Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks
Abstract
:1. Introduction
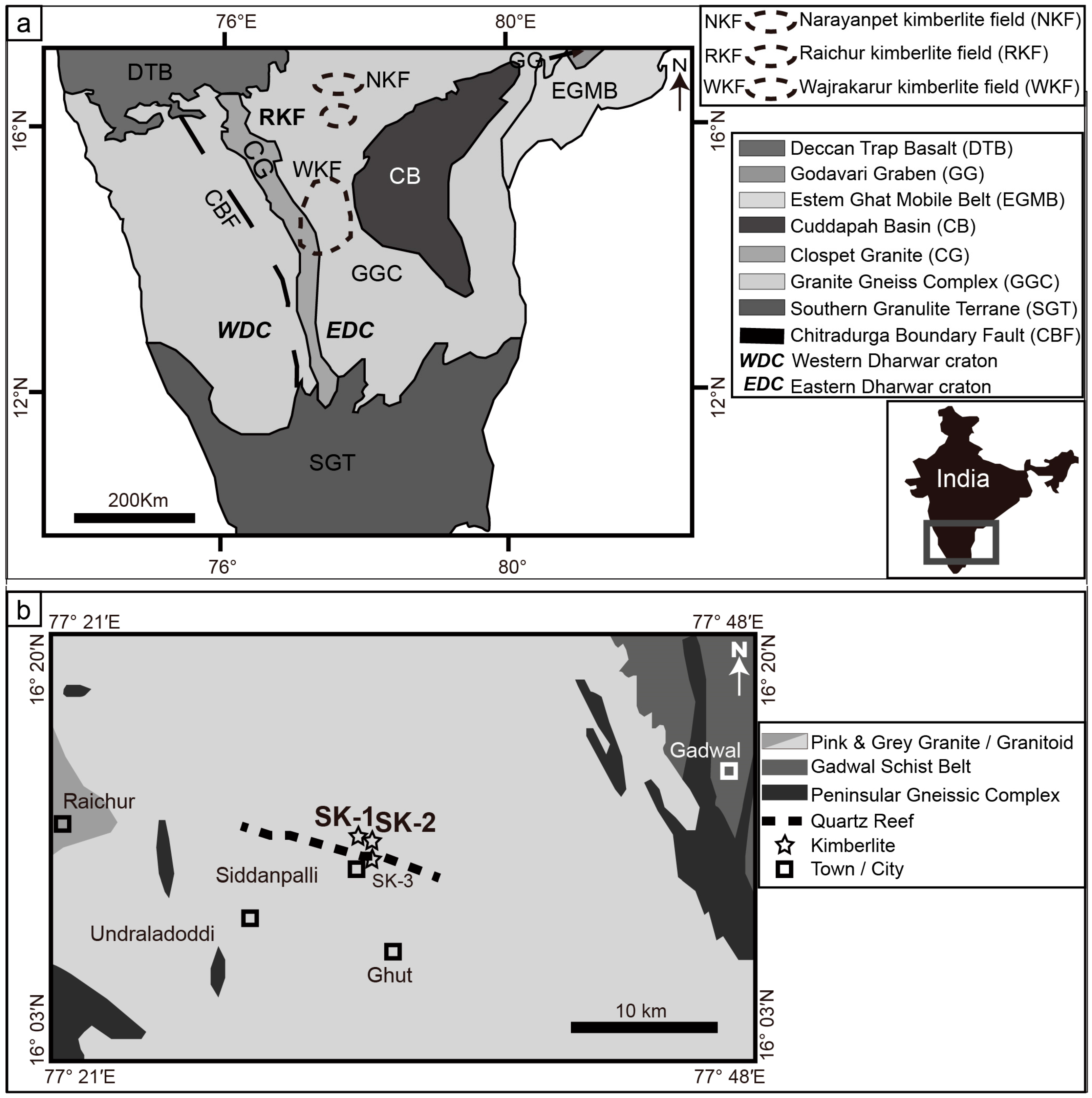
2. Geological Setting
3. Methods
4. Results
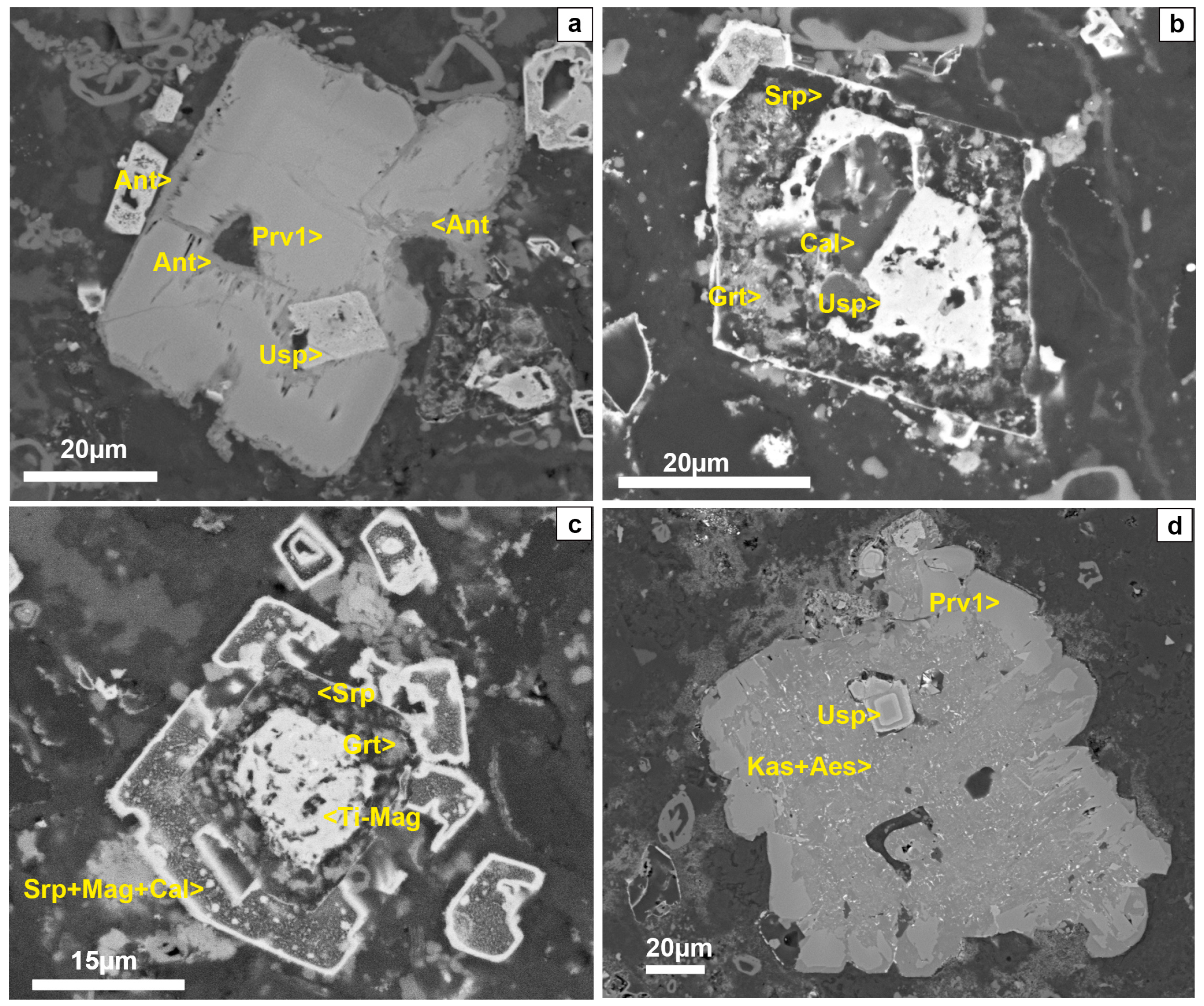
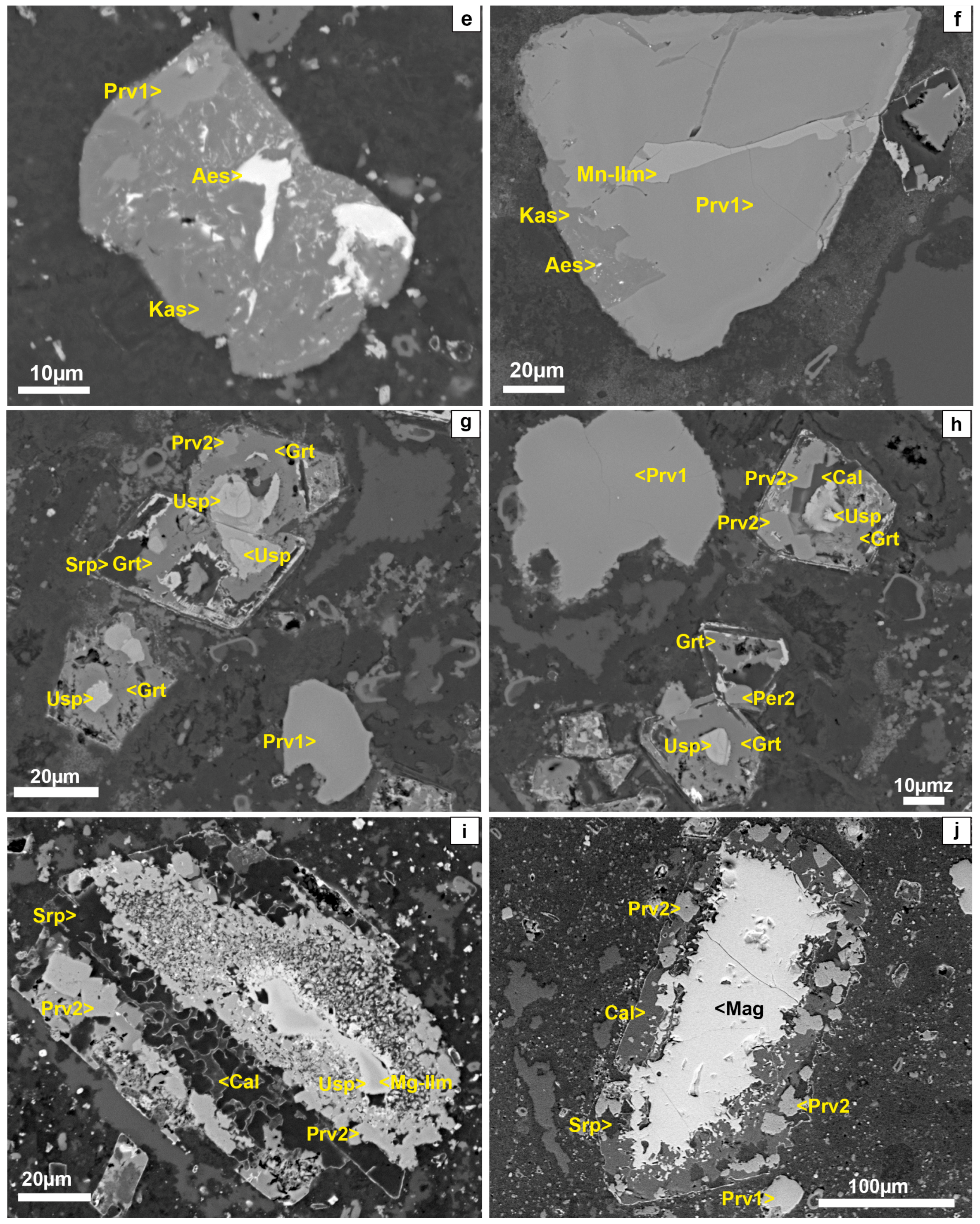
4.1. Mineral Textures
4.2. MicroRaman Study
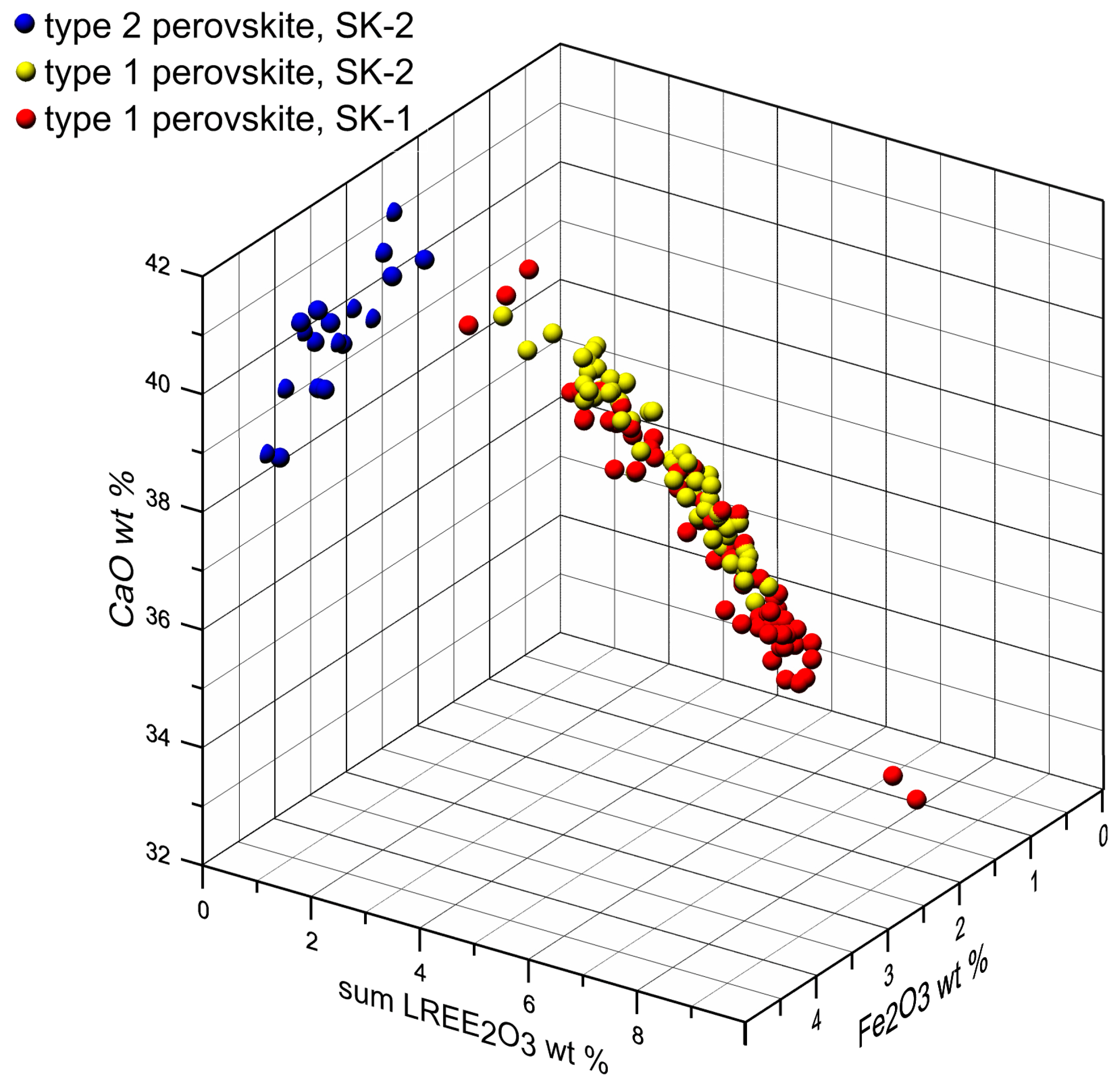
4.3. Mineral Chemistry
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rezvukhin, D.I.; Malkovets, V.G.; Sharygin, I.S.; Kuzmin, D.V.; Litasov, K.D.; Gibsher, A.A.; Pokhilenko, N.P.; Sobolev, N.V. Inclusions of Cr- and Cr-Nb-Rutile in pyropes from the Internatsionalnaya kimberlite pipe, Yakutia. Dokl. Earth Sci. 2016, 466, 173–176. [Google Scholar] [CrossRef]
- Ragozin, A.L.; Zedgenizov, D.A.; Shatskii, V.S.; Orihashi, Y.; Agashev, A.M.; Kagi, H. U-Pb age of rutile from the eclogite xenolith of the Udachnaya kimberlite pipe. Dokl. Earth Sci. 2014, 457, 861–864. [Google Scholar] [CrossRef]
- Roeder, P.L.; Schulze, D.J. Crystallization of groundmass spinel in kimberlite. J. Petrol. 2008, 49, 1473–1495. [Google Scholar] [CrossRef]
- Bellis, A.; Canil, D. Ferric Iron in CaTiO3 perovskite as an oxygen barometer for kimberlitic magmas I: Experimental calibration. J. Petrol. 2007, 48, 219–230. [Google Scholar] [CrossRef]
- Castillo-Oliver, M.; Galí, S.; Melgarejo, J.C.; Griffin, W.L.; Belousova, E.; Pearson, N.J.; Watangua, M.; O’Reilly, S.Y. Trace-element geochemistry and U-Pb dating of perovskite in kimberlites of the Lunda Norte province (NE Angola): Petrogenetic and tectonic implications. Chem. Geol. 2016, 426, 118–134. [Google Scholar] [CrossRef]
