Hemimorphite Flotation with 1-hydroxydodecylidene-1,1-diphosphonic acid and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Flotation Tests
2.3. Zeta Potential Measurement
2.4. Contact Angle Measurements
2.5. FTIR Measurement
2.6. XPS Measurement
3. Results
3.1. Preparation of HDDPA
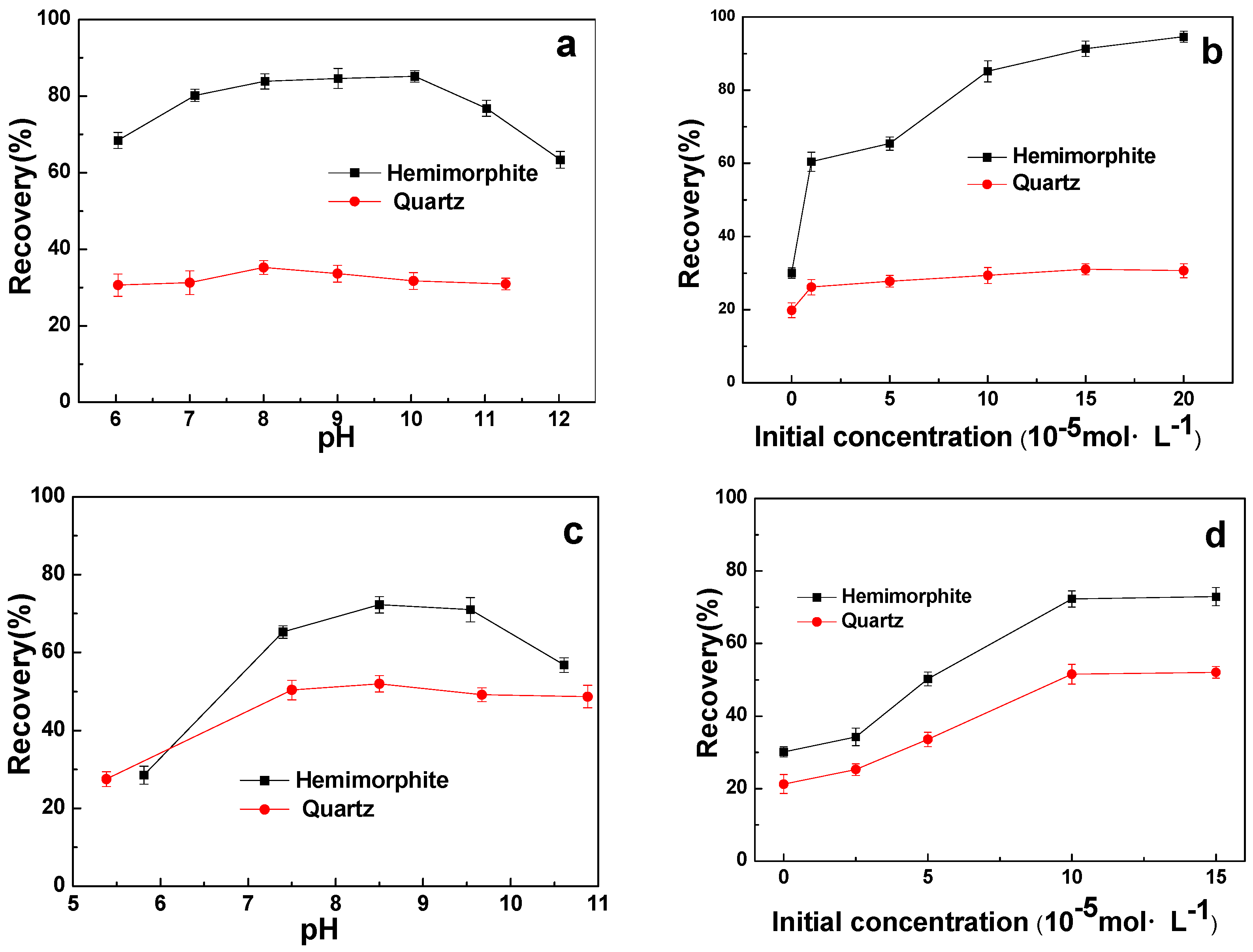
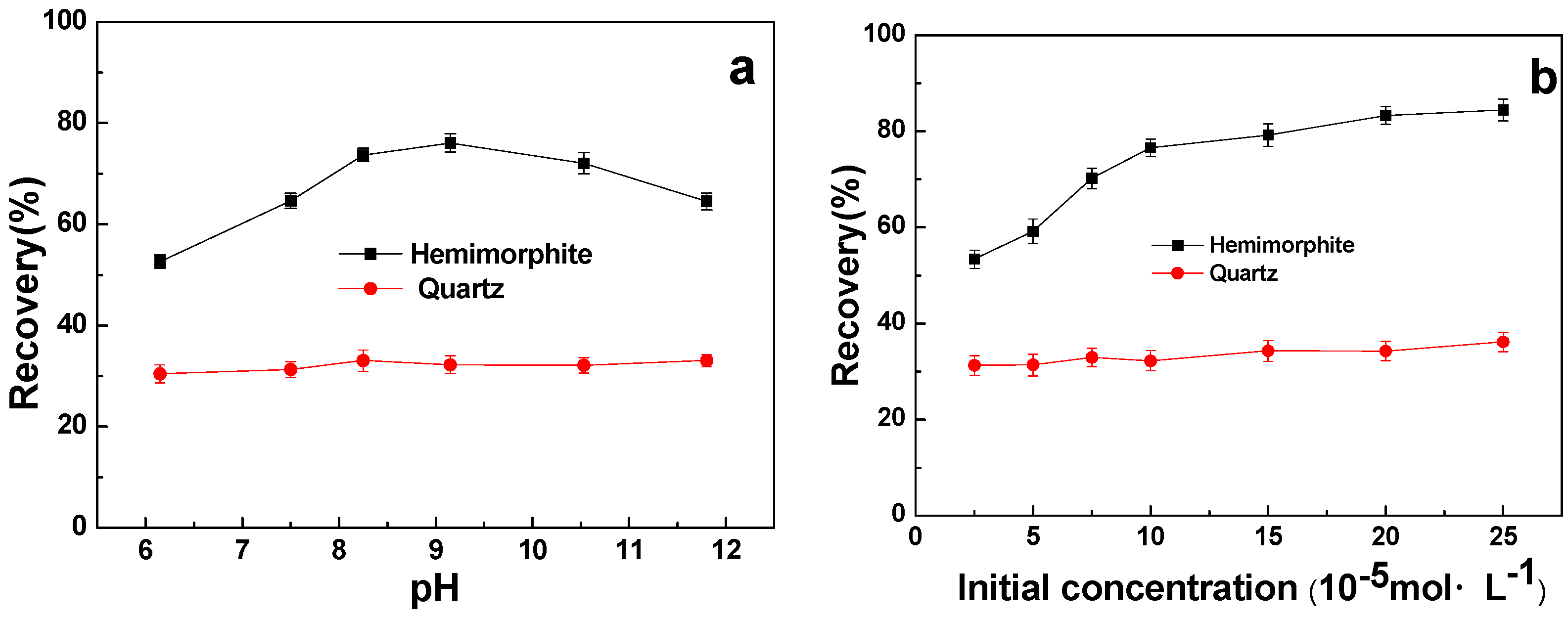
3.2. Micro-Flotation Results
3.3. Flotation of the Artificially Mixed Minerals
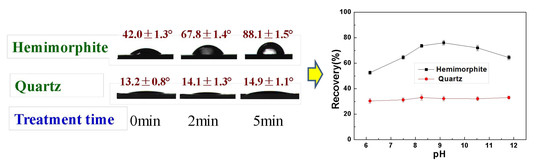
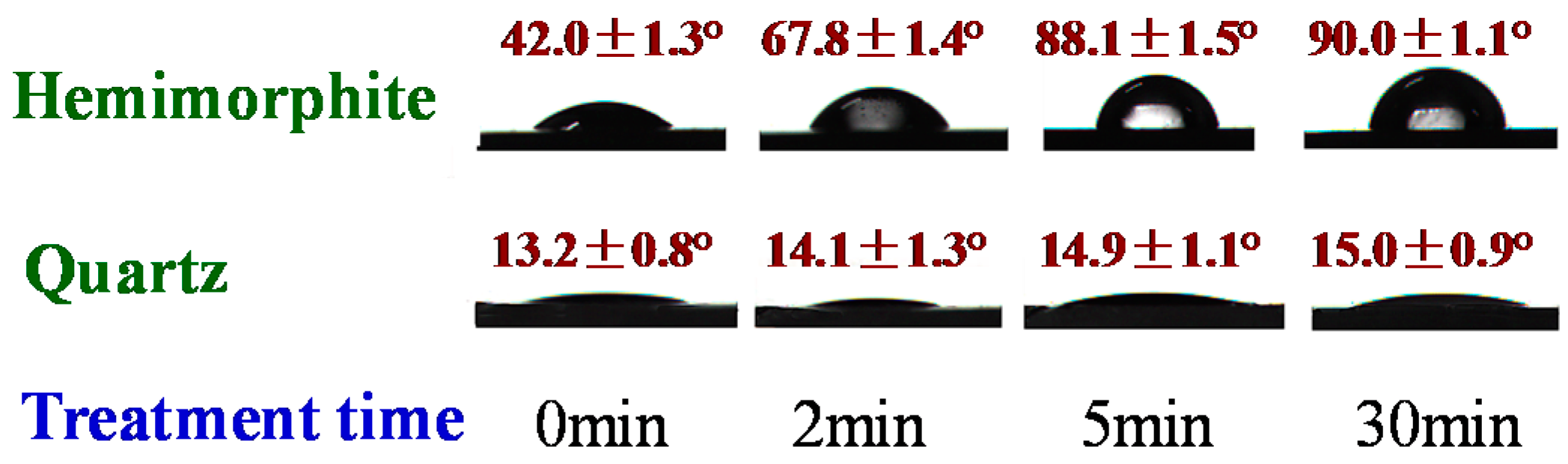
3.4. Contact Angle Results
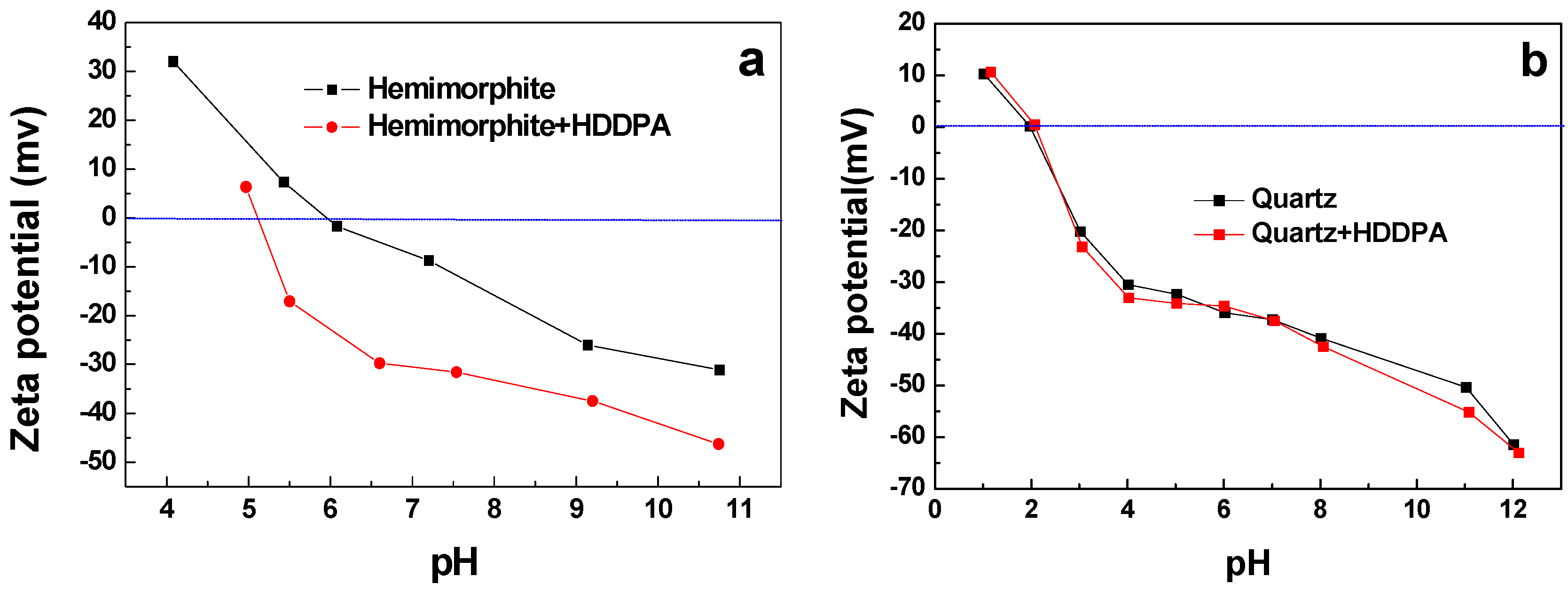
3.5. Zeta Potential Results
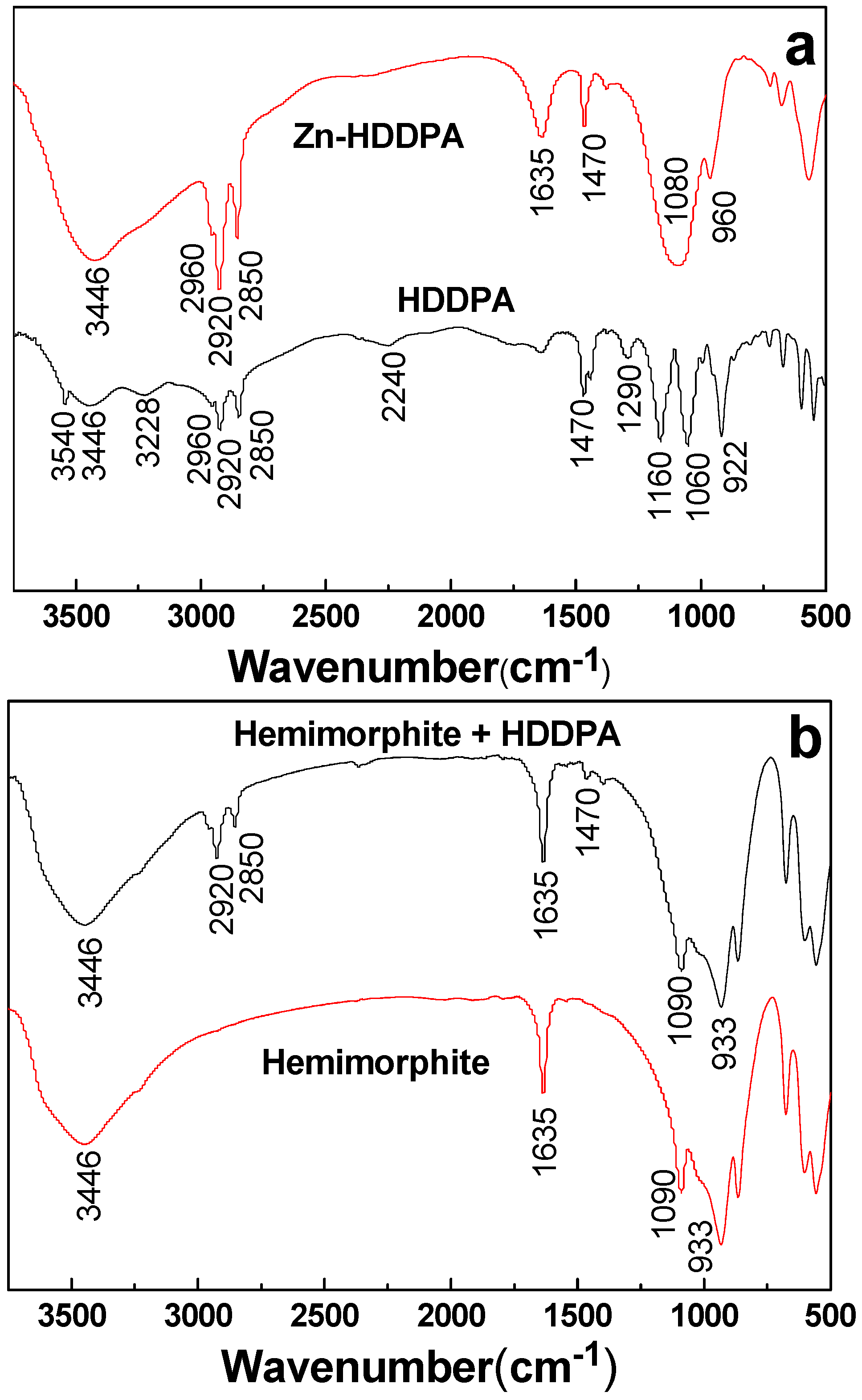
3.6. FTIR Analyses
3.7. XPS Analyses
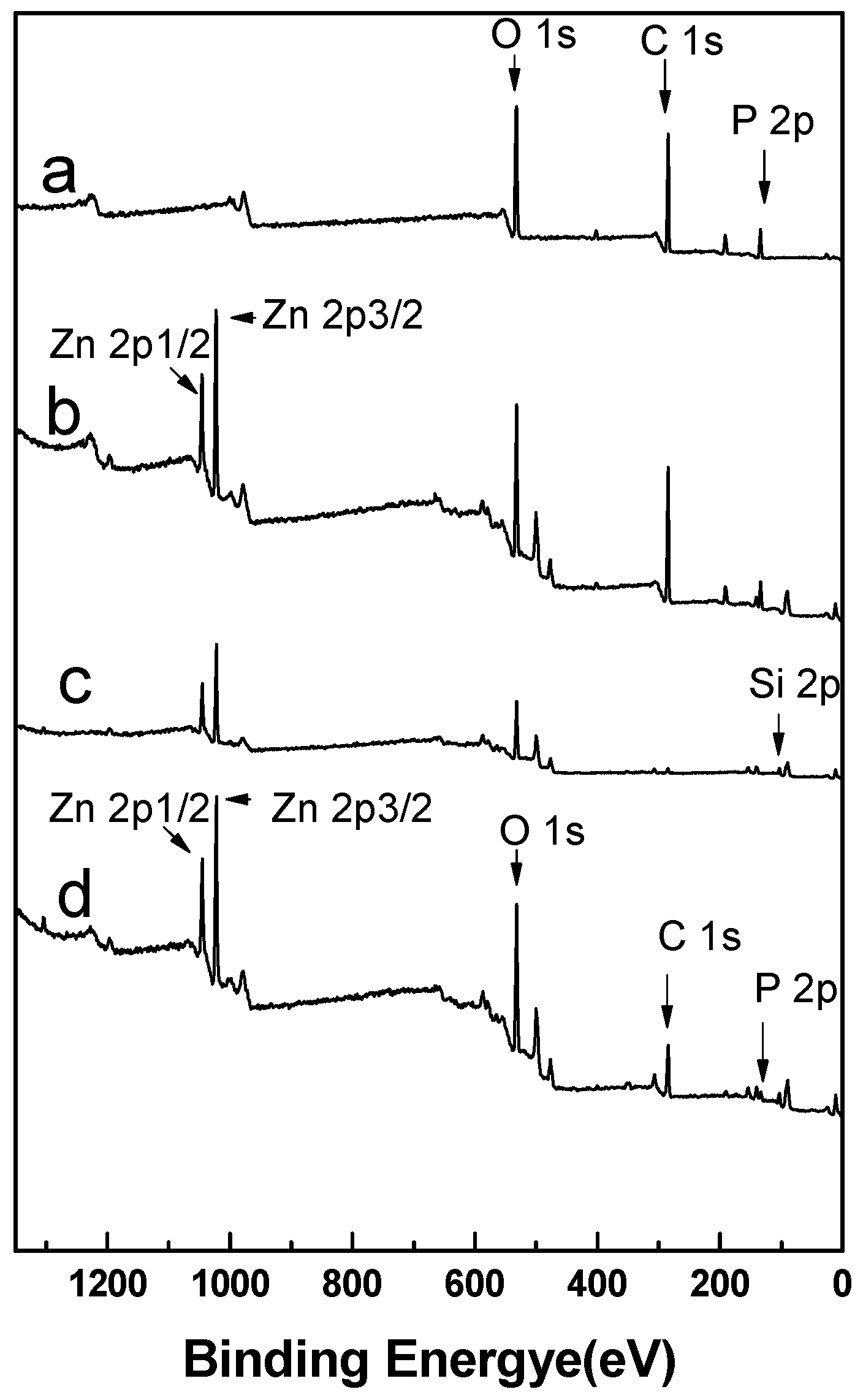
3.7.1. Survey XPS
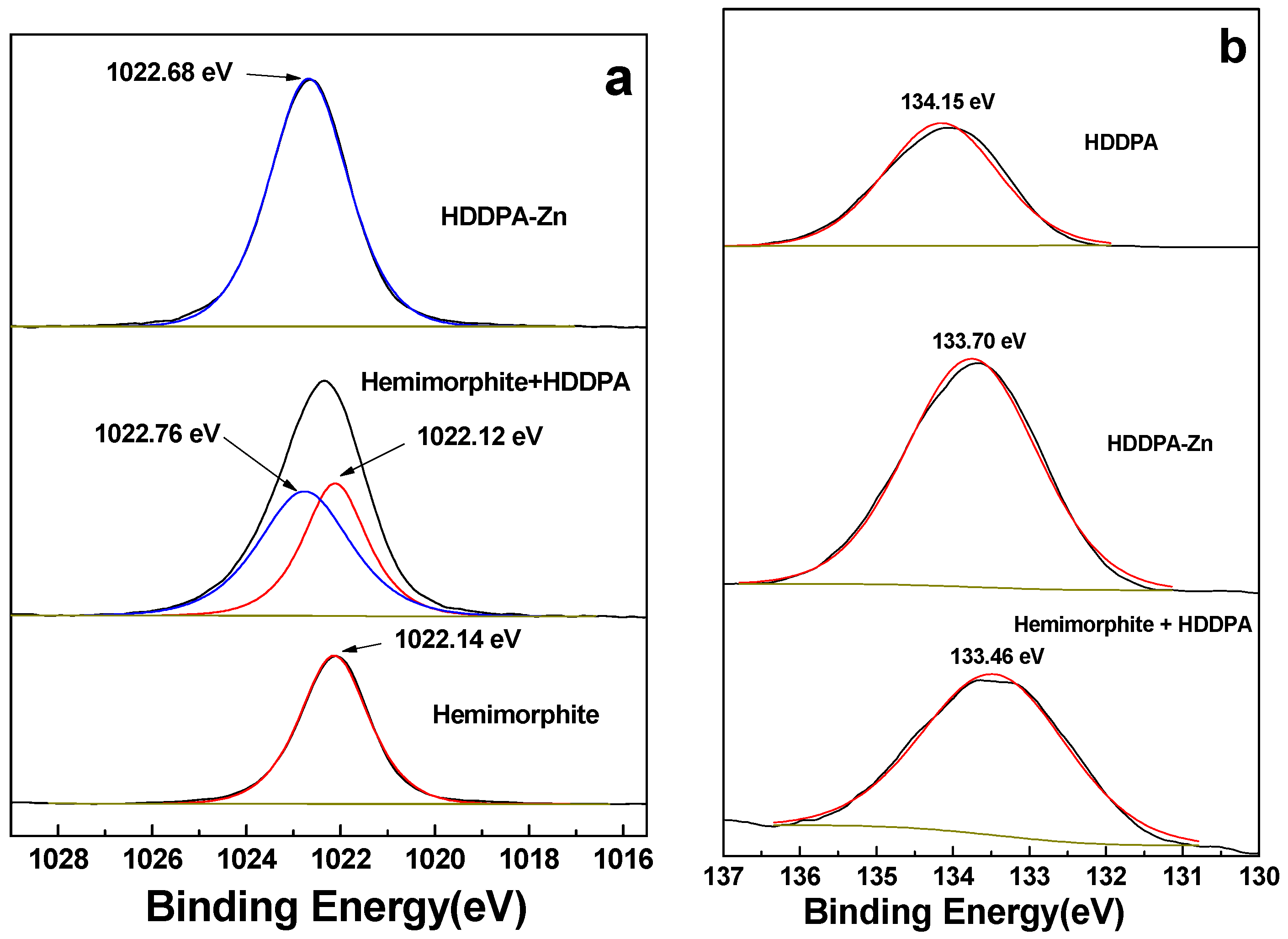
3.7.2. High-Resolution XPS
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seifert, S.; Simon, F.; Baumann, G.; Hietschold, M.; Seifert, A.; Spange, S. Adsorption of poly(vinyl formamide-co-vinyl amine) (PVFA-co-PVAm) polymers on zinc, zinc oxide, iron, and iron oxide surfaces. Langmuir 2011, 27, 14279–14289. [Google Scholar] [CrossRef] [PubMed]
- Fogliatti, D.P.; Kemppainen, S.A.; Kalnes, T.N.; Fan, J.; Shonnard, D.R. Life cycle carbon footprint of linear alkylbenzenesulfonate from coconut oil, palm kernel oil, and petroleum-based paraffins. ACS Sustain. Chem. Eng. 2014, 2, 1828–1834. [Google Scholar] [CrossRef]
