Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sulfidogenic Bioreactor
2.2. Formation of Copper Sulfide Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Physicochemical and Microbiological Analysis
3. Results
3.1. Operation of a Low Sulfidogenic Bioreactor
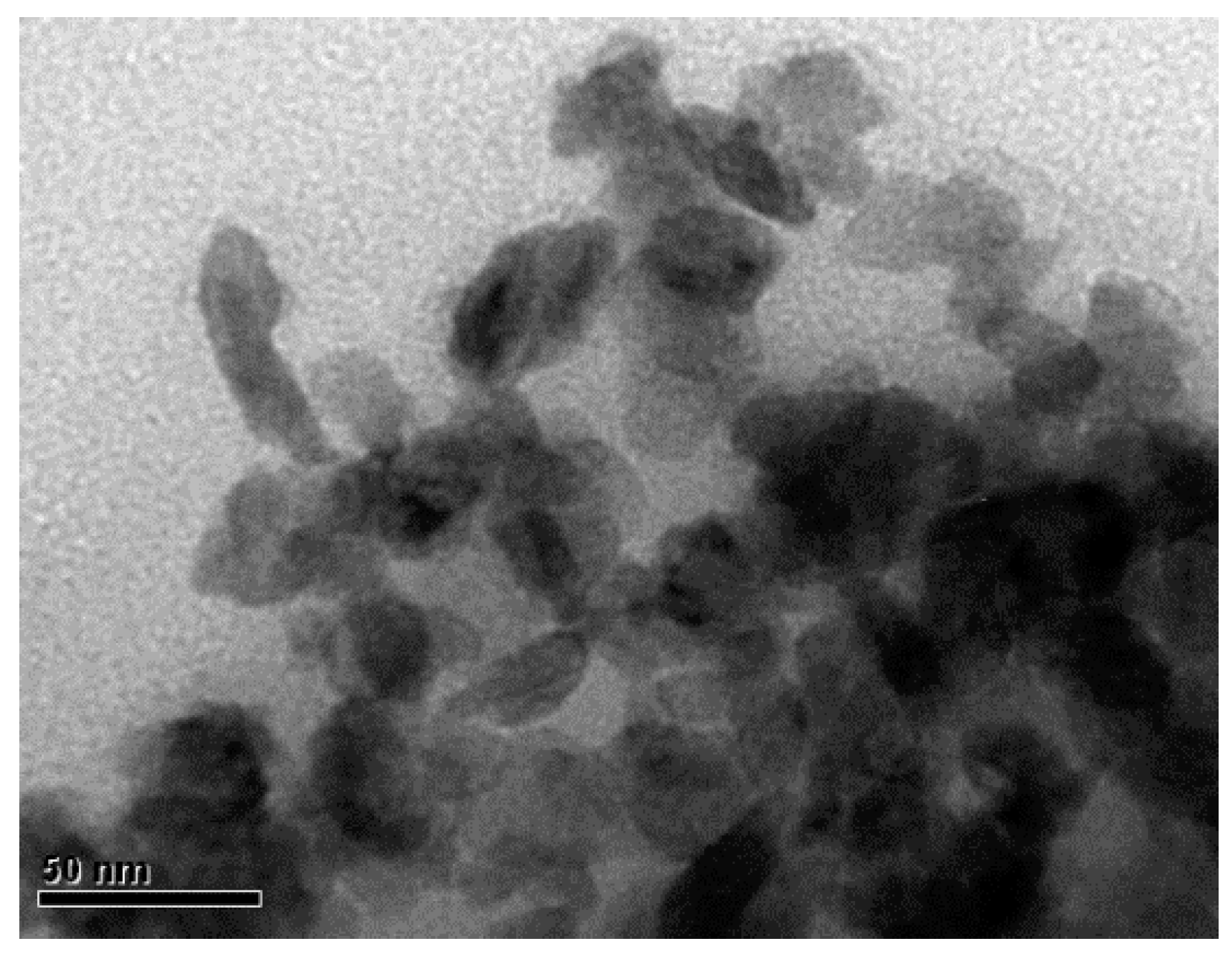
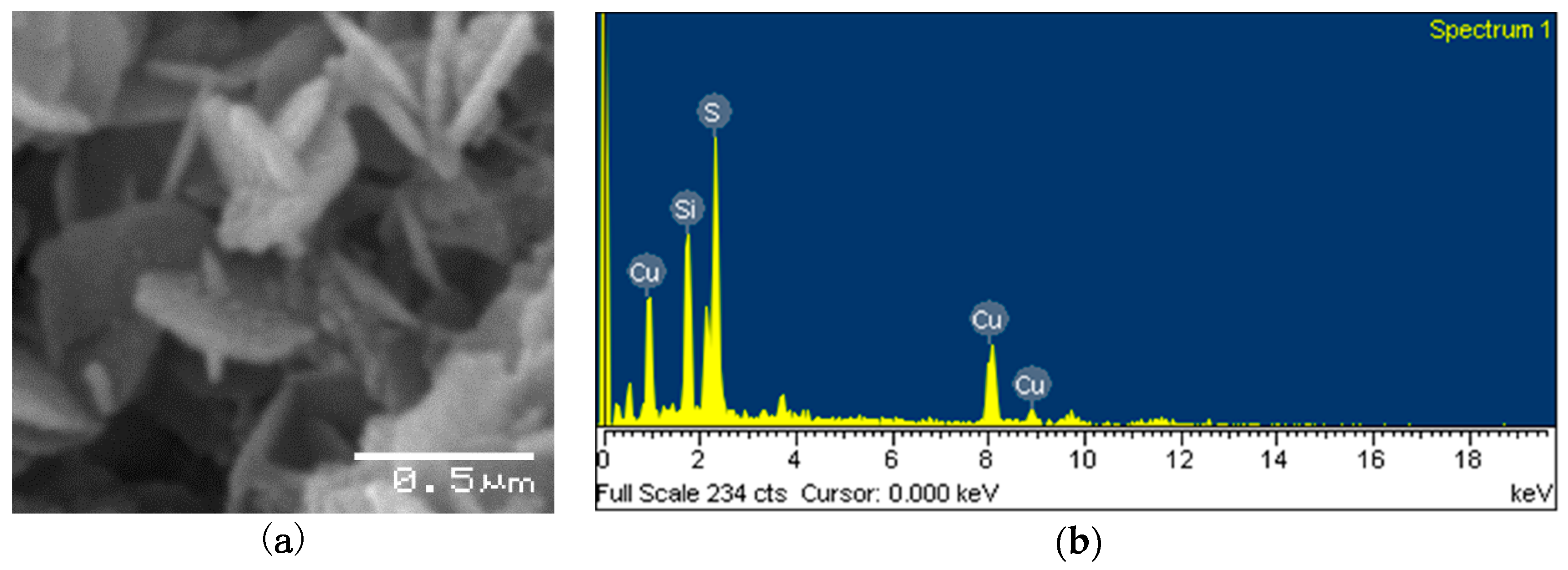
3.2. H2S Production and Formation of Copper Sulfide Nanoparticles
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, D.B.; Hallberg, K.B. The microbiology of acidic mine waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef]
- Nancucheo, I.; Bitencourt, J.A.P.; Sahoo, P.K.; Alves, J.O.; Siqueira, J.O.; Oliveira, G. Recent developments for remediating acidic mine waters using sulfidogenic bacteria. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Roussel, J.; Rolley, J.; Woodhall, F.; Mikheenko, I.P.; Johnson, D.B.; Gomez-Bolivar, J.; Merroun, M.L.; Macaskie, L.E. Biosynthesis of zinc sulfide quantum dots using waste off-gas from a metal bioremediation process. RSC Adv. 2017, 7, 21484–21491. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. 2012, 5, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Widdel, F. Microbiology and ecology of sulfate-and sulfur-reducing bacteria. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1988; pp. 469–585. ISBN 0471882267. [Google Scholar]
- Hedrich, S.; Johnson, D.B. Remediation and selective recovery of metals from acidic mine waters using novel modular bioreactors. Environ. Sci. Technol. 2014, 48, 12206–12212. [Google Scholar] [CrossRef] [PubMed]
- Nancucheo, I.; Rowe, O.F.; Hedrich, S.; Johnson, D.B. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol. Lett. 2016, 363, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schaffie, M.; Hosseini, M.R. Biological process for synthesis of semiconductor copper sulfide nanoparticle from mine wastewaters. J. Environ. Chem. Eng. 2014, 2, 386–391. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Botha, N.L. Synthesis and structural studies of copper sulfide nanocrystals. Results Phys. 2016, 6, 581–589. [Google Scholar] [CrossRef]
