The Effect of Seaweed Glue in the Separation of Copper–Molybdenum Sulphide Ore by Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Response Surface Methodology
2.3. Flotation Procedure
2.4. Adsorption Experiments
2.5. Zeta Potential Measurements
2.6. FT-IR Spectroscopy Experiments
3. Results and Discussion
3.1. Model Fittings and Statistical Analysis
3.2. Interactions among the Factors and Optimization Results
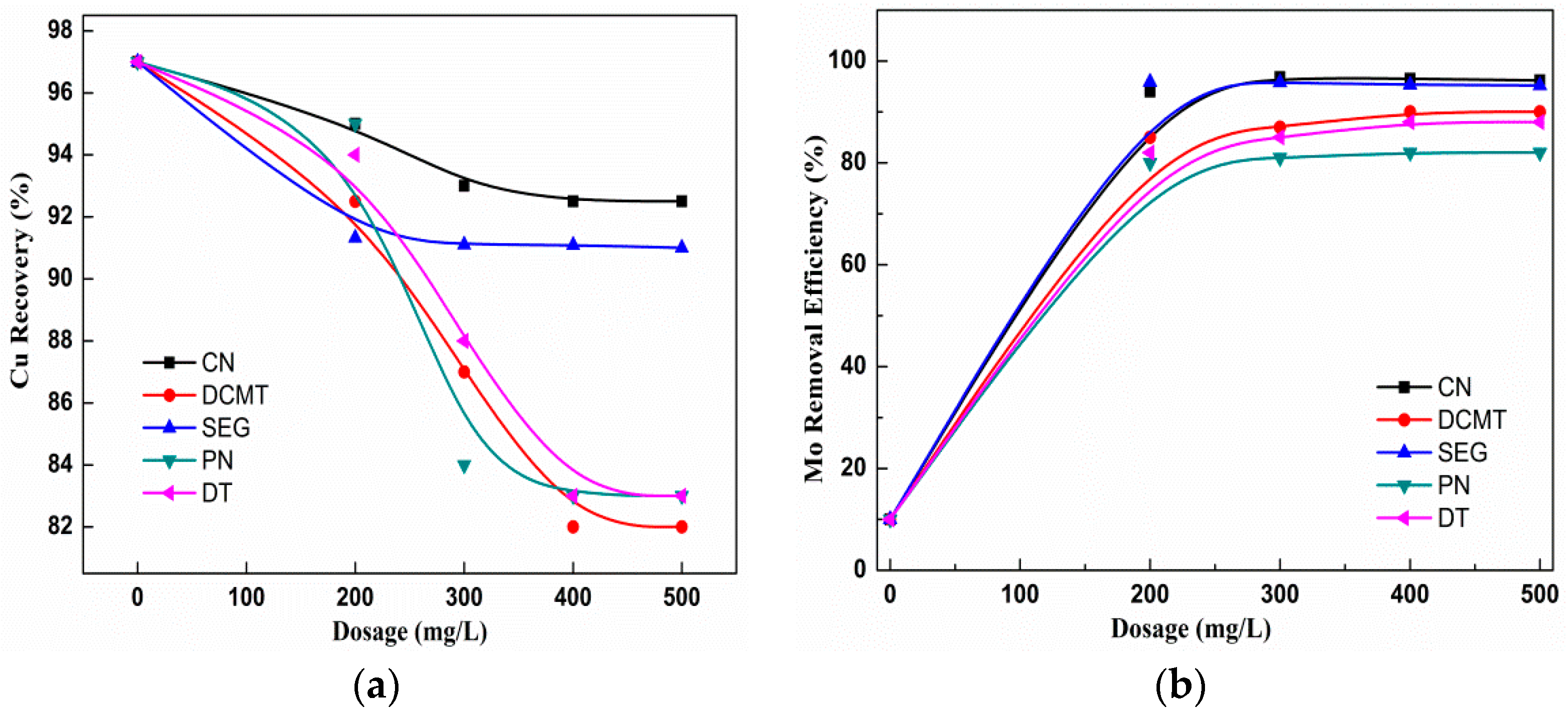
3.3. Contrast Experiments of Depressant
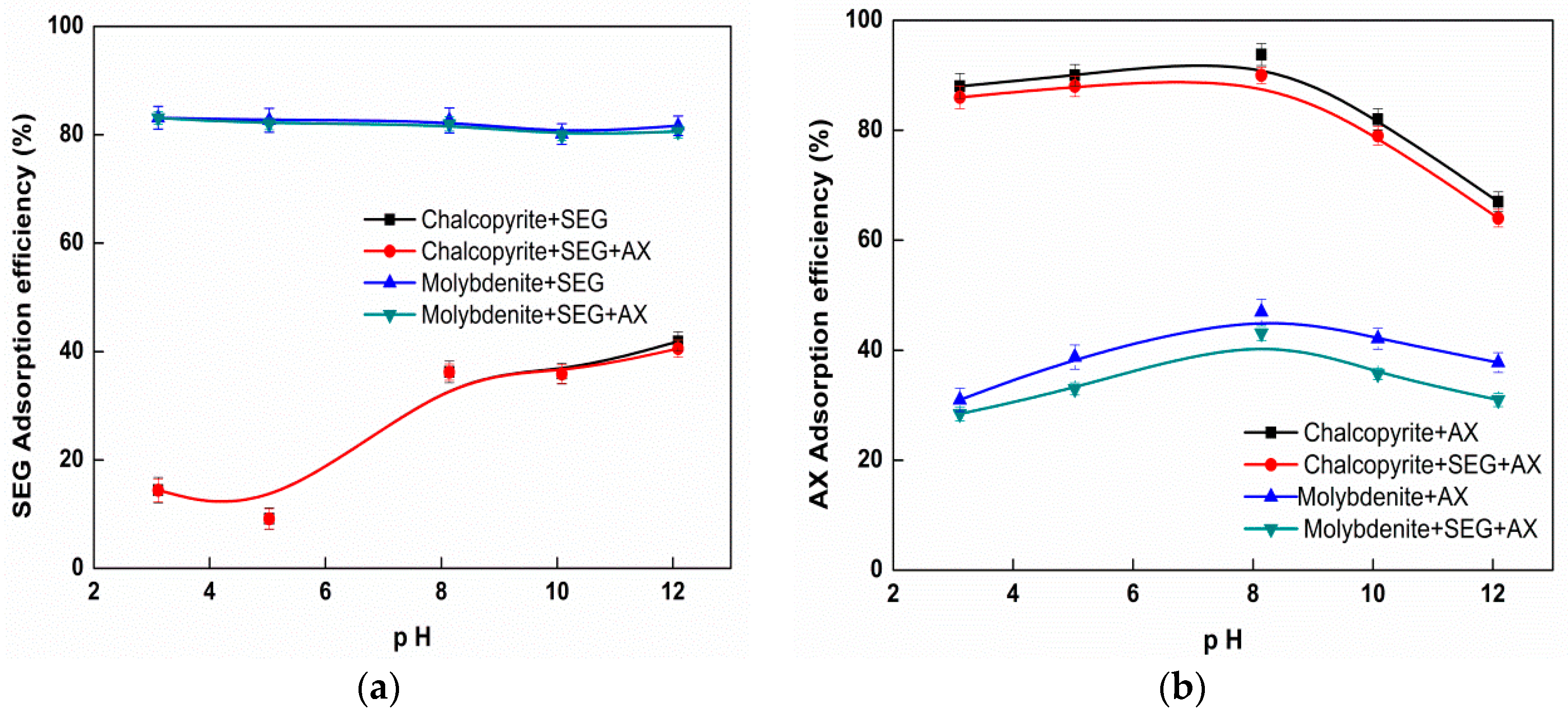
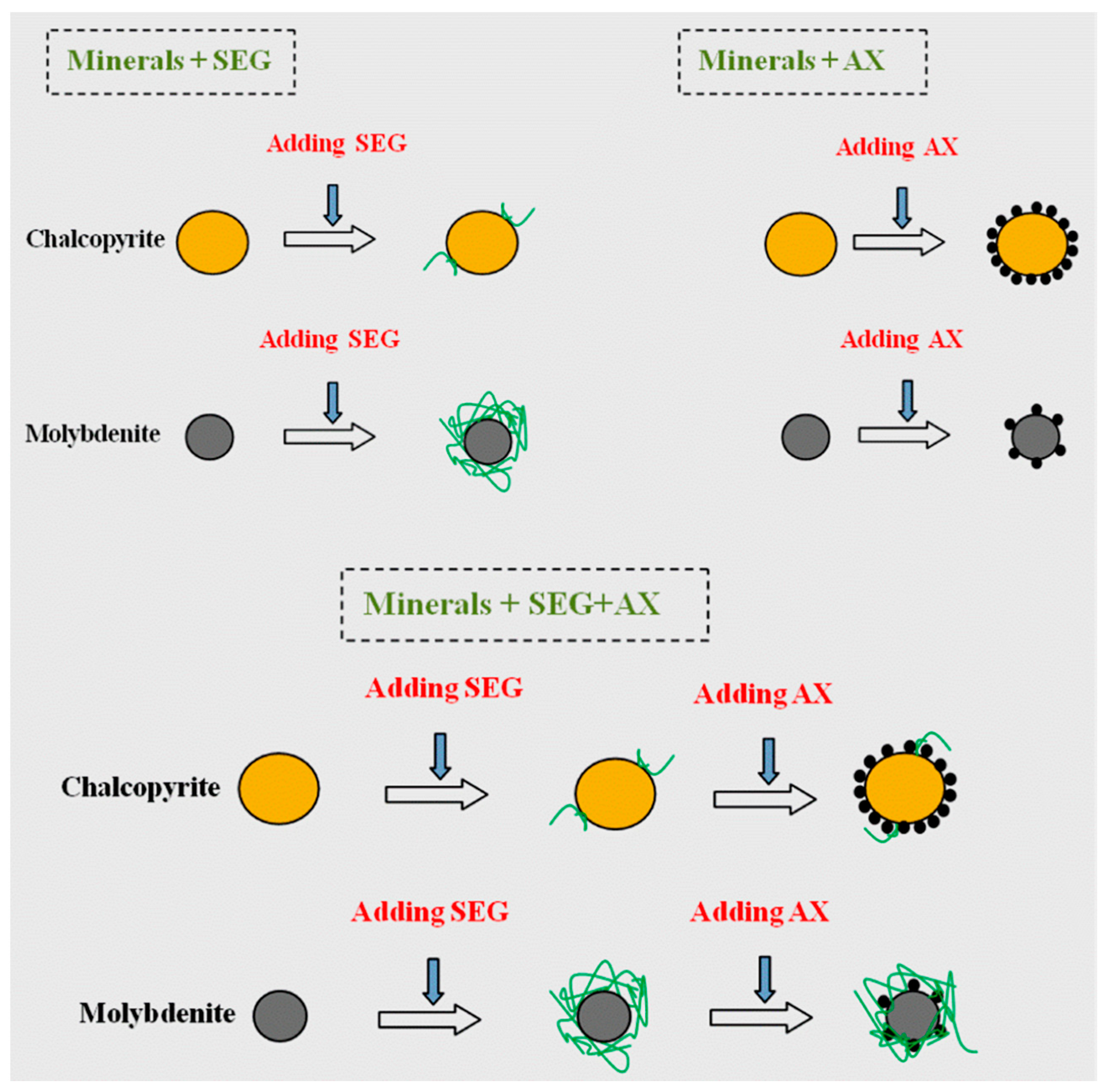
3.4. Adsorption Studies
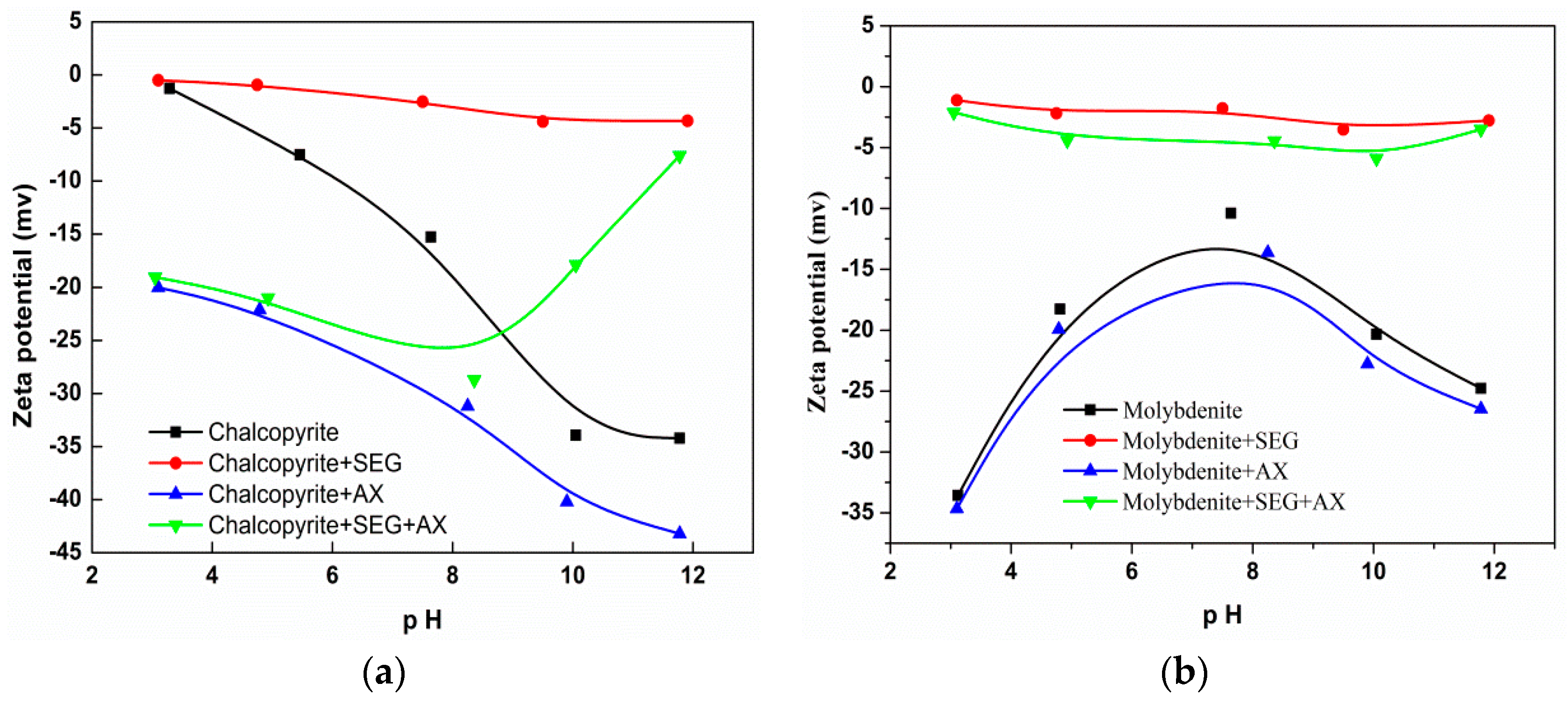
3.5. Zeta Potential Tests
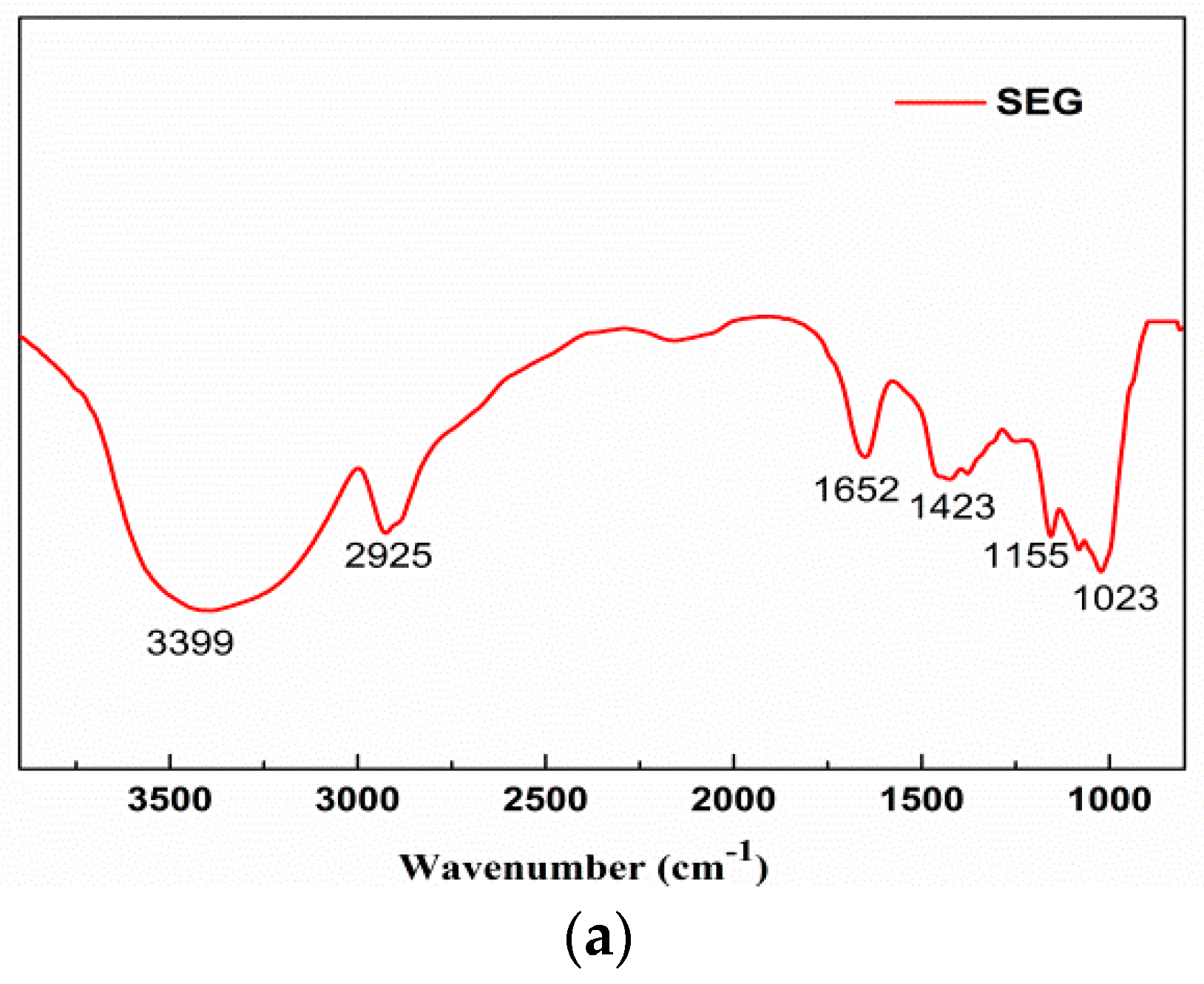
3.6. FT-IR Spectra Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.M.; Lu, Y.P. A novel method to limit the detrimental effect of serpentine on the flotation of pentlandite. Int. J. Miner. Process. 2012, 114, 11–13. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Li, M.Y.; Wei, D.; Cui, B. Flotation Behavior of Chalcopyrite and Molybdenite in the Presence of 2,3-Disulfanylbutanedioic Acid. J. Northeast. Univ. Nat. Sci. 2015, 36, 1020–1024. [Google Scholar]
