scholar] [CrossRef]
- Cathelineau, M.; Marignac, C.; Boiron, M.C.; Gianelli, G.; Puxeddu, M. Evidence for Li-rich brines and early magmatic fluid rock interaction in the Larderello geothermal system. Geochim. Cosmochim. Acta 1994, 58, 1083–1099. [Google Scholar] [CrossRef]
- Dini, A.; Gianelli, G.; Puxeddu, M.; Ruggieri, G. Origin and evolution of Pliocene–Pleistocene granites from the Larderello geothermal field (Tuscan Magmatic Province, Italy). Lithos 2005, 81, 1–31. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Prokofev, V.Y.; Zagorskii, V.E.; Smirnov, S.Z. Role of Boric Acids in the Formation of Pegmatite and Hydrothermal Minerals: Petrologic Consequences of Sassolite (H3BO3) Discovery in Fluid Inclusions. Petrol. C/C Petrol. 2000, 8, 214–237. [Google Scholar]
- Frondel, C.; Collette, R.L. Synthesis of tourmaline by reaction of mineral grains with NaCl-H3BO3 solution, and its implicatioins in rock metamorphism. Am. Mineral. 1957, 42, 754–758. [Google Scholar]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newslett. CPD 1999, 21, 14–15. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. Quantitative analysis of homogeneous or stratified microvolumes applying the model “PAP”. In Electron Probe Quantitation; Springer: Berlin, Germany, 1991; pp. 31–75. [Google Scholar]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, O.; Pezzotta, F. Nomenclature of the tourmaline-super- group minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Selway, J.B.; Xiong, J. Tourmaline-Recalculation Software Quoted in Tindle et al. 2002. Available online: http://www.open.ac.uk/earth-research/tindle/ (accessed on 23 August 2017).
- Neusser, G.; Abart, R.; Fischer, F.D.; Harlov, D.; Norberg, N. Experimental Na/K exchange between alkali feldspar and NaCl–KCl salt melt: Chemically induced fracturing and element partitioning. Contrib. Mineral. Petrol. 2012, 164, 341–358. [Google Scholar] [CrossRef]
- Chae, G.; Yun, S.-T.; Kwon, M.-J.; Kim, Y.-S.; Mayer, B. Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochem. J. 2006, 40, 95–102. [Google Scholar] [CrossRef]
- Morgan, G.B.; London, D. Experimental reactions of amphibolite with boron-bearing aqueous fluids at 200 MPa: Implications for tourmaline stability and partial melting in mafic rocks. Contrib. Mineral. Petrol. 1989, 102, 281–297. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Seward, T.M. A spectrophotometric study of aqueous iron (II) chloride complexing from 25 to 200 °C. Geochim. Cosmochim. Acta 1990, 54, 2207–2221. [Google Scholar] [CrossRef]
