Formation and Aggregation of Gold (Electrum) Nanoparticles in Epithermal Ores
Abstract
:1. Introduction
2. Results
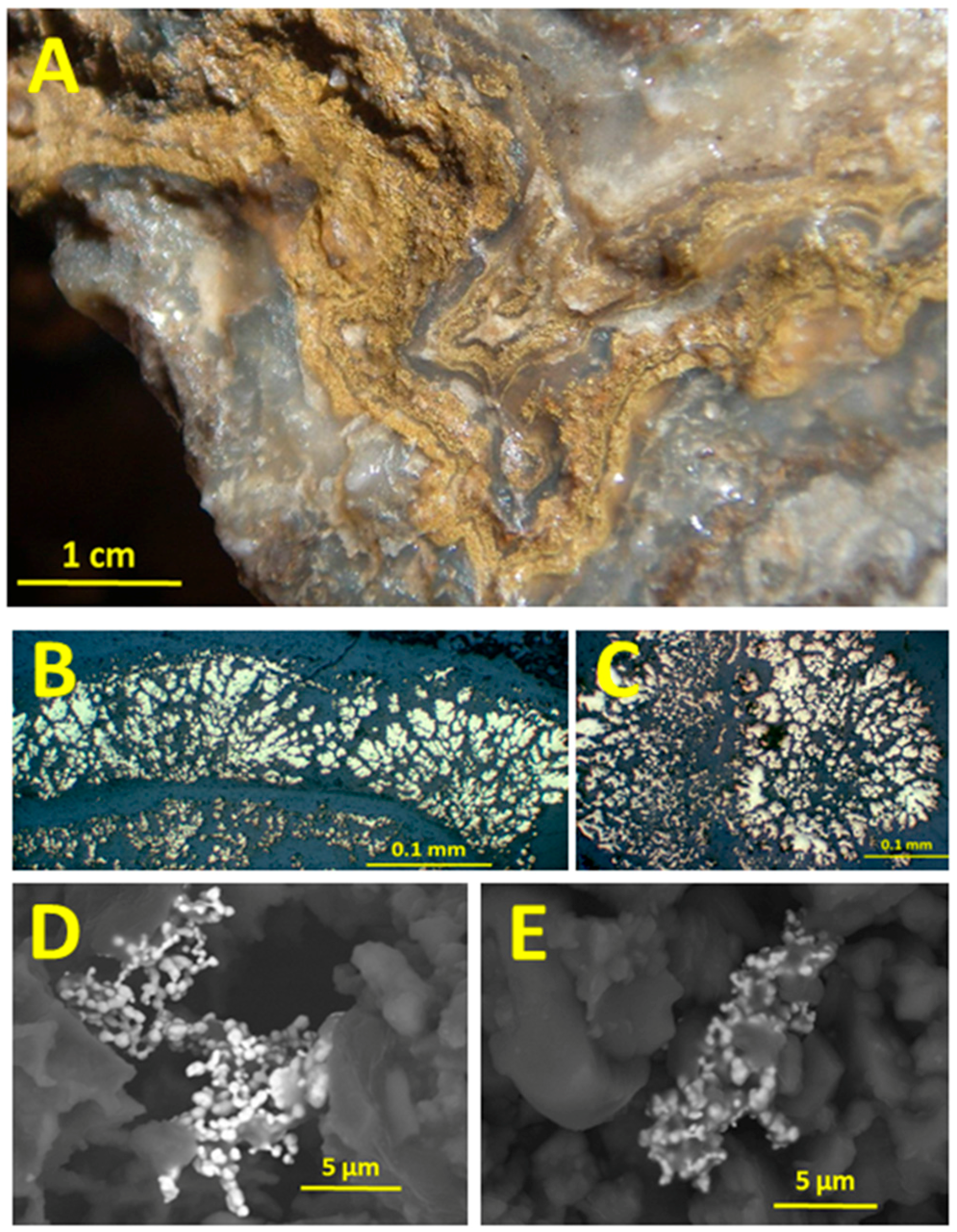
2.1. Bonanza Epithermal Ore Characteristics
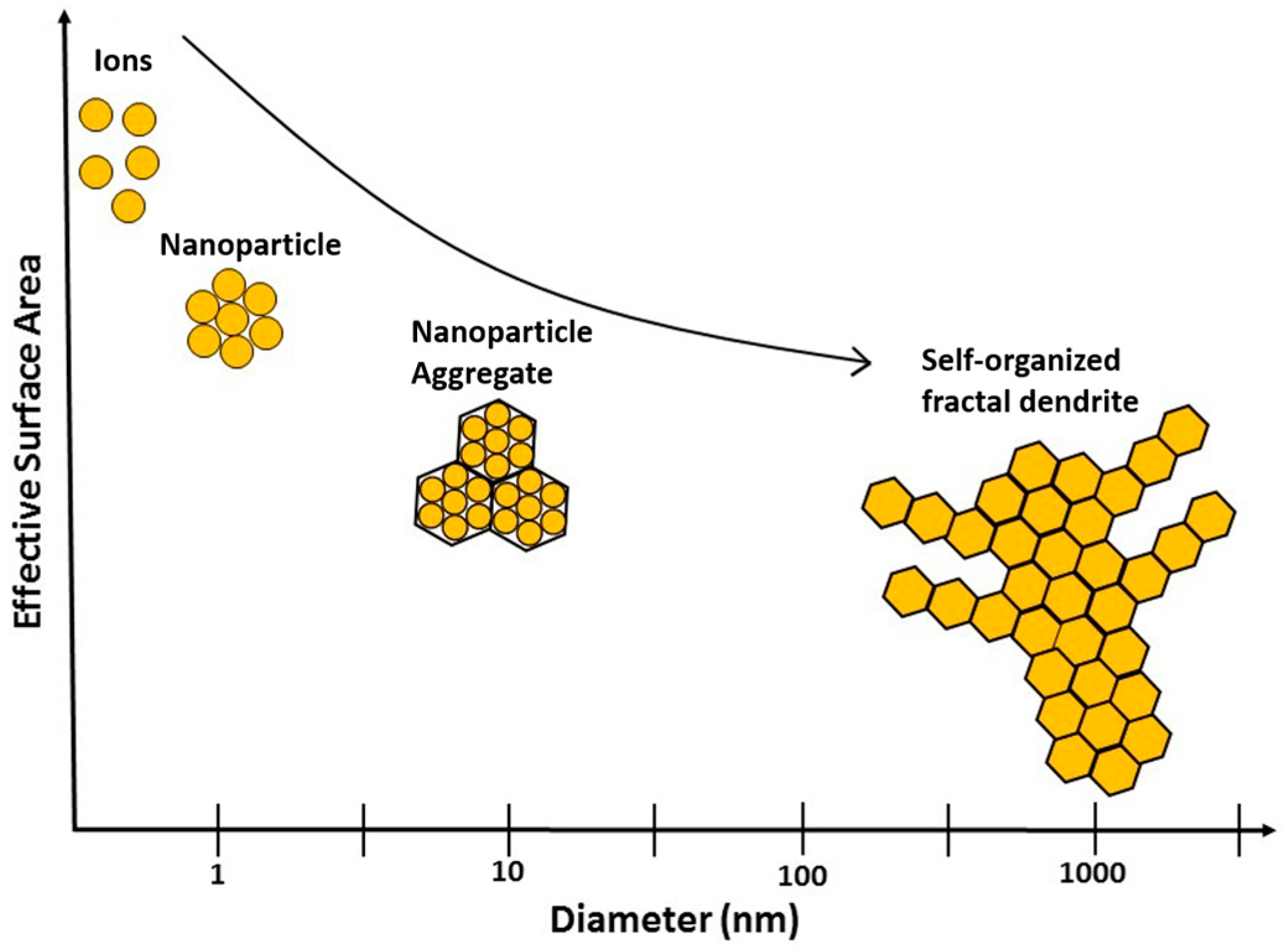
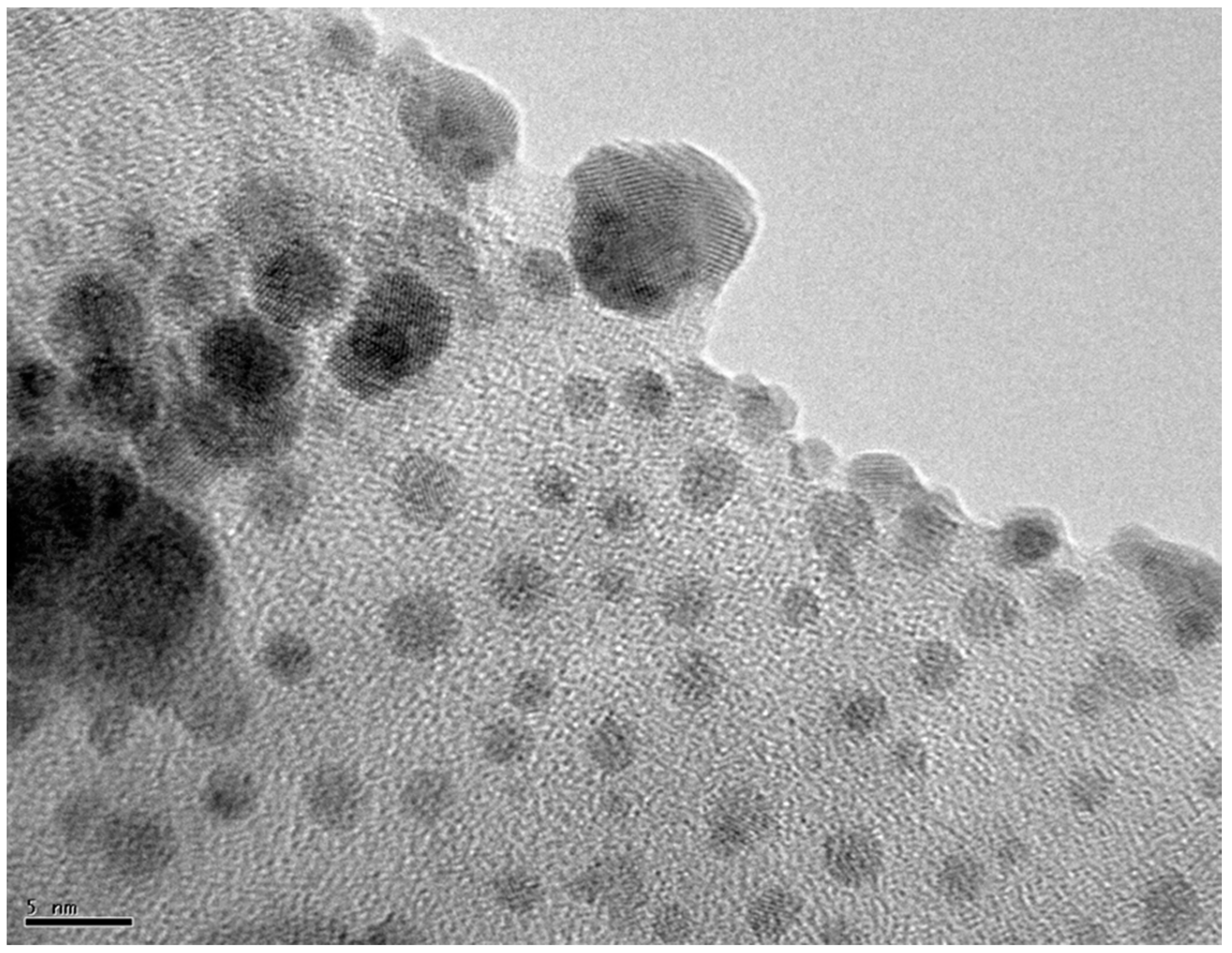
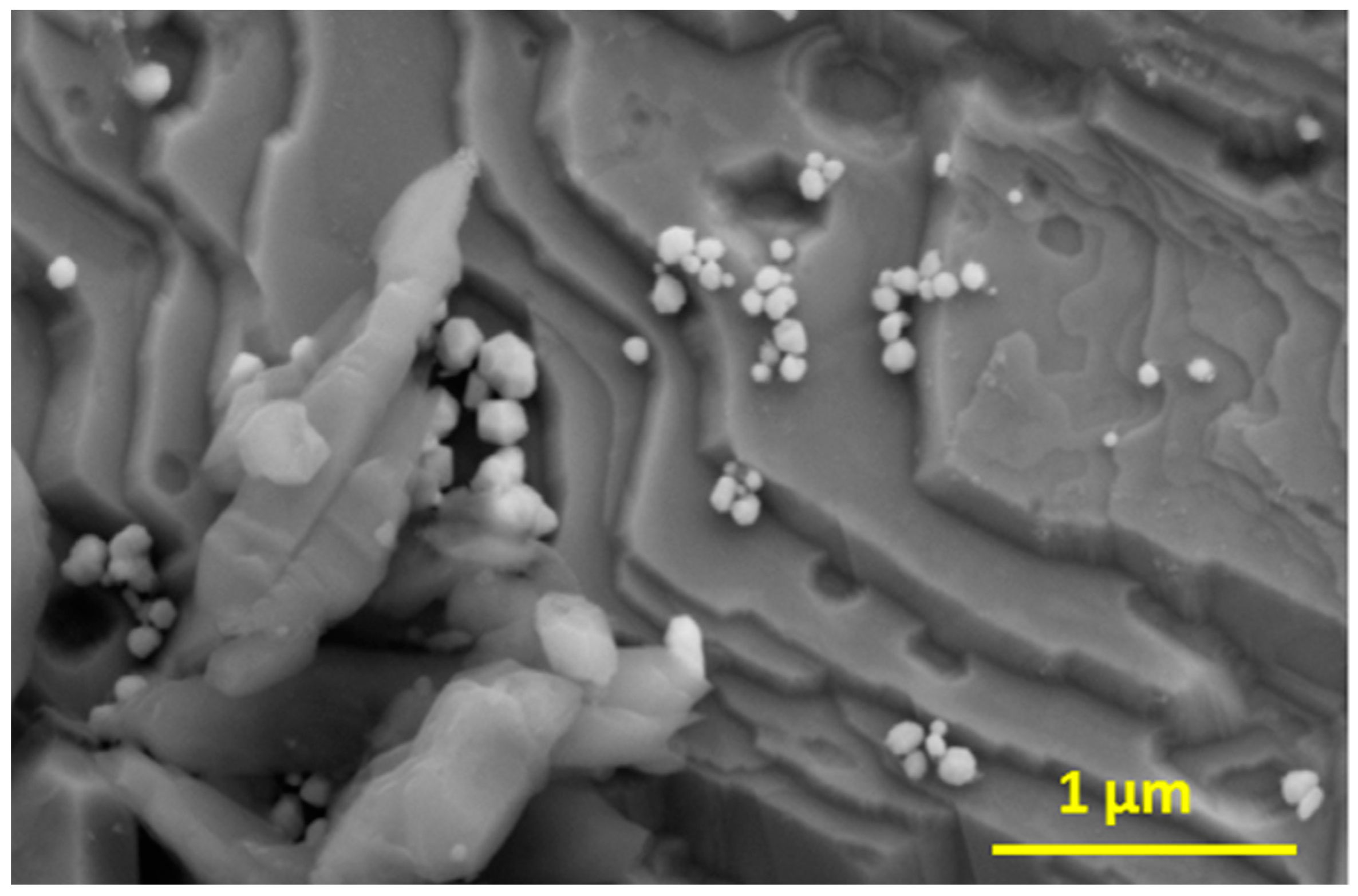
2.2. Aggregation of Metallic Nanoparticles
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hough, R.; Reich, M.; Noble, R. Noble metal nanoparticles in ore systems. In Nature’s Nanostructures; Barnard, A.S., Guo, H., Eds.; Pan Stanford Publishing Pte. Ltd. (CRC Press): Boca Raton, FL, USA, 2012; pp. 141–167. ISBN 978-981-4316-82-8. [Google Scholar]
- Hannington, M.; Harðardóttir, V.; Garbe-Schönberg, D.; Brown, K.L. Gold enrichment in active geothermal systems by accumulating colloidal suspensions. Nat. Geosci. 2016, 9, 299–303. [Google Scholar] [CrossRef]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.C.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Personick, M.L.; Mirkin, C.A. Making sense of the mayhem behind shape control in the synthesis of gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fernandes, A.A.; Erbe, A. Control of shape and surface crystallography of gold nanocrystals for electrochemical applications. Electrochim. Acta 2013, 113, 810–816. [Google Scholar] [CrossRef]
- Boydell, H.C. The role of colloidal solutions in the formation of mineral deposits. Inst. Min. Metall. Trans. 1924, 34, 145–337. [Google Scholar]
- Lindgren, W. Mineral Deposits, 4th ed.; McGraw Hill: New York, NY, USA, 1933; p. 237. [Google Scholar]
- Frondel, C. Stability of colloidal gold under hydrothermal conditions. Econ. Geol. 1938, 33, 1–20. [Google Scholar] [CrossRef]