- Harvey, T.J.; Yen, W.T. The influence of chalcopyrite, galena and pyrite on the selective extraction of zinc from base metal sulphide concentrates. Miner. Eng. 1998, 11, 1–21. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, H.; Wang, S.; Xia, L.; Zou, W.; Liu, G. Investigations on reverse cationic flotation of iron ore by using a gemini surfactant: Ethane-1,2-bis(dimethyl-dodecyl-ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Fairthorne, G.; Brinen, J.S.; Fornasiero, D.; Nagaraj, D.R.; Ralston, J. Spectroscopic and electrokinetic study of the adsorption of butyl ethoxycarbonyl thiourea on chalcopyrite. Int. J. Miner. Process. 1998, 54, 147–163. [Google Scholar] [CrossRef]
- Güler, T.; Hiçyilmaz, C.; GökagˇAç, G.; Ekmeçi, Z. Adsorption of dithiophosphate and dithiophosphinate on chalcopyrite. Miner. Eng. 2006, 19, 62–71. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 2, p. 68. ISBN 978-04-4-453082-0. [Google Scholar]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Mehdilo, A.; Zarei, H.; Irannajad, M.; Arjmandfar, H. Flotation of zinc oxide ores by cationic and mixed collectors. Miner. Eng. 2012, 36–38, 331–334. [Google Scholar] [CrossRef]
- Keqing, F.A.; Miller, J.D.; Jiang, T.; Li, G. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores. Trans. Nonferrous Met. Soc. China 2005, 15, 1138–1144. [Google Scholar]
