Bioelectrochemical Changes during the Early Stages of Chalcopyrite Interaction with Acidithiobacillus Thiooxidans and Leptospirillum sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis and Obtainment of RSS on Chalcopyrite
2.2. Biofilm Formation
2.3. Electrochemical Assessment of the MCE*-RSS Reactivity
2.4. Surficial Analyses after the Biooxidation of MCE*-RSS
3. Results
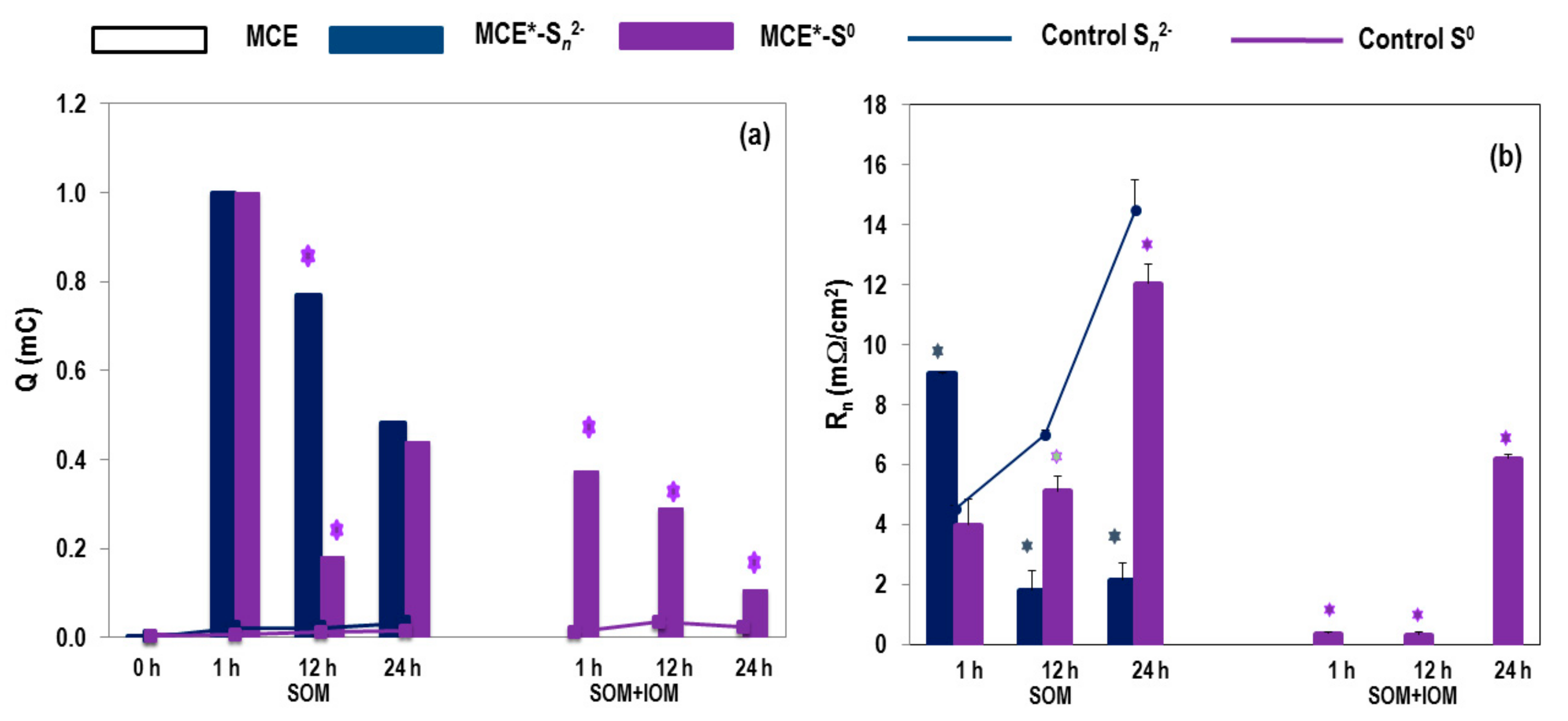
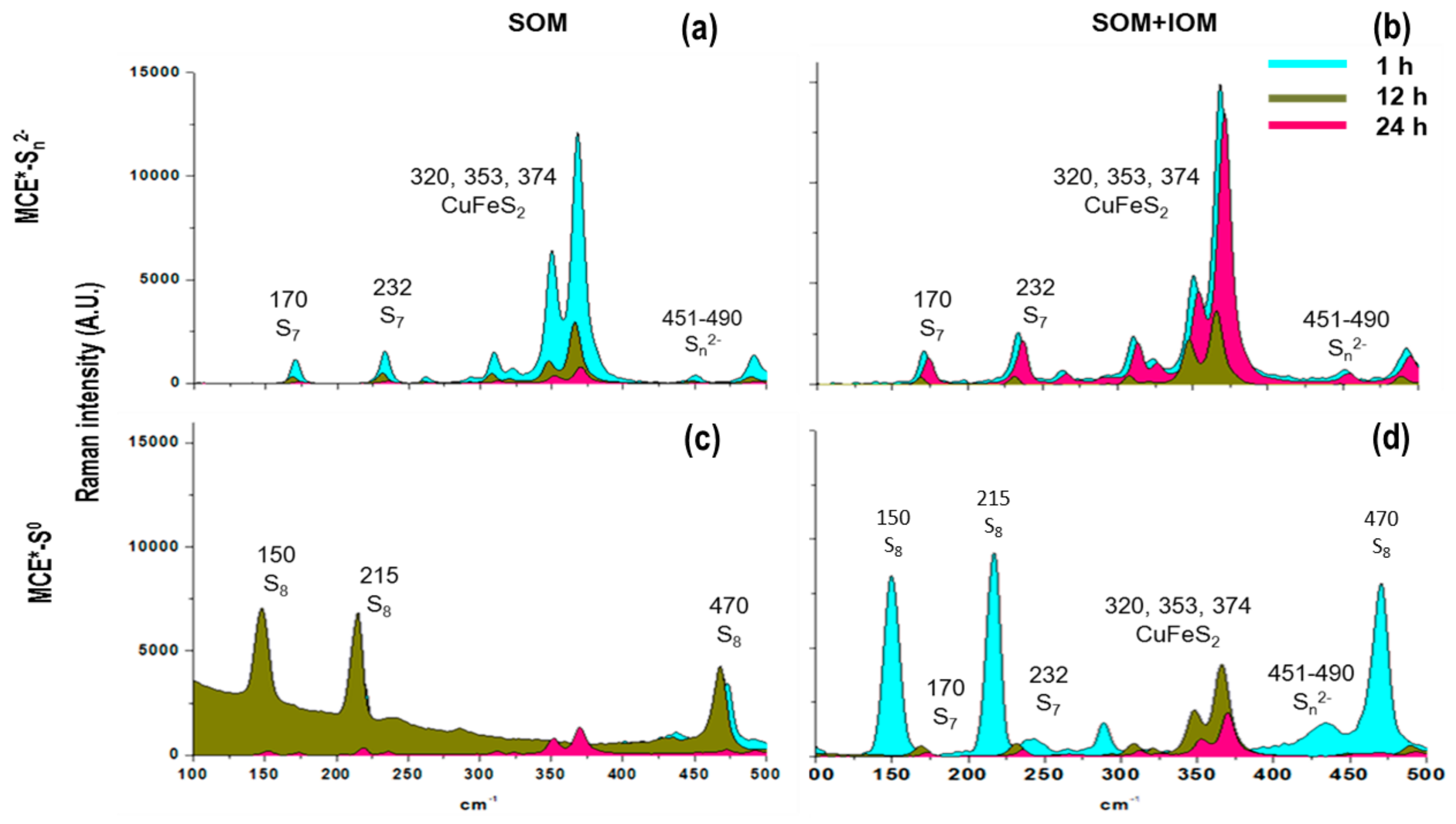
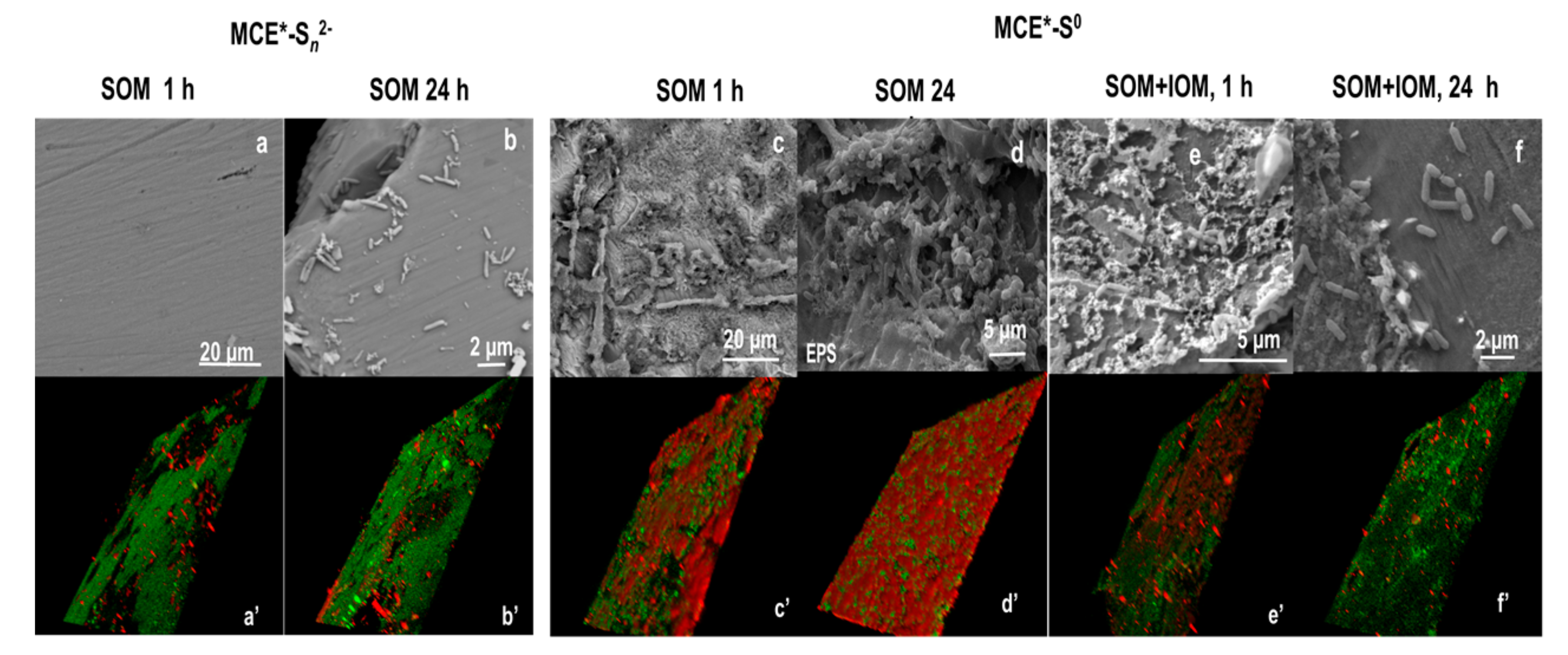
3.1. SOM Biofilms and Biooxidation of Sn2− and S0
3.2. Biooxidation of MCE*-S0 by SOM and IOM
3.3. EIS Analyses of MCE*-S0 with SOM or SOM+IOM Biofilms
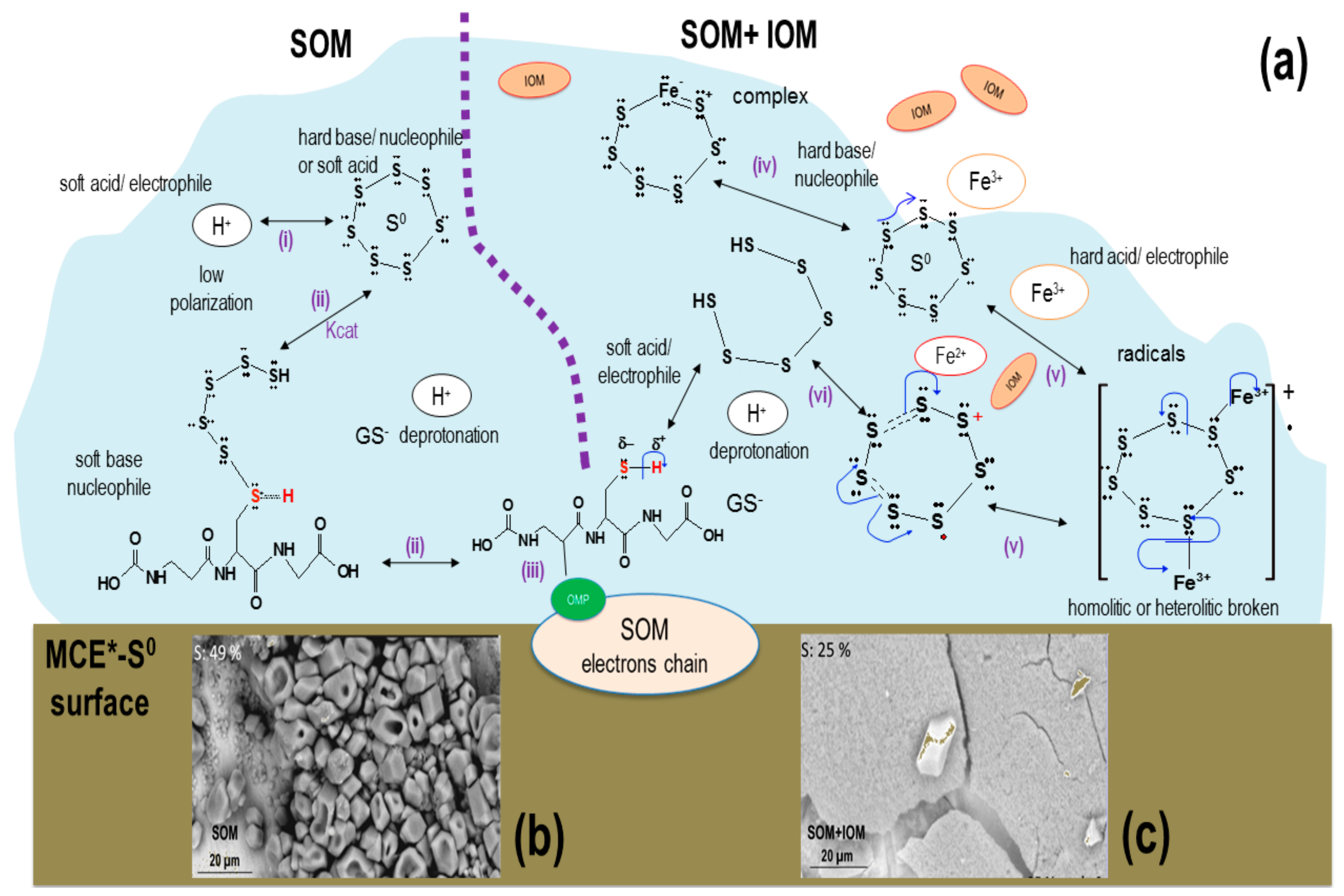
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Crundwell, F.K. The semiconductor mechanism of dissolution and the pseudo-passivation of chalcopyrite. Can. Metall. Q. 2015, 54, 279–288. [Google Scholar] [CrossRef]
- Crundwell, F.K. The dissolution and leaching of minerals: Mechanisms, myths and misunderstandings. Hydrometallurgy 2013, 139, 132–148. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Acciari, H.A.; Benedetti, A.V.; Garcia, O., Jr. Electrochemical techniques used to study bacterial-metal sulfides interactions in acidic environments. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 59–76. ISBN 978-1-4020-5588-1. [Google Scholar]
- García-Meza, J.V.; Fernández, J.; Lara, R.; González, I. Changes in biofilm structure during the colonization of chalcopyrite by Acidithiobacillus thiooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 6065–6075. [Google Scholar] [CrossRef] [PubMed]