- Fein, J.B.; Hemley, J.J.; D’Angelo, W.M.; Komninou, A.; Sverjensky, D.A. Experimental study of iron-chloride complexing in hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3179–3190. [Google Scholar] [CrossRef]
- Ding, K.; Seyfried, W.E., Jr. Determination of Fe-Cl complexing in the low pressure supercritical region (NaCl fluid): Iron solubility constraints on pH of subseafloor hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3681–3692. [Google Scholar] [CrossRef]
- Seward, T.M.; Barnes, H.L. Metal transport by hydrothermal ore fluids. Geochem. Hydrotherm. Ore Depos. 1997, 3, 435–486. [Google Scholar]
- Stefánsson, A.; Lemke, K.H.; Seward, T.M. Iron (III) complexation in hydrothermal solutions—An experimental and theoretical study. In Proceedings of the 15th International Conference on the Properties of Water and Steam, Berlin, Germany, 2008; pp. 7–11. [Google Scholar]
- Puxeddu, M.; Saupé, F.; Déchomets, R.; Gianelli, G.; Moine, B. Geochemistry and stratigraphic correlations—Application to the investigation of geothermal and mineral resources of Tuscany, Italy. Chem. Geol. 1984, 43, 77–113. [Google Scholar] [CrossRef]
- Von Goerne, G.; Franz, G.; Heinrich, W. Synthesis of tourmaline in the system Na2O-MgO-Al2O3-SiO2-B2O3-HCl and the distribution of Na between tourmaline and fluid at 300 to 700 °C and 200 MPa. Contrib. Mineral. Petrol. 2001, 141, 160–173. [Google Scholar] [CrossRef]
- Pesquera, A.; Torres-Ruiz, J.; García-Casco, A.; Gil-Crespo, P.P. Evaluating the controls on tourmaline formation in granitic systems: A case study on peraluminous granites from the Central Iberian Zone (CIZ), Western Spain. J. Petrol. 2013, 54, 609–634. [Google Scholar] [CrossRef]
- Mlynarczyk, M.S.J.; Williams-Jones, A.E. Zoned tourmaline associated with cassiterite: Implications for fluid evolution and tin mineralization in the San Rafael Sn-Cu deposit, southeastern Peru. Can. Mineral. 2006, 44, 347–365. [Google Scholar] [CrossRef]
- Dini, A. Ore deposits, industrial minerals and geothermal resources. Period. Mineral. 2003, 72, 41–52. [Google Scholar]
- Dünkel, I. The Genesis of East Elba Iron ore Deposits and Their Interrelation with Messinian Tectonics. Ph.D. Thesis, Institut und Museum für Geologie und Paläontologie der Universität Tübingen, Tübingen, Germany, 2002; 143p. [Google Scholar]
- Gillieron, F. Osservazioni sulla geologia dei giacimenti di ferro dell’Elba orientale. L’Ind. Min. 1959, 10, 1–10. (In Italian) [Google Scholar]