- Liu, C.; Feng, Q.; Zhang, G.; Ma, W.; Meng, Q.; Chen, Y. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 89, 163–167. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Jing, M.; Rao, F.; Wu, L.; Li, K.; Cao, S. A review of forming process and flotation mechanism of hemimorphite. Chin. J. Process Eng. 2017, 17, 903–910. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid. Miner. Metall. Process. 2006, 23, 87–96. [Google Scholar]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]
- Hosseini, S. Selective flotation of angooran oxidised zinc ore using mercaptans. In Proceedings of the International Mineral Processing Symposium, Belek-Antalya, Turkey, 21–23 October 2008; Turkish Mining Development Foundation: Ankara, Turkey, 2008; pp. 367–376. [Google Scholar]
- Marabini, A.M.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating reagents for flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Han, C.; Wei, D.Z.; Shen, Y.B.; Liu, W.G. Flotation behavior of hemimorphite and smithsonite in dodecylamine system. J. Northeast. Univ. Nat. Sci. 2016, 37, 1582–1587. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Grimm, R.; Kolařík, Z. Acidic organophosphorus extractants-XIX: Extraction of Cu(II), Ni(II), Zn(II) and Cd(II) by di(2-ethylhexyl) phosphoric acid. J. Inorg. Nucl. Chem. 1974, 36, 189–192. [Google Scholar] [CrossRef]
- Owusu, G. Selective extractions of Zn and Cd from Zn-Cd-Co-Ni sulphate solution using di-2-ethylhexyl phosphoric acid extractant. Hydrometallurgy 1998, 47, 205–215. [Google Scholar] [CrossRef]