- Barton, P.B., Jr.; Bethke, P.M.; Toulmin, P., III. An attempt to determine the vertical component of flow rate of ore-forming solutions in the OH vein, Creede, Colorado. In Proceedings of the IAGOD 1970 Meeting, Kyoto, Japan, 28 August–2 September 1971; pp. 132–136. [Google Scholar]
- Boyle, R.W. The Geochemistry of Gold and Its Deposits; Geological Survey of Canada: Ottawa, ON, Canada, 1979; Volume 280, p. 584. [Google Scholar]
- Saunders, J.A. Colloidal transport of gold and silica in epithermal precious metal systems: Evidence from the Sleeper deposit, Humboldt County, Nevada. Geology 1990, 18, 757–760. [Google Scholar] [CrossRef]
- Saunders, J.A. Silica and gold textures at the Sleeper deposit, Humboldt County, Nevada: Evidence for colloids and implications for ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Saunders, J.A.; Schoenly, P.A. Boiling, colloid nucleation and aggregation, and the genesis of bonanza gold mineralization at the Sleeper Deposit, Nevada. Miner. Depos. 1995, 30, 199–211. [Google Scholar] [CrossRef]
- Herrington, R.J.; Wilkinson, J.J. Colloidal gold and silica in mesothermal vein systems. Geology 1993, 21, 539–542. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Hitchen, G.J.; Hart, R.; Reddy, S.M.; Saunders, M.; Clode, P.; Vaughan, D.; Lowe, J.; Anand, R.R.; et al. Naturally occurring gold nanoparticles and nanoplates. Geology 2008, 36, 571–574. [Google Scholar] [CrossRef]
- Hough, R.M.; Butt, C.R.M.; Buhner, J.F. The mineralogy, crystallography and metallography of gold. Elements 2009, 5, 297–302. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P. Colloidal gold nanoparticles in ore systems. Geochim. Cosmochim. Acta 2010, 74, A420. [Google Scholar]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Osovetsky, B.M. Aggregation of nanogold particles in the environment. Nat. Resour. Res. 2015, 25, 241–253. [Google Scholar] [CrossRef]