- Murakami, T.; Ito, J.-I.; Utsunomiya, S.; Kasama, T.; Kozai, N.; Ohnuki, T. Anoxic dissolution processes of biotite: Implications for Fe behavior during Archean weathering. Earth Planet. Sci. Lett. 2004, 224, 117–129. [Google Scholar] [CrossRef]
- Eugster, H.P.; Wilson, G.A. Transport and deposition of ore-forming elements in hydrothermal systems associated with granites. In High Heat Production (HHP) Granites, Hydrothermal Circulation and Ore Genesis; Halls, C., Ed.; Institution of Mining and Metallurgy: London, UK, 1985; pp. 87–98. [Google Scholar]
- Müller, B.; Seward, T.M. Spectrophotometric determination of the stability of tin (II) chloride complexes in aqueous solution up to 300 °C. Geochim. Cosmochim. Acta 2001, 65, 4187–4199. [Google Scholar] [CrossRef]
- Benvenuti, M.; Dini, A.; D’Orazio, M.; Chiarantini, L.; Corretti, A.; Costagliola, P. The tungsten and tin signature of iron ores from Elba Island (Italy): A tool for provenance studies of iron production in the mediterranean region. Archaeometry 2013, 55, 479–506. [Google Scholar] [CrossRef]
- Bertini, G.; Casini, M.; Gianelli, G.; Pandeli, E. Geological structure of a long-living geothermal system, Larderello, Italy. Terra Nova 2006, 18, 163–169. [Google Scholar] [CrossRef]
- Dini, A.; Brogi, A.; Liotta, D.; Rimondi, V.; Ruggieri, G.; Zucchi, M. The Eastern Elba Island Magmatic-Hydrothermal Complex: A Proxy for the Supercritical Roots of Larderello Geothermal System (Italy). In Proceedings of the Goldschmidt 2016, Yokohama, Japan, 26 June–1 July 2016; Volume 580. [Google Scholar]
- Von Goerne, G.; Franz, G.; van Hinsberg, V.J. Experimental determination of Na–Ca distribution between tourmaline and fluid in the system CaO–Na2O–MgO–Al2O3–SiO2–B2O3–H2O. Can. Mineral. 2011, 49, 137–152. [Google Scholar] [CrossRef]

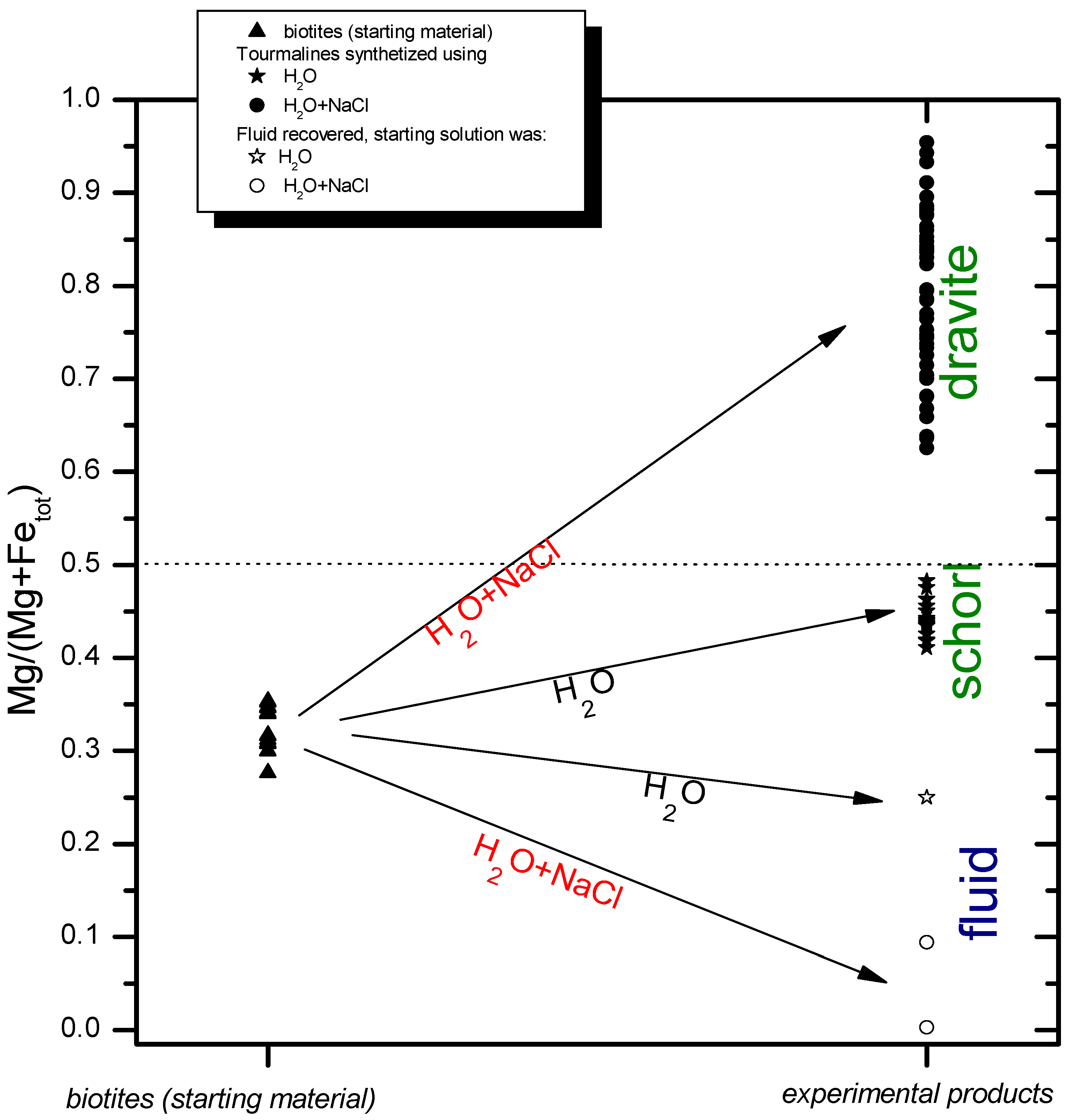
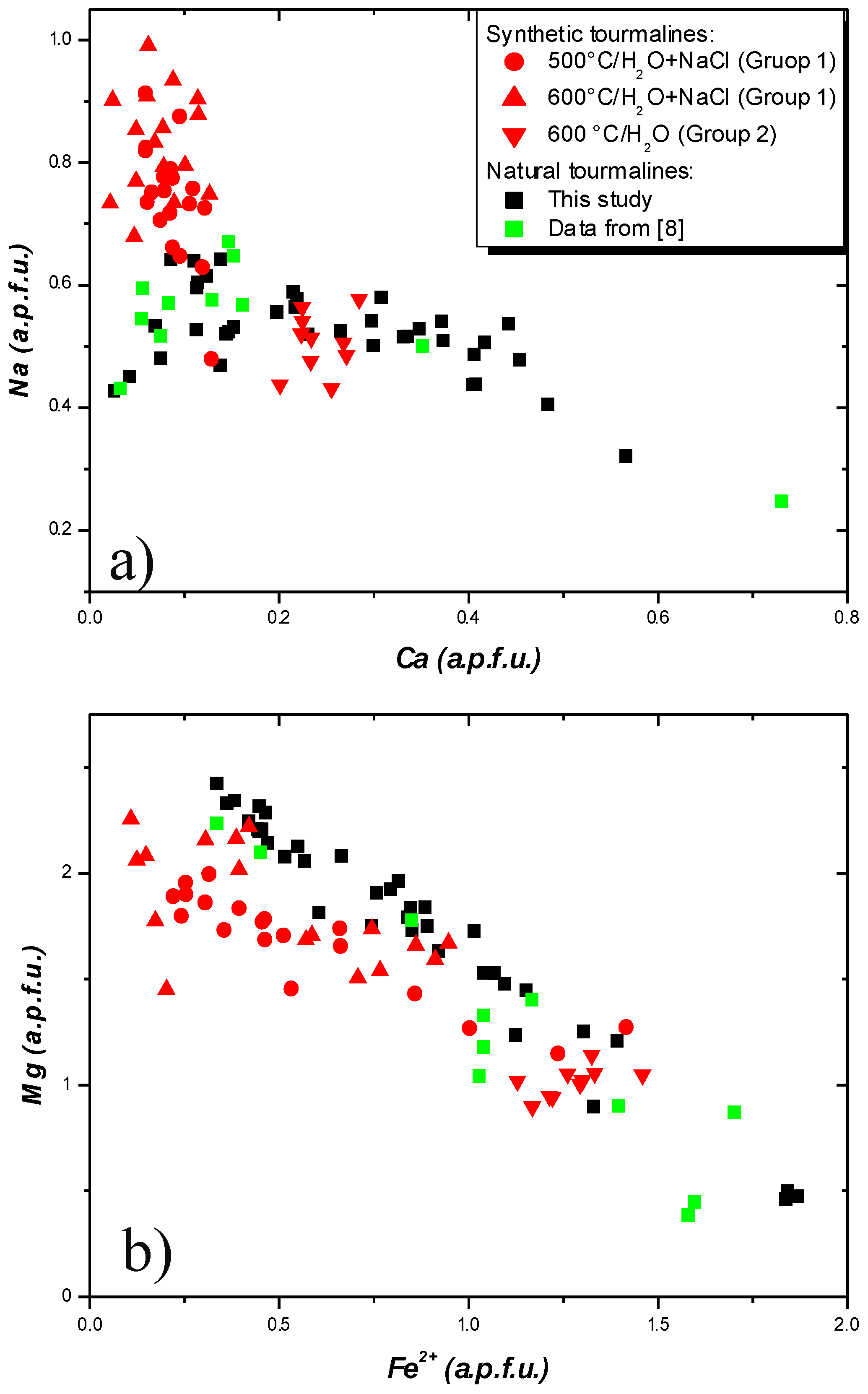
| Sample | GN1B | GN1B | GN1B | GN1B | GN1B | GN1B | GN1B | GN1B | GN1B | Sample | GN1B | GN1B | GN1B | GN1B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | biotite | biotite | biotite | biotite | biotite | biotite | biotite | biotite | biotite | Phase | plagioclase | plagioclase | plagioclase | plagioclase |
| SiO2 | 33.39 | 32.70 | 33.19 | 32.91 | 33.33 | 33.42 | 32.87 | 33.37 | 32.79 | SiO2 | 64.73 | 66.82 | 66.90 | 66.43 |
| TiO2 | 3.02 | 2.69 | 3.14 | 3.31 | 2.75 | 3.37 | 3.49 | 2.93 | 0.71 | TiO2 | 0.03 | 0.06 | bdl | 0.23 |