- Miralles, N.; Sastre, A.M. Comparative study of the distribution equilibria of Zn(II) in chloride medium by organophosphoric,-phosphonic and -phosphinic acids. Monatsh. Chem. 1993, 124, 987–994. [Google Scholar] [CrossRef]
- Sainz-Diaz, C.I.; Klocker, H.; Marr, R.; Bart, H.J. New approach in the modelling of the extraction equilibrium of zinc with bis-(2-ethylhexyl) phosphoric acid. Hydrometallurgy 1996, 42, 1–11. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y. The development of a composite collector for the flotation of rutile. Miner. Eng. 1999, 12, 1419–1430. [Google Scholar] [CrossRef]
- Gruner, H.; Bilsing, U. Cassiterite flotation using styrene phosphonic acid to produce high-grade concentrates at high recoveries from finely disseminated ores- comparison with other collectors and discussion of effective circuit configurations. Miner. Eng. 1992, 5, 429–434. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Flotation performances and adsorption mechanism of α-hydroxyoctyl phosphinic acid to cassiterite. Appl. Surf. Sci. 2015, 353, 856–864. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Xu, H.; Jia, H.; Liu, G. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite. Miner. Eng. 2015, 71, 188–193. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Wang, S.; Liu, G. The activation mechanism of Cu(II) to ilmenite and subsequent flotation response to α-hydroxyoctyl phosphinic acid. Ind. Eng. Chem. 2016, 37, 123–130. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Adsorption of α-hydroxyoctyl phosphonic acid to ilmenite/water interface and its application in flotation. Colloids Surf. A 2016, 490, 67–73. [Google Scholar] [CrossRef]