- Peng, H.; Wu, D.; Abdalla, M. Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite. Minerals 2017, 7, 51. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Liu, J. Investigation into the flotation response of refractory molybdenum ore to depressant mixtures: A case study. Int. J. Min. Sci. Technol. 2016, 26, 1089–1094. [Google Scholar] [CrossRef]
- Sun, W.; Yin, Z.; Hu, Y. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar]
- Lin, Q.Q.; Gu, G.H.; Wang, H. Recovery of molybdenum and copper from porphyry ore via iso-flotability flotation. Trans. Nonferrous Met. Soc. China 2017, 27, 2260–2271. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Chen, H.T.; Ravishankar, S.A.; Farinato, R.S. Rational polymer design for solid–liquid separations in mineral processing applications. Int. J. Miner. Process. 2003, 72, 75–86. [Google Scholar] [CrossRef]
- Gustafsson, J.O.; Lannefors, C.J. Polyquaternary Polymer as a Depressant in a Method for Froth Flotation of Potash Ores. WO/2014/095797. 26 June 2014. [Google Scholar]
- Pearse, M.J. Historical use and future development of chemicals for solid–liquid separation in the mineral processing industry. Miner. Eng. 2003, 16, 103–108. [Google Scholar] [CrossRef]
- Andreeva, N.; Ishizaki, T.; Baroch, P. Creating Biointerface on Polymer by Plasma-Initiated Graft Polymerization. Trans. Mater. Res. Soc. Jpn. 2011, 36, 549–552. [Google Scholar] [CrossRef]
- Badiger, H.; Shukla, S.; Kalyani, S. Characterization of sodium alginate membrane crosslinked with glutaraldehyde in pervaporation separation. J. Appl. Polym. Sci. 2014, 131, 209–219. [Google Scholar]
- Rhim, J.W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Harnsilawat, T.; Pongsawatmanit, R.; Mcclements, D.J. Characterization of β-lactoglobulin-sodium alginate interactions in aqueous solutions: A calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocoll. 2006, 20, 577–585. [Google Scholar] [CrossRef]
- Vikartovská, A.; Bučko, M.; Mislovičová, D. Improvement of the stability of glucose oxidase via, encapsulation in sodium alginate–cellulose sulfate–poly(methylene-co-guanidine) capsules. Enzym. Microb. Technol. 2007, 41, 748–755. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Sasaki, T.; Machida, I.; Ishiwata, S. Macromolecular ion flotation of Fe3+, Cu2+, and Ni2+ ions by combined use of macromolecular anions and cationic surfactant. Bull. Chem. Soc. Jpn. 1982, 55, 3109–3112. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydro-xyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Asanov, I.P.; Okotrub, A.V.; Varnek, V.A.; Vyalikh, D.V. Spectroscopic and electrochemical characterization of the surface layers of chalcopyrite (CuFeS2) reacted in acidic solutions. Appl. Surf. Sci. 2004, 225, 395–409. [Google Scholar] [CrossRef]
- Triffett, B.; Veloo, C.; Adair, B.J.I. An investigation of the factors affecting the recovery of molybdenite in the Kennecott Utah Copper bulk flotation circuit. Miner. Eng. 2008, 21, 832–840. [Google Scholar] [CrossRef]
- Zhang, M. Study on reagent-making of P-nokes. China Mol. Ind. 2002, 38, 28–36. [Google Scholar]
- Kondrat’ev, S.A.; Burdakova, E.A.; Konovalov, I.A. Collectability of physically adsorbed xanthate ion–dixanthogen associates. J. Min. Sci. 2016, 52, 541–550. [Google Scholar] [CrossRef]
- Song, M.M.; Branfordwhite, C.; Nie, H.L. Optimization of adsorption conditions of BSA on thermosensitive magnetic composite particles using response surface methodology. Colloids Surf. B Biointerfaces 2011, 84, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.A.; Hami, Z.; Mirzaei, N. N-doped TiO2, nanosheets for photocatalytic degradation and mineralization of diazinon under simulated solar irradiation: Optimization and modeling using a response surface methodology. J. Mol. Liq. 2016, 22, 183–191. [Google Scholar] [CrossRef]