| Al2O3 | 19.74 | 19.88 | 19.45 | 18.93 | 19.70 | 19.19 | 19.03 | 19.80 | 21.16 | Al2O3 | 18.79 | 19.10 | 19.82 | 19.21 |
| Cr2O3 | 0.49 | 0.31 | 0.24 | 0.18 | 0.23 | 0.58 | 0.07 | 0.35 | 0.18 | Cr2O3 | 0.42 | 0.92 | 0.25 | bdl |
| FeO | 23.80 | 23.97 | 23.49 | 24.15 | 22.50 | 22.90 | 24.26 | 23.24 | 22.49 | FeO | 0.78 | 0.75 | 0.87 | 0.17 |
| MnO | 0.70 | 0.96 | 1.15 | 0.64 | 0.96 | 0.68 | 1.10 | 0.91 | 0.84 | MnO | 1.40 | 0.78 | 0.94 | 0.70 |
| MgO | 5.73 | 5.97 | 6.12 | 6.06 | 6.90 | 6.65 | 5.84 | 6.70 | 6.87 | MgO | bdl | bdl | 0.09 | 0.02 |
| CaO | bdl | 0.07 | bdl | bdl | 0.20 | bdl | 0.01 | bdl | bdl | CaO | 0.45 | 0.44 | 0.87 | 0.39 |
| Na2O | 0.13 | 0.18 | 0.31 | 0.20 | 0.20 | 0.14 | 0.29 | 0.23 | 0.22 | Na2O | 12.16 | 11.69 | 11.59 | 11.49 |
| K2O | 9.56 | 9.32 | 9.51 | 10.19 | 10.10 | 10.42 | 10.04 | 9.62 | 10.37 | K2O | 0.13 | 0.21 | 0.05 | 0.37 |
| sum | 96.55 | 96.05 | 96.60 | 96.57 | 96.86 | 97.35 | 96.99 | 97.17 | 95.62 | sum | 98.89 | 100.76 | 101.38 | 99.01 |
| Cations per 22 O | Cations per 8 O | |||||||||||||
| Si | 5.170 | 5.104 | 5.146 | 5.136 | 5.139 | 5.143 | 5.116 | 5.129 | 5.121 | Si | 2.942 | 2.965 | 2.939 | 2.972 |
| IVAl | 2.830 | 2.896 | 2.854 | 2.864 | 2.861 | 2.857 | 2.884 | 2.871 | 2.879 | Al | 1.007 | 0.999 | 1.026 | 1.013 |
| Fe3+ | 0.033 | 0.031 | 0.036 | 0.007 | ||||||||||
| sum | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | sum | 3.981 | 3.995 | 4.000 | 3.992 |
| VIAl | 0.772 | 0.761 | 0.699 | 0.618 | 0.718 | 0.622 | 0.607 | 0.715 | 1.015 | |||||
| Ti | 0.352 | 0.316 | 0.366 | 0.389 | 0.319 | 0.391 | 0.408 | 0.339 | 0.083 | Ca | 0.022 | 0.021 | 0.041 | 0.019 |
| Fe2+ | 3.085 | 3.131 | 3.048 | 3.154 | 2.904 | 2.950 | 3.160 | 2.989 | 2.940 | Na | 1.072 | 1.006 | 0.987 | 0.996 |
| Mn | 0.092 | 0.127 | 0.151 | 0.084 | 0.125 | 0.088 | 0.145 | 0.119 | 0.111 | K | 0.008 | 0.012 | 0.003 | 0.021 |
| Mg | 1.323 | 1.391 | 1.415 | 1.410 | 1.587 | 1.525 | 1.356 | 1.535 | 1.600 | sum | 1.101 | 1.039 | 1.030 | 1.036 |
| Cr | 0.060 | 0.038 | 0.030 | 0.023 | 0.029 | 0.071 | 0.009 | 0.042 | 0.022 | |||||
| sum | 5.683 | 5.764 | 5.709 | 5.678 | 5.683 | 5.647 | 5.684 | 5.740 | 5.772 | sum cations | 5.083 | 5.033 | 5.030 | 5.028 |
| Ca | 0.000 | 0.012 | 0.000 | 0.000 | 0.032 | 0.000 | 0.001 | 0.000 | 0.000 | End-members (mol %) | ||||
| Na | 0.040 | 0.054 | 0.094 | 0.062 | 0.060 | 0.042 | 0.086 | 0.070 | 0.066 | anorthite | 2.0 | 2.0 | 4.0 | 1.8 |
| K | 1.889 | 1.857 | 1.881 | 2.028 | 1.987 | 2.046 | 1.993 | 1.887 | 2.066 | albite | 97.3 | 96.8 | 95.8 | 96.2 |