- Jurisson, S.; Berning, D.; Jia, W.; Ma, D. Coordination compounds in nuclear medicine. Chem. Rev. 1993, 93, 1137–1156. [Google Scholar] [CrossRef]
- Rizkalla, E.N.; Zaki, M.T.; Ismail, M.I. Metal chelates of phosphonate-containing ligands-V stability of some 1-hydroxyethane-1,1-diphosphonic acid metal chelates. Talanta 1980, 27, 715–719. [Google Scholar] [CrossRef]
- Kotlyarevsky, I.L.; Alferiev, I.S.; Krasnukhina, A.V.; Pomazov, V.D.; Egorov, N.V. New phospho-organic collectors for flotation of non-sulphide minerals. In Reagents in Mineral Technology; Jones, M.J., Oblatt, R., Eds.; Institution of Mining and Metallurgy: London, UK, 1984; pp. 173–179. ISBN 978-08-2-477715-9. [Google Scholar]
- Houot, R. Is the cassiterite contained in complex sulphide polymetallic ore recoverable? Int. J. Miner. Process. 1991, 32, 45–57. [Google Scholar] [CrossRef]
- Lavrinenko, A.A.; Shrader, E.A.; Kharchikov, A.N.; Kunilova, I.V. Apatite flotation from brazilite-apatite-magnetite ore. J. Min. Sci. 2013, 49, 811–818. [Google Scholar] [CrossRef]
- Chaudhuri Pradip, N.C. Flotation of degana wolframite. Trans. Indian Inst. Met. 1997, 50, 383–390. [Google Scholar]
- Chen, G.L.; Tao, D.; Ren, H.; Ji, F.F.; Qiao, J.K. An investigation of niobite flotation with octyl diphosphonic acid as collector. Int. J. Miner. Process. 2005, 76, 111–122. [Google Scholar] [CrossRef]