- Shuster, J.; Reith, F.; Cornelis, G.; Parson, J.E.; Parson, J.M.; Southam, G. Secondary gold structures: Relics of past biogeochemical transformations and implications for colloidal gold dispersion in subtropical environments. Chem. Geol. 2017, 450, 154–164. [Google Scholar] [CrossRef]
- Taran, Y.A.; Bernard, A.; Gavilanes, J.-C.; Africano, F. Native gold in mineral precipitates from high-temperature volcanic gases of Colima volcano, Mexico. Appl. Geochem. 2000, 15, 337–346. [Google Scholar] [CrossRef]
- Zelenski, M.; Kamenetsky, V.S.; Hedenquist, J. Gold recycling and enrichment beneath volcanoes: A case study of Tolbachik, Kamchatka. Earth Planet. Sci. Lett. 2016, 43, 735–746. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C. “Invisible” gold revealed: Imaging of gold nanoparticles in a Carlin-type deposit. Am. Mineral. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Pope, J.G.; Brown, K.L.; McConchie, D.M. Gold Concentrations in springs at Waiotapu, New Zealand: Implications for precious metal deposition in geothermal systems. Econ. Geol. 2005, 100, 677–687. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Mitropoulos, P.; Valsami-Jones, E.; Voudouris, P. A new terrestrial active mineralizing hydrothermal system associated with ore-bearing travertines in Greece (northern Euboea Island and Sperchios area). J. Geochem. Explor. 2017, 179, 9–24. [Google Scholar] [CrossRef]
- Gartman, A.; Hannington, M.; Jamieson, J.W.; Peterkin, B.; Garbe-Schönberg, D.; Findlay, A.J.; Fuchs, S.; Kwasnitschka, T. Boiling-induced formation of colloidal gold in black smoker hydrothermal fluids. Geology 2017. under review. [Google Scholar]