| sum | 1.929 | 1.923 | 1.975 | 2.090 | 2.079 | 2.088 | 2.081 | 1.957 | 2.132 | orthoclase | 0.7 | 1.2 | 0.3 | 2.1 |
| sum cations | 15.612 | 15.688 | 15.684 | 15.768 | 15.762 | 15.735 | 15.766 | 15.697 | 15.904 | |||||
| Mg # | 0.30 | 0.31 | 0.32 | 0.31 | 0.35 | 0.34 | 0.30 | 0.34 | 0.35 | |||||
| H2O calc | 3.88 | 3.85 | 3.87 | 3.84 | 3.89 | 3.91 | 3.85 | 3.91 | 3.84 | |||||
| sum calc | 100.4 | 99.9 | 100.5 | 100.4 | 100.8 | 101.3 | 100.8 | 101.1 | 99.5 | |||||
| Experiment | 2 | 4 | 5 | 6 | 8 | 9 | 10 | 11 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting Material | ||||||||||||||
| Rock/sep. | GN1B sep. | GN1B sep. | GN1B sep. | GN1B sep. | GN1B | GN1B | GN1B | GN1B | GN1B sep. | GN1B sep. | GN1B sep. | GN1B sep. | GN1B | GN1B sep. |
| Fluid | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O | H2O | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O | H2O |
| Rock/sep. (g) | 0.0311 | 0.0275 | 0.0408 | 0.3164 | 0.0421 | 0.0523 | 0.0539 | 0.0486 | 0.0276 | 0.031 | 0.3284 | 0.0451 | 0.0486 | 0.2504 |
| Fluid (g) | 0.0786 | 0.0394 | 0.0514 | 0.6593 | 0.0831 | 0.0839 | 0.0836 | 0.0854 | 0.0404 | 0.0759 | 0.6487 | 0.0919 | 0.0867 | 0.5215 |
| H3BO3 (g) | 0.0078 | 0.0055 | 0.0102 | 0.0791 | 0.0114 | 0.0005 | 0.0001 | 0.0005 | 0.00002 | 0.0073 | 0.0657 | 0.0134 | 0.0115 | 0.0626 |
| H3BO3 (M) | 1.6 | 2.3 | 3.2 | 1.9 | 2.2 | 0.1 | 0.01 | 0.1 | 0.01 | 1.6 | 1.6 | 2.4 | 2.1 | 1.9 |
| Solid/Fluid | 0.5 | 0.8 | 1.0 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.5 | 0.6 | 0.6 | 0.7 | 0.6 |
| P-T Conditions | ||||||||||||||
| T (°C) | 500 | 500 | 500 | 600 | 600 | 600 | 600 | 600 | 600 | 500 | 500 | 600 | 600 | 500 |
| P (MPa) | 130 | 130 | 130 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Note | long capsule | long capsule | long capsule | |||||||||||
| Results | ||||||||||||||
| tourmaline | yes | yes | yes | yes | yes | no | no | no | no | yes | yes | yes | yes | yes |
| XMg | 0.63–0.90 | 0.71–0.88 | 0.68–0.85 | 0.66–0.88 | 0.44–0.45 | 0.73–0.74 | 0.64–0.95 | 0.42–0.47 | ||||||
| Rietveld Refinement (wt %) | ||||||||||||||
| biotite | 22 (47) | 3 (27) | 15 (47) | 21 (47) | ||||||||||
| plagioclase | 49 (44) | 29 (41) | 59 (44) | 47 (44) | ||||||||||
| tourmaline | 25 | 43 | 20 | 29 | ||||||||||
| K-feldspar | 2 | 17 | 5 | |||||||||||
| quartz | 2 (4) | 8 (25) | 1 (4) | 4 (4) | ||||||||||