- Batumike, J.M.; Griffin, W.L.; Belousova, E.A.; Pearson, N.J.; O’Reilly, S.Y.; Shee, S.R. LAM-ICPMS U-Pb dating of kimberlitic perovskite: Eocene-Oligocene kimberlites from the Kundelungu Plateau, D.R. Congo. Earth Planet. Sci. Lett. 2008, 267, 609–619. [Google Scholar] [CrossRef]
- Tappe, S.; Kjarsgaard, B.A.; Kurszlaukis, S.; Nowell, G.M.; Phillips, D. Petrology and Nd-Hf Isotope Geochemistry of the Neoproterozoic Amon Kimberlite Sills, Baffin Island (Canada): Evidence for Deep Mantle Magmatic Activity Linked to Supercontinent Cycles. J. Petrol. 2014, 55, 2003–2042. [Google Scholar] [CrossRef]
- Malkovets, V.G.; Rezvukhin, D.I.; Belousova, E.A.; Griffin, W.L.; Sharygin, I.S.; Tretiakova, I.G.; Gibsher, A.A.; O’Reilly, S.Y.; Kuzmin, D.V.; Litasov, K.D.; et al. Cr-rich rutile: A powerful tool for diamond exploration. Lithos 2016, 265, 304–311. [Google Scholar] [CrossRef]
- Mitchell, R.H. Perovskites Modern and Ancient; Almaz Press: Thunder Bay, ON, Canada, 2002. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. Three compositional varieties of perovskite from kimberlites of the Lac de Gras field (Northwest Territories, Canada). Mineral. Mag. 2001, 65, 133–148. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Lehmann, B.; Mainkar, D.; Belyatsky, B. Petrogenesis of the end-Cretaceous diamondiferous Behradih orangeite pipe: Implication for mantle plume-lithosphere interaction in the Bastar craton, Central India. Contrib. Mineral. Petrol. 2011, 161, 721–742. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Occurrence, alteration patterns and compositional variation of perovskite in kimberlites. Can. Mineral. 2000, 38, 975–994. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chakhmouradian, A.R. Instability of perovskite in a CO2-rich environment: Examples from carbonatite and kimberlite. Can. Mineral. 1998, 36, 939–952. [Google Scholar]
- Pereira, V.P.; Conceicao, R.V.; Formoso, M.L.L.; Pires, A.C. Alteration of perovskite to anatase in silica-undersaturated rocks of the Catalao I carbonatite complex, Brazil; a Raman study. Rev. Bras. Geociênc. 2005, 35, 239–244. [Google Scholar]
- Martins, T.; Chakhmouradian, A.R.; Medici, L. Perovskite alteration in kimberlites and carbonatites: The role of kassite, CaTi2O4(OH)2. Phys. Chem. Miner. 2014, 41, 473–484. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A. Timing of eastern North American kimberlite magmatism: Continental extension of the Great Meteor hotspot track? Earth Planet. Sci. Lett. 2000, 178, 253–268. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Wu, F.Y.; Mitchell, R.H.; Li, Q.L.; Lehmann, B. Mesoproterozoic U-Pb ages, trace element and Sr-Nd isotopic composition of perovskite from kimberlites of the Eastern Dharwar craton, southern India: Distinct mantle sources and a widespread 1.1Ga tectonomagmatic event. Chem. Geol. 2013, 353, 48–64. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A.; Creaser, R.A. The temporal evolution of North American kimberlites. Lithos 2004, 76, 377–397. [Google Scholar] [CrossRef]