- Riyaz, S.; Parveen, A.; Azam, A. Microstructural and optical properties of CuS nanoparticles prepared by sol—Gel route. Perspect. Sci. 2016, 8, 632–635. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Sanchez-Mora, E.; Pal, U.; Paraguay-Delgado, F.; Mathew, X. Synthesis of CuS nanoparticles by a wet chemical route and their photocatalytic activity. J. Nanopart. Res. 2015, 17, 301. [Google Scholar] [CrossRef]
- Zhou, N.Q.; Tian, L.J.; Wang, Y.C.; Li, D.B.; Li, P.P.; Zhang, X.; Yu, H.Q. Extracellular biosynthesis of copper sulfide nanoparticles by Shewanella oneidensis MR-1 as a photothermal agent. Enzyme Microb. Technol. 2016, 95, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Leiva, E.D.; Rámila, C.D.P.; Vargas, I.T.; Escauriaza, C.R.; Bonilla, C.A.; Pizarro, G.E.; Regan, J.M.; Pasten, P.A. Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed. Sci. Total Environ. 2014, 466–467, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Zamanova, M.K.; Glotova, V.N.; Izhenbina, T.N.; Krutas, D.S.; Novikov, V.T. Simultaneous HPLC-UV determination of lactic acid, glycolic acid, glycolide, lactide and ethyl acetate in monomers for producing biodegradable polymers. Procedia Chem. 2014, 10, 244–251. [Google Scholar] [CrossRef]
- Oliveira, B.M.; Barrio, E.; Querol, A.; Pérez-Torrado, R. Enhanced enzymatic activity of glycerol-3-phosphate dehydrogenase from the cryophilic Saccharomyces kudriavzevii. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Rowe, O.F.; Sánchez-España, J.; Hallberg, K.B.; Johnson, D.B. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 2007, 9, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Stams, A.J.M.; Hedrich, S.; Nancucheo, I.; Johnson, D.B. Desulfosporosinus acididurans sp. nov.: An acidophilic sulfate-reducing bacterium isolated from acidic sediments. Extremophiles 2015, 19, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Anandham, R.; Indiragandhi, P.; Kwon, S.W.; Sa, T.M.; Jeon, C.O.; Kim, Y.K.; Jee, H.J. Pandoraea thiooxydans sp. nov., a facultatively chemolithotrophic, thiosulfate-oxidizing bacterium isolated from rhizosphere soils of sesame (Sesamum indicum L.). Int. J. Syst. Evol. Microbiol. 2010, 60, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Zepeda, V.J.; Nancucheo, I.; Guillen, M.; Becerra, E.; Escuti, C.; Cautivo, D.; González, D.; Colipai, C.; Demergasso, C.; Galleguillos, P.A. Biological production of copper sulfide concentrate from flotation tailings and low Grade ore. Solid State Phenom. 2017, 262, 202–206. [Google Scholar] [CrossRef]