| Synthetic Tourmalines | Natural Tourmalines (Cala Stagnone) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Exp2 | Exp2 | Exp2 | Exp2 | Exp2 | Exp4 | Exp4 | Exp5 | Exp6 | Exp6 | Exp18 | Exp8 | Exp8 | Exp19 | Exp19 | Exp19 | Exp19 | Exp19 | F1ST1A | F2ST3A | F2ST3A | F2ST3A | F1ST4C | F1ST4C |
| Label | 6 | 11 | 16 | 18 | 19 | 16 | 19 | 17 | 7 | 13 | 1 | 4 | 10 | 2 | 3 | 4 | 5 | 8 | 14 | 1 | 2 | 3 | 4 | 5 |
| Fluid | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O-NaCl | H2O | H2O | H2O | H2O | H2O | H2O | H2O | ||||||
| SiO2 | 35.54 | 35.75 | 35.44 | 35.10 | 34.52 | 37.14 | 35.68 | 34.86 | 34.28 | 32.49 | 31.49 | 33.72 | 35.07 | 32.87 | 34.70 | 33.38 | 35.97 | 32.82 | 36.10 | 36.01 | 35.04 | 35.56 | 36.00 | 35.68 |
| TiO2 | 0.39 | 0.48 | 0.50 | 0.41 | 0.56 | 0.41 | 0.43 | 0.94 | 1.32 | 1.06 | 1.16 | 0.65 | 0.87 | 0.72 | 0.72 | 0.81 | 0.62 | 0.49 | 0.77 | 0.26 | 0.98 | 0.42 | 1.81 | 0.63 |
| Al2O3 | 34.66 | 35.51 | 34.89 | 34.76 | 34.49 | 34.02 | 35.27 | 32.23 | 33.51 | 33.98 | 35.88 | 33.91 | 33.95 | 34.04 | 33.55 | 36.37 | 34.20 | 35.42 | 29.78 | 31.28 | 32.18 | 34.63 | 28.28 | 32.29 |
| Cr2O3 | 0.02 | bdl | 0.16 | 0.31 | bdl | 0.07 | 0.05 | 0.09 | 0.09 | 0.02 | 0.06 | bdl | 0.14 | 0.10 | bdl | bdl | bdl | 0.02 | 0.18 | 0.54 | 0.34 | 0.19 | 0.21 | 0.65 |
| FeO | 3.72 | 1.87 | 3.33 | 2.30 | 4.77 | 1.78 | 1.86 | 8.71 | 3.05 | 6.71 | 5.00 | 9.22 | 9.15 | 9.11 | 8.69 | 8.17 | 8.88 | 8.22 | 3.25 | 5.91 | 9.36 | 13.46 | 7.47 | 5.40 |
| MnO | 0.08 | 0.04 | 0.03 | bdl | 0.07 | bdl | 0.09 | 0.04 | bdl | bdl | 0.11 | 0.03 | bdl | 0.10 | 0.08 | 0.06 | 0.03 | bdl | 0.52 | 0.60 | 0.51 | 0.43 | 0.56 | 0.49 |
| MgO | 6.97 | 8.10 | 7.28 | 8.17 | 7.07 | 7.41 | 7.82 | 4.55 | 9.04 | 6.64 | 5.97 | 4.06 | 4.29 | 3.97 | 3.76 | 4.14 | 3.89 | 3.54 | 10.70 | 7.99 | 5.05 | 2.05 | 8.02 | 7.14 |
| CaO | 0.42 | 0.55 | 0.45 | 0.37 | 0.60 | 0.48 | 0.50 | 0.71 | 0.51 | 0.38 | 0.48 | 1.48 | 1.27 | 1.56 | 1.51 | 1.32 | 1.33 | 1.40 | 2.32 | 0.78 | 0.64 | 0.43 | 1.88 | 1.12 |
| Na2O | 2.22 | 2.06 | 2.46 | 2.37 | 2.29 | 2.28 | 2.09 | 1.46 | 2.30 | 2.55 | 2.85 | 1.55 | 1.69 | 1.75 | 1.49 | 1.60 | 1.50 | 1.31 | 1.38 | 2.01 | 1.87 | 1.52 | 1.60 | 1.74 |
| K2O | bdl | 0.03 | 0.07 | 0.10 | bdl | 0.18 | bdl | 0.07 | 0.03 | 0.07 | bdl | bdl | bdl | 0.31 | bdl | 0.07 | 0.66 | 0.40 | bdl | 0.07 | bdl | bdl | bdl | 0.29 |
| Sum | 84.01 | 84.40 | 84.60 | 83.89 | 84.37 | 83.76 | 83.80 | 83.67 | 84.13 | 83.89 | 82.99 | 84.62 | 86.43 | 84.53 | 84.49 | 85.94 | 87.08 | 83.63 | 84.99 | 85.46 | 85.96 | 88.69 | 85.82 | 85.43 |