- Zheng, X.P.; Misra, M.; Smith, R.W.; Qiao, J.K. Fersmite flotation with diphosphonic acid and other collectors. Miner. Eng. 1996, 9, 331–341. [Google Scholar] [CrossRef]
- Kieczykowski, G.R.; Jobson, R.B.; Melillo, D.G.; Reinhold, D.F.; Grenda, V.J.; Shinkai, I. Preparation of (4-amino-1-hydroxybutylidene)bisphosphonic acid sodium salt, MK-217 (alendronate aodium). An improved procedure for the preparation of 1-hydroxy-1,1-bisphosphonic acids. J. Org. Chem. 1995, 27, 8310–8312. [Google Scholar] [CrossRef]
- Ozdemir, O.; Karaguzel, C.; Nguyen, A.V.; Celik, M.S.; Miller, J.D. Contact angle and bubble attachment studies in the flotation of trona and other soluble carbonate salts. Miner. Eng. 2009, 22, 168–175. [Google Scholar] [CrossRef]
- Pereira, C.A.; Peres, A.E.C. Reagents in calamine zinc ores flotation. Miner. Eng. 2005, 18, 275–277. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite and quartz using anionic collectors. Int. J. Miner. Process. 2016, 158, 55–62. [Google Scholar] [CrossRef]
- Jingzun, W.; Ting, W. How to explain the infrared spectrum. Univ. Chem. 2016, 31, 90–97. [Google Scholar] [CrossRef]
- Elias, A.; Didi, M.A.; Villemin, D.; Semaoune, T.; Ouattas, S. Synthesis of mono-and dialkylphosphates by the reactions of hydroxycompounds with the phosphorus pentaoxide under microwave irradiation. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 2599–2607. [Google Scholar] [CrossRef]