- Gelman, A. Analysis of variance. Qual. Contr. Appl. Stat. 2005, 20, 295–300. [Google Scholar]
- Tybout, A.; Sternthal, B.; Keppel, G.; Verducci, J.; Meyers-Levy, J.; Barnes, J.; Maxwell, S.; Allenby, G.; Gupta, S.; Steenkamp, J.B.; et al. Analysis of Variance. J. Consum. Psychol. 2016, 10, 5–35. [Google Scholar]
- Shaykh, Z.M.; Zinatizadeh, A.A.L. Statistical modeling of photocatalytic degradation of synthetic amoxicillin wastewater (SAW) in an immobilized TiO2 photocatalytic reactor using response surface methodology (RSM). J. Taiwan Inst. Chem. Eng. 2014, 45, 1717–1726. [Google Scholar] [CrossRef]
- Schenone, A.V.; Conte, L.O.; Botta, M.A.; Alfano, O.M. Modeling and optimization of photo-Fenton degradation of 2,4-D using ferrioxalate complex and response surface methodology (RSM). Environ. Manag. 2015, 155, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Fayazi, M.; Afzali, D.; Taher, M.A.; Mostafavi, A.; Gupta, V.K. Removal of Safranin dye from aqueous solution using magnetic mesoporous clay: Optimization study. J. Mol. Liq. 2015, 212, 675–685. [Google Scholar] [CrossRef]
- Eslami, A.; Asadi, A.; Meserghani, M.; Bahrami, H. Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J. Mol. Liq. 2016, 222, 739–744. [Google Scholar] [CrossRef]