| H2O * | 3.6 | 3.7 | 3.7 | 3.7 | 3.6 | 3.7 | 3.7 | 3.5 | 3.6 | 3.6 | 3.5 | 3.6 | 3.6 | 3.5 | 3.6 | 3.6 | 3.7 | 3.5 | 3.7 | 3.6 | 3.6 | 3.7 | 3.6 | 3.6 |
| B2O3 * | 10.6 | 10.7 | 10.6 | 10.6 | 10.5 | 10.7 | 10.7 | 10.3 | 10.5 | 10.3 | 10.3 | 10.3 | 10.5 | 10.2 | 10.3 | 10.5 | 10.6 | 10.2 | 10.6 | 10.5 | 10.4 | 10.6 | 10.4 | 10.6 |
| Total | 98.24 | 98.84 | 98.92 | 98.15 | 98.52 | 98.12 | 98.14 | 97.46 | 98.32 | 97.74 | 96.80 | 98.48 | 100.62 | 98.30 | 98.40 | 100.09 | 101.38 | 97.40 | 99.22 | 99.63 | 100.00 | 102.98 | 99.87 | 99.63 |
| Structural formula based on 31 anions (O, OH, F) and 3 B | ||||||||||||||||||||||||
| Si | 5.84 | 5.79 | 5.79 | 5.75 | 5.70 | 6.05 | 5.82 | 5.91 | 5.65 | 5.49 | 5.33 | 5.69 | 5.78 | 5.58 | 5.83 | 5.51 | 5.88 | 5.58 | 5.93 | 5.94 | 5.83 | 5.82 | 5.99 | 5.87 |
| Al | 0.16 | 0.21 | 0.21 | 0.25 | 0.30 | 0.00 | 0.18 | 0.09 | 0.35 | 0.51 | 0.67 | 0.31 | 0.22 | 0.42 | 0.17 | 0.49 | 0.12 | 0.42 | 0.07 | 0.06 | 0.17 | 0.18 | 0.01 | 0.13 |
| sum T | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.05 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Al | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 5.70 | 6.00 | 6.00 | 6.00 | 5.54 | 6.00 |
| Mg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 | 0.00 | 0.00 | 0.00 | 0.46 | 0.00 |
| sum Z | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Al | 0.55 | 0.57 | 0.50 | 0.47 | 0.42 | 0.53 | 0.59 | 0.35 | 0.15 | 0.25 | 0.49 | 0.43 | 0.37 | 0.39 | 0.48 | 0.59 | 0.47 | 0.67 | 0.00 | 0.02 | 0.15 | 0.49 | 0.00 | 0.14 |
| Ti | 0.05 | 0.06 | 0.06 | 0.05 | 0.07 | 0.05 | 0.05 | 0.12 | 0.16 | 0.13 | 0.15 | 0.08 | 0.11 | 0.09 | 0.09 | 0.10 | 0.08 | 0.06 | 0.09 | 0.03 | 0.12 | 0.05 | 0.23 | 0.08 |
| Cr | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.07 | 0.04 | 0.02 | 0.03 | 0.08 |
| Mg | 1.71 | 1.96 | 1.77 | 2.00 | 1.74 | 1.80 | 1.90 | 1.15 | 2.22 | 1.67 | 1.51 | 1.02 | 1.05 | 1.00 | 0.94 | 1.02 | 0.95 | 0.90 | 2.32 | 1.96 | 1.25 | 0.50 | 1.53 | 1.75 |
| Mn | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.07 | 0.08 | 0.07 | 0.06 | 0.08 | 0.07 |
| Fe2+ | 0.51 | 0.25 | 0.45 | 0.32 | 0.66 | 0.24 | 0.25 | 1.24 | 0.42 | 0.95 | 0.71 | 1.30 | 1.26 | 1.29 | 1.22 | 1.13 | 1.21 | 1.17 | 0.45 | 0.81 | 1.30 | 1.84 | 1.04 | 0.74 |
| sum Y | 2.83 | 2.84 | 2.81 | 2.87 | 2.90 | 2.63 | 2.82 | 2.87 | 2.97 | 3.00 | 2.88 | 2.84 | 2.81 | 2.81 | 2.74 | 2.85 | 2.71 | 2.80 | 2.95 | 2.99 | 2.95 | 2.97 | 2.90 | 2.87 |
| Ca | 0.07 | 0.10 | 0.08 | 0.07 | 0.11 | 0.08 | 0.09 | 0.13 | 0.09 | 0.07 | 0.09 | 0.27 | 0.22 | 0.28 | 0.27 | 0.23 | 0.23 | 0.26 | 0.41 | 0.14 | 0.11 | 0.07 | 0.34 | 0.20 |