- Florian, B.; Noël, N.; Thyssen, C.; Felschau, I.; Sand, W. Some quantitative data on bacterial attachment to pyrite. Miner. Eng. 2011, 24, 1132–1138. [Google Scholar] [CrossRef]
- Dopson, M.; Halinen, A.K.; Rahunen, N.; Ozkaya, B.; Sahinkaya, E.; Kaksonen, A.H.; Lindström, E.B.; Puhakka, J.A. Mineral and iron oxidation at low temperatures by pure and mixed cultures of acidophilic microorganisms. Biotechnol. Bioengineer. 2007, 97, 1205–1215. [Google Scholar] [CrossRef]
- He, H.; Jin, G.; Wang, H.; Huang, X.; Chen, Z.; Sun, D.; Tang, Y. Annealed NaV3O8 nanowires with good cycling stability as a novel cathode for Na-ion batteries. J. Mater. Chem. A 2014, 2, 3563–3570. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, M.; Jin, Q.; Zhao, J. On-line detection of Cu(II) in bioleaching system by anodic stripping differential pulse voltammetry. Trans. Nonferrous Met. Soc. China 2014, 24, 582–587. [Google Scholar] [CrossRef]
- Greenspan, P.; Fowler, S.D. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 1985, 26, 781–789. [Google Scholar] [PubMed]
- Mycroft, J.R.; Bancroft, G.M.; McIntyre, N.S.; Lorimer, J.W.; Hill, I.R. Detection of sulphur and polysulphides on electrochemically oxidized pyrite surfaces by X-ray photoelectron spectroscopy and Raman spectroscopy. J. Electroanal. Chem. 1990, 292, 139–152. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakamuta, Y.; Hirajima, T.; Tuovinen, O.H. Raman characterization of secondary minerals formed during chalcopyrite leaching with Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 95, 153–158. [Google Scholar] [CrossRef]