- Senior, G.D.; Trahar, W.J. The influence of metal hydroxides and collector on the flotation of chalcopyrite. Int. J. Miner. Process. 1991, 33, 321–341. [Google Scholar] [CrossRef]
- Chulhyun, P.; Hoseok, J. The Effect of Sodium Silicate as pH Modifier and Depressant in the Froth Flotation of Molybdenite Ores. Mater. Trans. 2010, 51, 1367–1369. [Google Scholar]
- Mowla, D.; Karimi, G.; Ostadnezhad, K. Removal of hematite from silica and ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Zou, Y.R. Studies on Effect of Anionic Starch on the Floatability of Aluminum-Silicon Minerals and Its Mechanism. Master’s Thesis, Central South University, Changsha, China, 2008. [Google Scholar]
- Bellamy, L.J. Infrared spectroscopy of complex molecules. In The Infra-Red Spectra of Complex Molecules; Science Press: Beijing, China, 1980; pp. 123–126. [Google Scholar]
- Rath, R.K.; Subramanian, S.; Pradeepy, T. Surface chemical studies on pyrite in the presence of polysaccharide—Based flotation depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef] [PubMed]

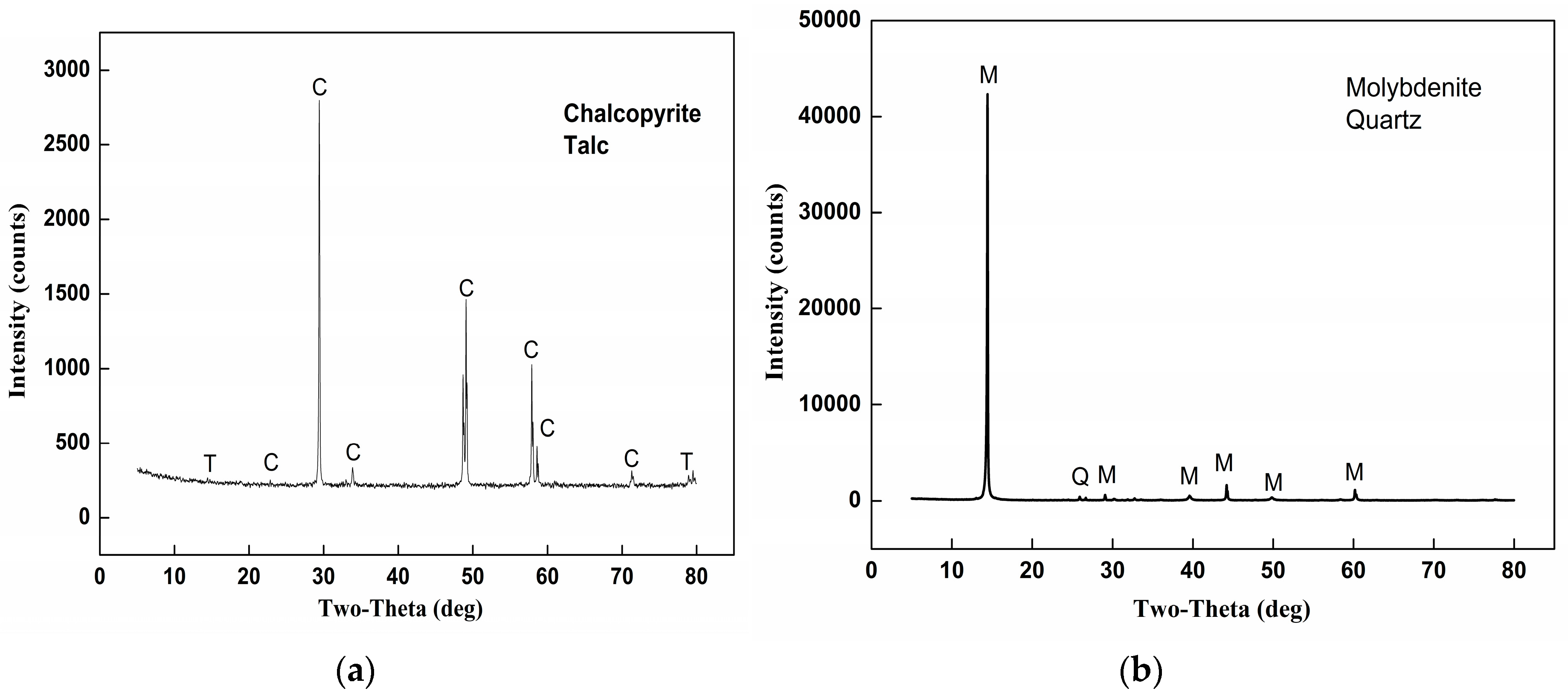

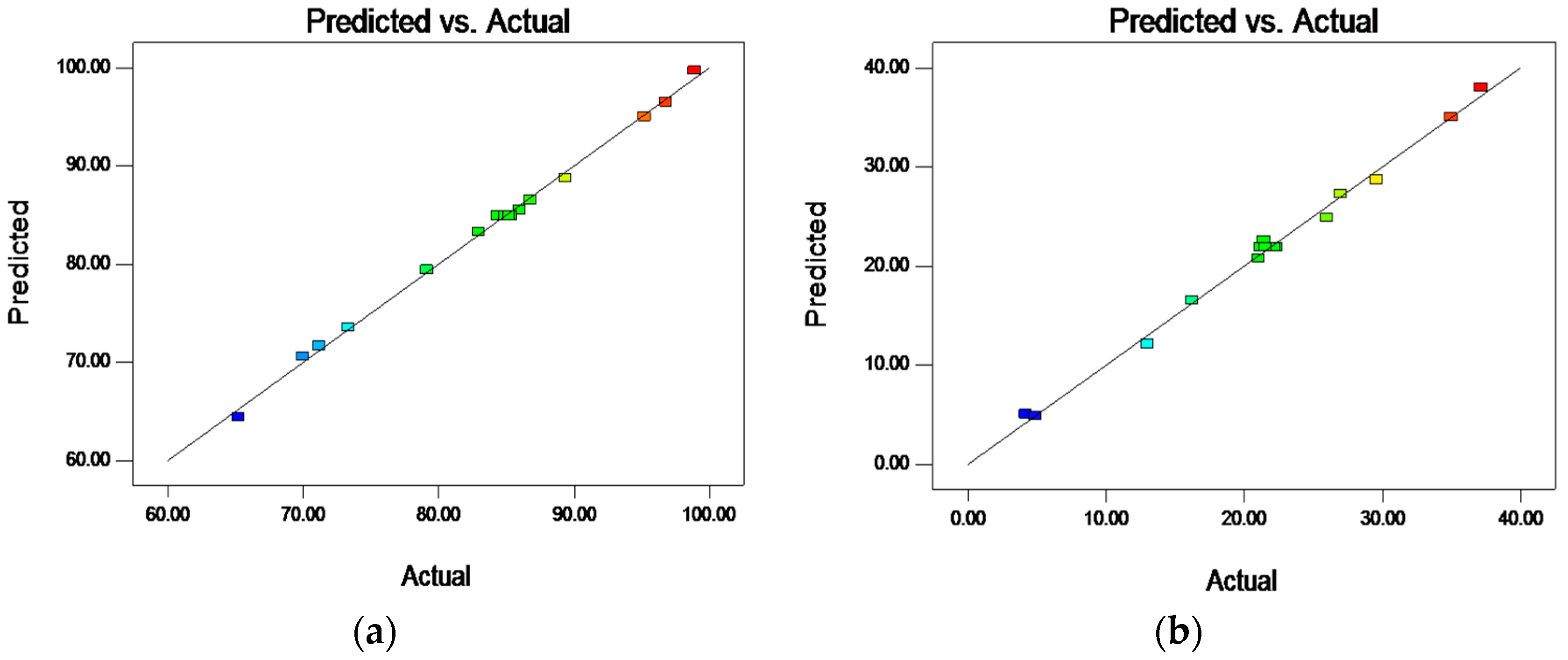