- Alazard, D.; Joseph, M.; Battaglia-Brunet, F.; Cayol, J.L.; Ollivier, B. Desulfosporosinus acidiphilus sp. nov.: A moderately acidophilic sulfate-reducing bacterium isolated from acid mining drainage sediments. Extremophiles 2010, 14, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Hallberg, K.B.; Johnson, D.B. Sulfidogenesis in low pH (3.8–4.2) media by a mixed population of acidophilic bacteria. Biodegradation 2006, 17, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Nancucheo, I.; Johnson, D.B. Removal of sulfate from extremely acidic mine waters using low pH sulfidogenic bioreactors. Hydrometallurgy 2014, 150, 222–226. [Google Scholar] [CrossRef]
- Boonstra, J.; van Lier, R.; Janssen, G.; Dijkman, H.; Buisman, C.J.N. Biological treatment of acid mine drainage. In Biohydrometallurgy and the Environment Toward the Mining of the 21st Century (Process Metallurgy 9B); Amils, R., Ballester, A., Eds.; Elsevier: Elsevier, Amsterdam, The Nederland, 1999; pp. 559–567. ISBN 978-0-444-50193-6. [Google Scholar]
- Riekkola-Vanhanen, M. Talvivaara mining company—From a project to a mine. Miner. Eng. 2013, 48, 2–9. [Google Scholar] [CrossRef]
- Liyanage, D.D.; Thamali, R.J.K.A.; Kumbalatara, A.A.K.; Weliwita, J.A.; Witharana, S. An analysis of nanoparticle settling times in liquids. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]



| pH | Analyte Concentration (mM) | ||||||
|---|---|---|---|---|---|---|---|
| Ca | Cu | Mg | Na | Co | Zn | SO42− | |
| 3.50 | 10.00 | 2.80 | 9.00 | 2.00 | 0.01 | 0.11 | 24.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colipai, C.; Southam, G.; Oyarzún, P.; González, D.; Díaz, V.; Contreras, B.; Nancucheo, I. Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor. Minerals 2018, 8, 35. https://doi.org/10.3390/min8020035
Colipai C, Southam G, Oyarzún P, González D, Díaz V, Contreras B, Nancucheo I. Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor. Minerals. 2018; 8(2):35. https://doi.org/10.3390/min8020035
Chicago/Turabian StyleColipai, Camila, Gordon Southam, Patricio Oyarzún, Daniella González, Víctor Díaz, Braulio Contreras, and Ivan Nancucheo. 2018. "Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor" Minerals 8, no. 2: 35. https://doi.org/10.3390/min8020035