| Na | 0.71 | 0.65 | 0.78 | 0.75 | 0.73 | 0.72 | 0.66 | 0.48 | 0.73 | 0.83 | 0.93 | 0.51 | 0.54 | 0.58 | 0.49 | 0.51 | 0.48 | 0.43 | 0.44 | 0.64 | 0.60 | 0.48 | 0.52 | 0.56 |
| K | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | 0.04 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.01 | 0.14 | 0.09 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.06 |
| sum X | 0.78 | 0.75 | 0.87 | 0.84 | 0.84 | 0.84 | 0.75 | 0.62 | 0.83 | 0.92 | 1.02 | 0.77 | 0.77 | 0.93 | 0.76 | 0.76 | 0.85 | 0.77 | 0.85 | 0.79 | 0.72 | 0.56 | 0.85 | 0.81 |
| Classif. | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Schorl | Dravite | Dravite | Dravite | Schorl | Schorl | Schorl | Schorl | Schorl | Schorl | Schorl | Dravite | Dravite | Schorl | Schorl | Dravite | Dravite |
| Experiment | 6 | 17 | 20 |
|---|---|---|---|
| T (°C) | 600 | 500 | 500 |
| P (MPa) | 100 | 100 | 100 |
| Fluid | H2O-NaCl | H2O-NaCl | H2O |
| Values in μg/g | |||
| ICP | |||
| Na | 57,446 | 64,636 | bdl |
| Li | 131 | 85 | 19 |
| K | 11,439 | 6438 | 529 |
| Mg | 185 | 8 | 104 |
| Ca | 229 | 326 | 85 |
| Fe | 1786 | 2709 | 312 |
| Mn | 237 | 164 | 1.9 |
| Si | na | 274 | 1994 |
| Ni | na | 1.7 | na |
| LC | |||
| Cl | 109,368 | 119,527 | 479 |
| F | 120 | 77 | 128 |
| SO4 | 95 | 442 | 95 |
| NO3 | bdl | 17 | 39 |
| NH4 | bdl | 28 | 52 |
| Starting Fluid Composition (LC) | |||
| Na (LC) | 87,856 | bdl | |
| Cl (LC) | 128,872 | bdl | |
| Li (ICP) | 28 | bdl | |
| K (ICP) | 281 | bdl | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, A.; Ruggieri, G.; Chiarantini, L.; Montegrossi, G.; Rimondi, V. Experimental Investigation of Biotite-Rich Schist Reacting with B-Bearing Fluids at Upper Crustal Conditions and Correlated Tourmaline Formation. Minerals 2017, 7, 155. https://doi.org/10.3390/min7090155
Orlando A, Ruggieri G, Chiarantini L, Montegrossi G, Rimondi V. Experimental Investigation of Biotite-Rich Schist Reacting with B-Bearing Fluids at Upper Crustal Conditions and Correlated Tourmaline Formation. Minerals. 2017; 7(9):155. https://doi.org/10.3390/min7090155
Chicago/Turabian StyleOrlando, Andrea, Giovanni Ruggieri, Laura Chiarantini, Giordano Montegrossi, and Valentina Rimondi. 2017. "Experimental Investigation of Biotite-Rich Schist Reacting with B-Bearing Fluids at Upper Crustal Conditions and Correlated Tourmaline Formation" Minerals 7, no. 9: 155. https://doi.org/10.3390/min7090155