- Yucel, M.; Gartman, A.; Chan, C.; Luther, G.W., III. Hydrothermal vents as a kinetically stable source of iron sulphide-bearing nanoparticles to the ocean. Nat. Geosci. 2011, 4, 367–371. [Google Scholar] [CrossRef]
- Gartman, A.; Findlay, A.J.; Luther, G.W., III. Nanoparticulate pyrite and other nanoparticles are a widespread component of hydrothermal vent black smoker emissions. Chem. Geol. 2014, 366, 32–41. [Google Scholar] [CrossRef]
- Gartman, A.; Luther, G.W., III. Comparison of pyrite (FeS2) synthesis mechanisms to reproduce natural FeS2 nanoparticles found at hydrothermal vents. Geochim. Cosmochim. Acta 2013, 120, 447–458. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and Earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, B.; Wang, P.; Dellago, C.; Wang, Y.; Fang, Y. Nanoparticle-based crystal growth via multistep self-assembly. CrystEngComm 2013, 15, 5114–5118. [Google Scholar] [CrossRef]
- Saunders, J.A.; Schoenly, P.A. Fractal structure of electrum dendrites in bonanza epithermal Au-Ag deposits. In Fractal Geometry and Its Use in the Earth Sciences; Barton, C.C., LaPointe, P.R., Eds.; Plenum Publishing: New York, NY, USA, 1995; pp. 251–261. [Google Scholar]
- Saunders, J.A.; Schoenly, P.A.; Cook, R.B. Electrum disequilibrium crystallization textures in volcanic-hosted bonanza epithermal gold deposits. In Geology and Ore Deposits of the America Cordillera Symposium Proceeding; Coyner, A.R., Fahey, P.L., Eds.; Geological Society of Nevada: Reno-Sparks, NV, USA, 1996; pp. 173–179. [Google Scholar]