- Paton, C.; Hergt, J.M.; Phillips, D.; Woodhead, J.D.; Shee, S.R. New insights into the genesis of Indian kimberlites from the Dharwar Craton via in situ Sr isotope analysis of groundmass perovskite. Geology 2007, 35, 1011–1014. [Google Scholar] [CrossRef]
- Woodhead, J.; Hergt, J.; Phillips, D.; Paton, C. African kimberlites revisited: In situ Sr-isotope analysis of groundmass perovskite. Lithos 2009, 112, 311–317. [Google Scholar] [CrossRef]
- Zurevinski, S.E.; Heaman, L.M.; Creaser, R.A. The origin of Triassic/Jurassic kimberlite magmatism, Canada: Two mantle sources revealed from the Sr-Nd isotopic composition of groundmass perovskite. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimski-Korsakov, O.M.; Nefedov, E.I.; Ilyinskiy, G.A.; Sergeev, A.B.; Abakumova, N.B. The Caledonian Ultramafic Alkaline and Carbonatite Complexes of the Kola Peninsula and Northern Karelia; Nedra: Moscow, Russia, 1965. [Google Scholar]
- Lepekhina, E.N.; Antonov, A.V.; Savva, E.V.; Belyatsky, B.V.; Sergeev, S.A. Perovskite from the Tiksheozero carbonatite: Age and genesis. In Perovskite from the Tiksheozero Carbonatite: Age and Genesis. Extended Abstract: Geochemistry of Magmatic Rocks—School Geochemistry of Alkaline Rocks; Vernadsky Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences: Moscow, Russia, 2009. [Google Scholar]
- Tappe, S.; Jenner, G.A.; Foley, S.F.; Heaman, L.; Besserer, D.; Kjarsgaard, B.A.; Ryan, B. Torngat ultramafic lamprophyres and their relation to the North Atlantic Alkaline Province. Lithos 2004, 76, 491–518. [Google Scholar] [CrossRef]