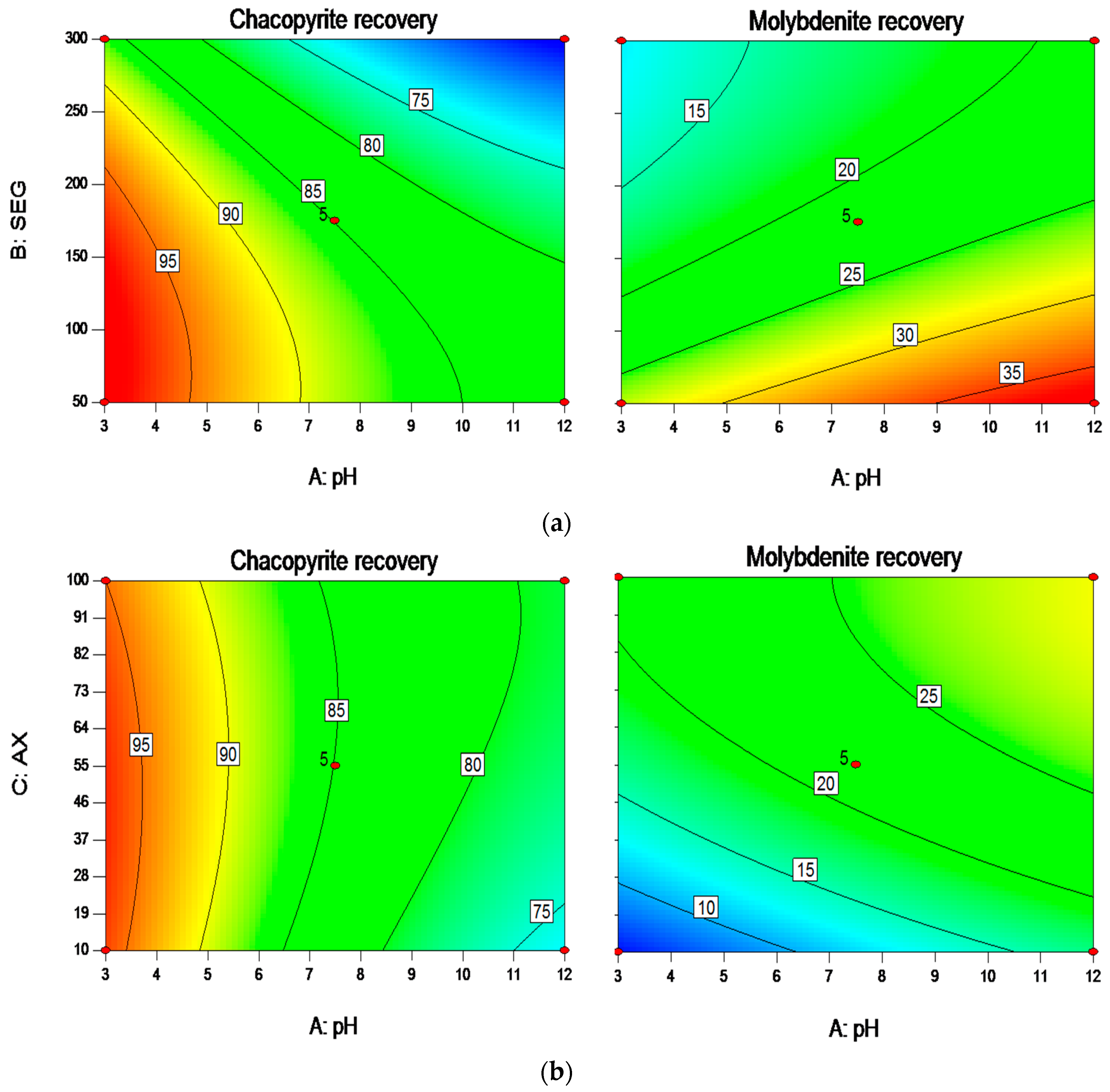
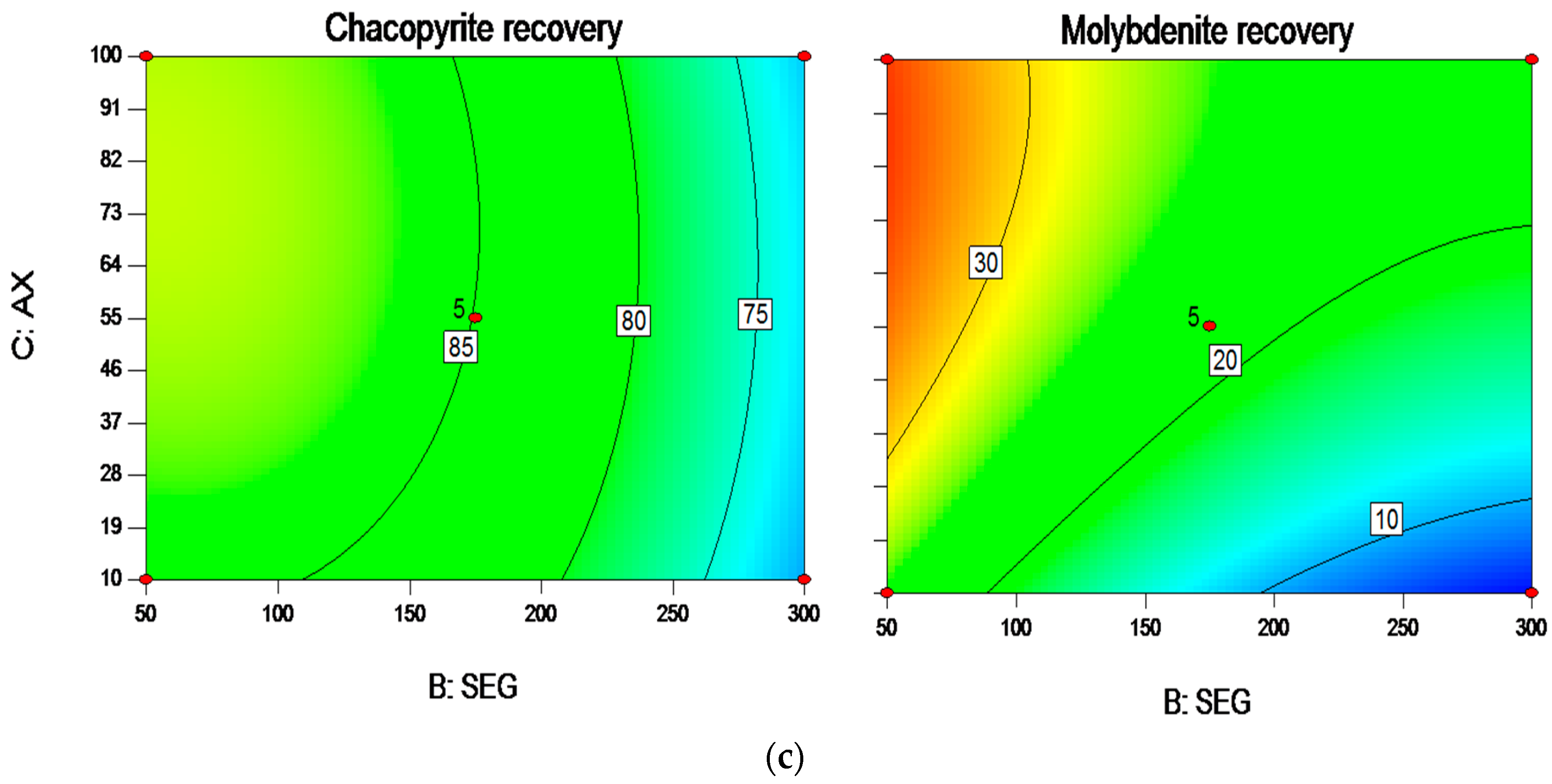

| Independent Variables | Codes | Ranges and Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Initial pH | X1 | 3 | 50 | 10 |
| SEG dosage (mg/L) | X2 | 7.5 | 175 | 55 |
| AX dosage (mg/L) | X3 | 12 | 300 | 100 |
| Run | Factor 1 A: pH | Factor 2 (mg/L) B: SEG | Factor 3 (mg/L) C: AX | Response 1 (%) Chalcopyrite Recovery | Response 2 (%) Molybdenite Recovery |
|---|---|---|---|---|---|
| 1 | 12 | 300 | 55 | 66.21 | 21.01 |
| 2 | 12 | 175 | 10 | 73.32 | 16.21 |
| 3 | 7.5 | 175 | 55 | 84.76 | 22.31 |
| 4 | 7.5 | 175 | 55 | 84.34 | 22.19 |
| 5 | 12 | 50 | 55 | 82.98 | 37.16 |
| 6 | 12 | 175 | 100 | 79.12 | 29.56 |
| 7 | 7.5 | 300 | 10 | 69.98 | 4.87 |
| 8 | 3 | 300 | 55 | 86.78 | 12.98 |
| 9 | 7.5 | 50 | 10 | 85.98 | 25.98 |
| 10 | 7.5 | 175 | 55 | 85.34 | 22.10 |
| 11 | 3 | 50 | 55 | 98.89 | 27.01 |
| 12 | 7.5 | 175 | 55 | 84.89 | 21.21 |
| 13 | 7.5 | 175 | 55 | 85.21 | 21.54 |
| 14 | 7.5 | 50 | 100 | 89.34 | 34.99 |
| 15 | 3 | 175 | 10 | 95.21 | 21.01 |
| 16 | 7.5 | 300 | 100 | 71.16 | 21.42 |
| 17 | 3 | 175 | 10 | 93.65 | 5.98 |
| Source | F Value (Cu/Mo) | Prob > F (Cu/Mo) | |
|---|---|---|---|
| Mode | 1313/138.0 | <0.0001 | |
| X1-pH | 1491/22.87 | <0.0001/0.002 | |