- Saunders, J.A.; Vikre, P.; Unger, D.L.; Beasley, L. Colloidal and physical transport textures exhibited by electrum and naumannite in bonanza epithermal veins from western USA, and their significance. In Great Basin Evolution and Metallogeny Symposium Proceedings; Steininger, R., Pennell, W., Eds.; Geological Society of Nevada: Reno-Sparks, NV, USA, 2011; pp. 825–832. [Google Scholar]
- Saunders, J.A. Textural evidence of episodic introduction of metallic nanoparticles into bonanza epithermal ores. Minerals 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Marinova, I.; Ganev, V.; Titorenkova, R. Colloidal origin of colloform-banded textures in the Paleogene low-sulfidation Khan Krum gold deposit, SE Bulgaria. Miner. Depos. 2014, 49, 49–74. [Google Scholar] [CrossRef]
- Burke, M. An Electron Microscopy Investigation of Gold and Associated Minerals from Round Mountain, Nevada. Master’s Thesis, Miami University, Oxford, OH, USA, 2017. [Google Scholar]
- Burke, M.; Rakovan, J.; Krekeler, M.P.S. A study by electron microscopy of gold and associated minerals from Round Mountain, Nevada. Ore Geol. Rev. 2017, 89. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Hedenquist, J.W. Linkages between volcanotectonic settings, ore-fluid compositions, and epithermal precious metal deposits. In Volcanic, Geothermal, and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth; Society of Economic Geologists: Littleton, CO, USA, 2003; pp. 315–343. [Google Scholar]
- Saunders, J.A.; Hofstra, A.H.; Goldfarb, R.J.; Reed, M.H. Geochemistry of hydrothermal gold deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 383–424. [Google Scholar]
- John, D.A. Miocene and early Pliocene epithermal gold silver deposits in the in the northern Great Basin, western USA: Characteristics, distribution, and relationship to magmatism. Econ. Geol. 2001, 96, 1827–1853. [Google Scholar]