- Dongre, A.N.; Viljoen, K.S.; Chalapathi Rao, N.V.; Gucsik, A. Origin of Ti-rich garnets in the groundmass of Wajrakarur field kimberlites, southern India: Insights from EPMA and Raman spectroscopy. Mineral. Petrol. 2016, 110, 295–307. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Heaman, L.M.; Kjarsgaard, B.A.; Romer, R.L.; Stracke, A.; Joyce, N.; Hoefs, J. Genesis of ultramafic lamprophyres and carbonatites at Aillik Bay, Labrador: A consequence of incipient lithospheric thinning beneath the North Atlantic Craton. J. Petrol. 2006, 47, 1261–1315. [Google Scholar] [CrossRef]
- Hammond, A.L.; Mitchell, R.H. Accessory mineralogy of orangeite from Swartruggens, South Africa. Mineral. Petrol. 2002, 76, 1–19. [Google Scholar] [CrossRef]
- Müntener, O.; Hermann, J. Titanian andradite in a metapyroxenite layer from the Malenco ultramafics (Italy): Implications for Ti-mobility and low oxygen fugacity. Contrib. Mineral. Petrol. 1994, 116, 156–168. [Google Scholar] [CrossRef]
- Amthauer, G.; Rossman, G.R. The hydrous component in andradite garnet. Am. Mineral. 1998, 83, 835–840. [Google Scholar] [CrossRef]
- Laverne, C.; Grauby, O.; Alt, J.C.; Bohn, M. Hydroschorlomite in altered basalts from Hole 1256D, ODP Leg 206: The transition from low-temperature to hydrothermal alteration. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef] [Green Version]
- Schingaro, E.; Lacalamita, M.; Mesto, E.; Ventruti, G.; Pedrazzi, G.; Ottolini, L.; Scordari, F. Crystal chemistry and light elements analysis of Ti-rich garnets. Am. Mineral. 2016, 101, 371–384. [Google Scholar] [CrossRef]