| X2-SEG | 1031/120.5 | <0.0001 | |
| X3-AX | 19.3/75.3 | 0.003/<0.0001 | |
| X12 | 16.1/1.25 | 0.005/0.32 | |
| X13 | 27.3/3.13 | 0.001/0.12 | |
| X23 | 2.39/14.43 | 0.166/0.006 | |
| Lack of fit (F) | 3.28/4.21 | Lack of fit (P) | 0.056/0.030 |
| R2 | 0.99/0.99 | Radj2 − Rpred2 | 0.027/0.066 |
| C.V.% | 0.84/4.60 | Adeq precisor | 65.21/43.73 |
| Initial pH | SEG Dosage (mg/L) | AX Dosage (mg/L) | Cu/Mo Recovery (%) | |
|---|---|---|---|---|
| Predicted Value | Experimental Value | |||
| 4.07 | 197.38 | 16.32 | 95.11/7.99 | 93.21/6.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Gu, G.; Li, S.; Wang, C.; Zhu, R. The Effect of Seaweed Glue in the Separation of Copper–Molybdenum Sulphide Ore by Flotation. Minerals 2018, 8, 41. https://doi.org/10.3390/min8020041
Chen Z, Gu G, Li S, Wang C, Zhu R. The Effect of Seaweed Glue in the Separation of Copper–Molybdenum Sulphide Ore by Flotation. Minerals. 2018; 8(2):41. https://doi.org/10.3390/min8020041
Chicago/Turabian StyleChen, Zhixiang, Guohua Gu, Shuangke Li, Chongqing Wang, and Renfeng Zhu. 2018. "The Effect of Seaweed Glue in the Separation of Copper–Molybdenum Sulphide Ore by Flotation" Minerals 8, no. 2: 41. https://doi.org/10.3390/min8020041