- Sander, M.V.; Einaudi, M.T. Epithermal deposition of gold during transition from propylitic to potassic alteration at Round Mountain, Nevada. Econ. Geol. 1990, 85, 285–311. [Google Scholar] [CrossRef]
- Saunders, J.A.; Unger, D.L.; Kamenov, G.D.; Fayek, M.; Hames, W.E.; Utterback, W.C. Genesis of Middle Miocene Yellowstone-hotspot-related bonanza epithermal Au-Ag deposits, Northern Great Basin Region, USA. Miner. Depos. 2008, 43, 715–734. [Google Scholar] [CrossRef]
- John, D.J.; Hofstra, A.H.; Fleck, R.F.; Brummer, J.E.; Saderholm, E.C. Geologic setting and genesis of the Mule Canyon low-sulfidation epithermal gold-silver deposit, north-central Nevada. Econ. Geol. 2003, 98, 425–463. [Google Scholar] [CrossRef]
- Leavitt, E.D.; Spell, T.L.; Goldstrand, P.M.; Arehart, G.G. Geochronology of the Midas low-sulfidation gold-silver deposit, Elko County Nevada. Econ. Geol. 2004, 99, 1665–1686. [Google Scholar] [CrossRef]
- Kamenov, G.D.; Saunders, J.A.; Hames, W.E.; Unger, D. Mafic magmas as sources for gold in middle-Miocene epithermal deposits of northern Great Basin, USA: Evidence from Pb isotopic compositions of native gold. Econ. Geol. 2007, 102, 1191–1195. [Google Scholar] [CrossRef]
- Saunders, J.A.; Mathur, R.; Kamenov, G.D.; Shimizu, T.; Brueseke, M.E. New isotopic evidence bearing on bonanza (Au-Ag) epithermal ore-forming processes. Miner. Depos. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Weitz, D.A.; Huang, J.S. Self-similar structures and the kinetics of aggregation of gold colloids. In Kinetics of Aggregation and Gelation; Family, F., Landau, D.P., Eds.; Elsevier: New York, NY, USA, 1984; pp. 19–27. [Google Scholar]