- Dongre, A.N.; Jacob, D.E.; Stern, R.A. Subduction-related origin of eclogite xenoliths from the Wajrakarur kimberlite field, Eastern Dharwar craton, Southern India: Constraints from petrology and geochemistry. Geochim. Cosmochim. Acta 2015, 166, 165–188. [Google Scholar] [CrossRef]
- Ghosh, B.; Morishita, T.; Ray, J.; Tamura, A.; Mizukami, T.; Soda, Y.; Ovung, T.N. A new occurrence of titanian (hydro)andradite from the Nagaland ophiolite, India: Implications for element mobility in hydrothermal environments. Chem. Geol. 2017, 457, 47–60. [Google Scholar] [CrossRef]
- Downs, R.T. The RRUFF Project: An integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. In Program and Abstracts of the 19th General Meeting of the International Mineralogical Association; International Mineralogical Association: Kobe, Japan, 2006; pp. O03–O13. [Google Scholar]
- Paschoal, C.W.A.; Moreira, R.L.; Fantini, C.; Pimenta, M.A.; Surendran, K.P.; Sebastian, M.T. Raman scattering study of RETiTaO6 dielectric ceramics. J. Eur. Ceram. Soc. 2003, 23, 2661–2666. [Google Scholar] [CrossRef]
- Tomašić, N.; Gajović, A.; Bermanec, V.; Rajić, M. Recrystallization of metamict Nb–Ta–Ti–REE complex oxides: A coupled X-Ray-Diffraction and Raman Spectroscopy study of aeschynite-(Y) and polycrase-(Y). Can. Mineral. 2004, 42, 1847–1857. [Google Scholar] [CrossRef]
- Nasir, S.; Theye, T.; Massonne, H.J. REE-Rich Aeschynite in Apatite-Dolomite Carbonatite, Eastern Oman Mountains. Open Mineral. J. 2009, 3, 17–27. [Google Scholar]