- Fowler, T.A.; Holmes, P.R.; Crundwell, F.K. The mechanism of the dissolution of pyrite in the presence of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 2987–2993. [Google Scholar] [CrossRef]
- Holmes, P.R.; Crundwell, F.K. Polysulfides do not cause passivation: Results from the dissolution of pyrite and implications for other sulfide minerals. Hydrometallurgy 2013, 139, 101–110. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–387. [Google Scholar] [CrossRef]
- Peng, A.; Xia, J.; Liu, H.; Nie, Z.; Yang, Y.; Zhu, W. Differential utilization of cyclic, orthorhombic α- and chain-like polymeric μ-sulfur by Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2014, 24, 1562–1570. [Google Scholar] [CrossRef]
- Echeverría-Vega, A.; Demergasso, C. Copper resistance, motility and the mineral dissolution behavior were assessed as novel factors involved in bacterial adhesion in bioleaching. Hydrometallurgy 2015, 157, 107–115. [Google Scholar] [CrossRef]
- Demergasso, C.; Galleguillos, F.; Soto, P.; Serón, M.; Iturriaga, V. Microbial succession during a heap bioleaching cycle of low grade cupper sulfides: Does this knowledge mean a real impute for industrial process design and control? Hydrometallurgy 2010, 104, 382–390. [Google Scholar] [CrossRef]
- Noël, N.; Florian, B.; Sand, W. AFM & EFM study on attachment of acidophilic leaching organisms. Hydrometallurgy 2010, 104, 370–375. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Ballester, A.; Blázquez, M.L.; González, F. Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy 1999, 51, 37–46. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Dreisinger, D.B.; Cooper, W.C.; Young, S.K. Silver-catalyzed bioleaching of low-grade copper ores. Part II: Stirred tank tests. Hydrometallurgy 2007, 88, 19–34. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Asselin, E.; Dixon, D.G. Electrochemical evaluation of the surface of chalcopyrite during dissolution in sulfuric acid solution. Electrochim. Acta 2010, 55, 5041–5056. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Acciari, H.A.; Arena, F.A.; Benedetti, A.V.; Fugivara, C.S.; Tremiliosi-Filho, G.; Garcia, O., Jr. Utilization of electrochemical impedance spectroscopy for monitoring bornite (Cu5FeS4) oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2009, 22, 254–262. [Google Scholar] [CrossRef]
- Rohwerder, T.; Sand, W. Mechanisms and biochemical fundamentals of bacterial metal sulfide oxidation. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 35–58. ISBN 978-1-4020-5588-1. [Google Scholar]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Li, X.; He, Z.; Liang, Y.; Guo, X.; Hu, Q.; Xiao, Y.; Cong, J.; Ma, L.; et al. Whole-genome sequencing reveals novel insights into sulfur oxidation in the extremophile Acidithiobacillus thiooxidans. BMC Microbiol. 2014, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Rohwerder, T.; Sand, W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology 2003, 149, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Steudel, R. Inorganic polysulfides Sn2− and radical anions Sn−. Top. Curr. Chem. 2003, 231, 127–152. [Google Scholar] [CrossRef]
- Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007, 5, 1505–1518. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Urich, T.; Gomes, C.M.; Kletzin, A.; Frazão, C. X-ray structure of a self-compartmentalizing sulfur cycle metalloenzyme. Science 2006, 311, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Blight, K.R.; Candy, R.M.; Ralph, D.E. The preferential oxidation of orthorhombic sulfur during batch culture. Hydrometallurgy 2009, 99, 100–104. [Google Scholar] [CrossRef]
- Tuovinen, O.H.; Särkijärvi, S.; Peuraniemi, E.; Junnikkala, S.; Puhakka, J.A.; Kaksonen, A.H. Acid leaching of Cu and Zn from a Smelter slag with a bacterial consortium. Adv. Mater. Res. 2015, 1130, 660–663. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Särkijärvi, S.; Peuraniemi, E.; Junnikkala, S.; Puhakka, J.A.; Tuovinen, O.H. Metal biorecovery in acid solutions from a copper smelter slag. Hydrometallurgy 2017, 168, 135–140. [Google Scholar] [CrossRef]
- Crundwell, F.K. How do bacteria interact with minerals? Hydrometallurgy 2003, 71, 75–81. [Google Scholar] [CrossRef]

| Assay | Time (h) | Rsol (Ω/cm2) | Rlayer (Ω/cm2) | Clayer (1 × 10−6) | Rct (Ω/cm2) | Cdl (1 × 10−6) | Z(ω) or W (kΩ/cm2s0.5) |
|---|---|---|---|---|---|---|---|
| SOM (control) | 1 | 20 ± 0.8 (12.3 ± 5.2) | 480 ± 13 (650 ± 0.7) | 5.14 (0.002) | 12,453 ± 30,992 (9445 ± 637) | 6200 (4.6) | 1.6 ± 0.03 (0.7 ± 0.01) |
| 12 | 20 ± 0.9 (9.3 ± 0.3) | 195 ± 16 (18.5 ± 2.4) | 2.1 (1.63) | 3110 ± 75 (1298 ± 20) | 0.93 (1.7) | 2.0 ± 0.01 (5.2 ± 0.02) | |
| 24 | 17.0 ± 0.5 (22.7 ± 1.3) | 3607 ± 120 (650 ± 5) | 18.4 (27) | 4930 ± 361 (12,930 ± 457) | 160 (39,000) | 0.7 (5.3 ± 0.15) | |