- Ren, H.; Ji, F.; Zhang, S. Effect and mechanism of diphosphonic acid on flotation characteristic of fersmite and intergrowth gangue. Trans. Nonferrous Met. Soc. China 2003, 13, 1013–1018. [Google Scholar]
- Andrews, B.; Almahdali, S.; James, K.; Ly, S.; Crowder, K.N. Copper oxide surfaces modified by alkylphosphonic acids with terminal pyridyl-based ligands as a platform for supported catalysis. Polyhedron 2016, 114, 360–369. [Google Scholar] [CrossRef]
- Chausov, F.F. Structurally selective protection of steel against oxygen corrosion by 1-hydroxyethylidenediphosphonatozincate. Bull. Russ. Acad. Sci. Phys. 2013, 77, 324–326. [Google Scholar] [CrossRef]
- Fu, R.B.; Hu, S.M.; Wu, X.T. Luminescent zinc phosphonates for ratiometric sensing of 2,4,6-trinitrophenol and temperature. Cryst. Growth Des. 2016, 16, 5074–5083. [Google Scholar] [CrossRef]
- Pilbáth, A.; Nyikos, L.; Bertóti, I.; Kálmán, E. Zinc corrosion protection with 1,5-diphosphono-pentane. Corros. Sci. 2008, 50, 3314–3321. [Google Scholar] [CrossRef]
- Pilbáth, A.; Bertóti, I.; Pfeifer, É.; Mink, J.; Nyikos, L.; Kálmán, E. Formation and characterization of 1, 5-diphosphono-pentane films on polycrystalline zinc substrates. Surf. Coat. Technol. 2009, 203, 1182–1192. [Google Scholar] [CrossRef]
- Ren, H.; Ji, F.; Zhang, S.; Bao, M.; Zhang, Q. Effects of diphosphonic acid on ilmenorutile collecting property and research of action mechanism. J. Univ. Sci. Technol. Beijing 2002, 9, 249–252. [Google Scholar]