- Weitz, D.A.; Oliveria, M. Fractal structures formed by kinetic aggregation of aqueous gold colloids. Phys. Rev. Lett. 1984, 52, 1433–1436. [Google Scholar] [CrossRef]
- Scott, M.C.; Chen, C.-C.; Mecklenburg, M.; Zhu, C.; Xu, R.; Ercius, P.; Dahmen, U. Electron tomography at 2.4-ångström resolution. Nature 2012, 483, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Seward, T.H.; Barnes, H.L. Metal transport by hydrothermal ore fluids. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley: New York, NY, USA, 1997; pp. 435–489. [Google Scholar]
- Heinrich, C.A.; Driesner, T.; Stefansson, A.; Seward, T.M. Magmatic vapor contraction and the transport of gold from the porphyry environment to epithermal ore deposits. Geology 2004, 32, 761–764. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Heinrich, C.A. Vapor transport of metals and the formation of magmatic-hydrothermal ore deposits. Econ. Geol. 2005, 100, 1287–1312. [Google Scholar] [CrossRef]
- Saunders, J.A.; Brueseke, M.E. Volatility of Se and Te during subduction-related “distillation” and the geochemistry of epithermal ores of western USA. Econ. Geol. 2012, 107, 165–172. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saunders, J.A.; Burke, M. Formation and Aggregation of Gold (Electrum) Nanoparticles in Epithermal Ores. Minerals 2017, 7, 163. https://doi.org/10.3390/min7090163
Saunders JA, Burke M. Formation and Aggregation of Gold (Electrum) Nanoparticles in Epithermal Ores. Minerals. 2017; 7(9):163. https://doi.org/10.3390/min7090163
Chicago/Turabian StyleSaunders, James A., and Michelle Burke. 2017. "Formation and Aggregation of Gold (Electrum) Nanoparticles in Epithermal Ores" Minerals 7, no. 9: 163. https://doi.org/10.3390/min7090163