| SOM+IOM (control) | 1 | 20 ± 0.8 (5.2 ± 1.3) | 2114 ± 62 (12 ± 2.39) | 11.8 (0.1) | 6830 ± 1628 (7307 ± 131) | 22,000 (0.13) | 2.0 ± 0.03 (1.6 ± 0.01) |
| 12 | 16.1 ± 0.3 (18.4 ± 1.5) | 141 ± 3.3 (396 ± 6) | 2.8 (1.04) | 701 ± 3 (9942 ± 102) | 0.19 (0.2) | 7.0 ± 0.01 (1.2) | |
| 24 | 15.3 ± 0.4 (37.2 ± 0.2) | 758 ± 46 (286 ± 25) | 28.4 (10.6) | 1815 ± 205 (6368 ± 1000) | 830 (0.5) | 3.5 ± 0.01 (0.3) |
| Time (h) | SOM | IOM+SOM | ||||
|---|---|---|---|---|---|---|
| 1 | 12 | 24 | 1 | 12 | 24 | |
| DH+ mm2/s (control) | 0.006 (0.001) | 0.004 (0.05) | 0.01 (0.04) | 0.003 ± 0.001 (0.001) | 0.34 (0.002) | 0.34 (0) |
| DFe2+ mm2/s (control) | 0 (0) | 0 (0) | 0 (0) | 0.001 (0) | 0.016 (0) | 0.007 (0) |
| DFe3+ mm2/s (control) | 0 (0) | 0 (0) | 0 (0) | 0.37 ± 0.15 (0.51 ± 0.004) | 6.20 (0.3) | 1.5 ± 0.002 (0.008) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cázares, I.; Patrón-Soberano, O.A.; García-Meza, J.V. Bioelectrochemical Changes during the Early Stages of Chalcopyrite Interaction with Acidithiobacillus Thiooxidans and Leptospirillum sp. Minerals 2017, 7, 156. https://doi.org/10.3390/min7090156
López-Cázares I, Patrón-Soberano OA, García-Meza JV. Bioelectrochemical Changes during the Early Stages of Chalcopyrite Interaction with Acidithiobacillus Thiooxidans and Leptospirillum sp. Minerals. 2017; 7(9):156. https://doi.org/10.3390/min7090156
Chicago/Turabian StyleLópez-Cázares, Irene, O. Araceli Patrón-Soberano, and J. Viridiana García-Meza. 2017. "Bioelectrochemical Changes during the Early Stages of Chalcopyrite Interaction with Acidithiobacillus Thiooxidans and Leptospirillum sp." Minerals 7, no. 9: 156. https://doi.org/10.3390/min7090156