- Chen, A.; Li, M.; Qian, Z.; Ma, Y.T.; Che, J.; Ma, Y.L. Hemimorphite ores: A review of processing technologies for zinc extraction. J. Miner. Met. Mater. Soc. 2016, 68, 2688–2697. [Google Scholar] [CrossRef]



| Composition | Zn | O | Si | Mg | Ca | Cu | P | Cl | S | Fe | Pb | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemimorphite | 57.53 | 31.00 | 10.92 | 0.218 | 0.115 | 0.08 | 0.04 | 0.01 | 0.02 | 0.01 | 0.02 | - |
| Quartz | - | 56.20 | 43.60 | 0.06 | 0.04 | - | - | - | - | 0.02 | - | 0.07 |
| Species | Atomic Concentration/% | ||||
|---|---|---|---|---|---|
| C 1s | O 1s | Si 2p | P 2p | Zn 2p | |
| HDDPA | 59.24 | 30.69 | - | 9.87 | - |
| Zn-HDDPA precipitate | 57.11 | 27.49 | - | 8.31 | 7.09 |
| Hemimorphite | 9.79 | 54.02 | 12.46 | - | 23.73 |
| Hemimorphite after HDDPA treatment | 39.12 | 41.74 | 5.51 | 3.44 | 10.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Liu, G.; Qin, J.; Fan, H. Hemimorphite Flotation with 1-hydroxydodecylidene-1,1-diphosphonic acid and Its Mechanism. Minerals 2018, 8, 38. https://doi.org/10.3390/min8020038
Tan W, Liu G, Qin J, Fan H. Hemimorphite Flotation with 1-hydroxydodecylidene-1,1-diphosphonic acid and Its Mechanism. Minerals. 2018; 8(2):38. https://doi.org/10.3390/min8020038
Chicago/Turabian StyleTan, Wen, Guangyi Liu, Jingqin Qin, and Hongli Fan. 2018. "Hemimorphite Flotation with 1-hydroxydodecylidene-1,1-diphosphonic acid and Its Mechanism" Minerals 8, no. 2: 38. https://doi.org/10.3390/min8020038