- Macdonald, R.; Bagiński, B.; Kartashov, P.M.; Zozulya, D.; Dzierżnowski, P. Hydrothermal alteration of chevkinite-group minerals. Part 2. Metasomatite from the Keivy massif, Kola Peninsula, Russia. Mineral. Mag. 2015, 79, 1039–1059. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. IMA report: Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–810. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites: Mineralogy, Geochemistry, and Petrology; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Cox, R.A.; Wilton, D.H.C. U–Pb dating of perovskite by LA-ICP-MS: An example from the Oka carbonatite, Quebec, Canada. Chem. Geol. 2006, 235, 21–32. [Google Scholar] [CrossRef]
- Simonetti, A.; Heaman, L.M.; Chacko, T. Use of discrete-dynode secondary electron multipliers with Faradays—A “reduced volume” approach for in-situ U-Pb dating of accessory minerals within petrographic thin sections by LA-MC-ICP-MS. In Laser Ablation-ICP-MS in the Earth Sciences; Short Course Series 40; Ylvester, P., Ed.; Mineralogical Association of Canada: Quebec, QC, Canada, 2008; pp. 24–264. [Google Scholar]
- Yang, Y.H.; Wu, F.Y.; Wilde, S.A.; Liu, X.M.; Zhang, Y.B.; Xie, L.W.; Yang, J.H. In situ perovskite Sr-Nd isotopic constraints on the petrogenesis of the Ordovician Mengyin kimberlites in the North China Craton. Chem. Geol. 2009, 264, 24–42. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Kjarsgaard, B.A. Integrating ultramafic lamprophyres into the IUGS classification of igneous rocks: Rationale and implications. J. Petrol. 2005, 46, 1893–1900. [Google Scholar] [CrossRef]

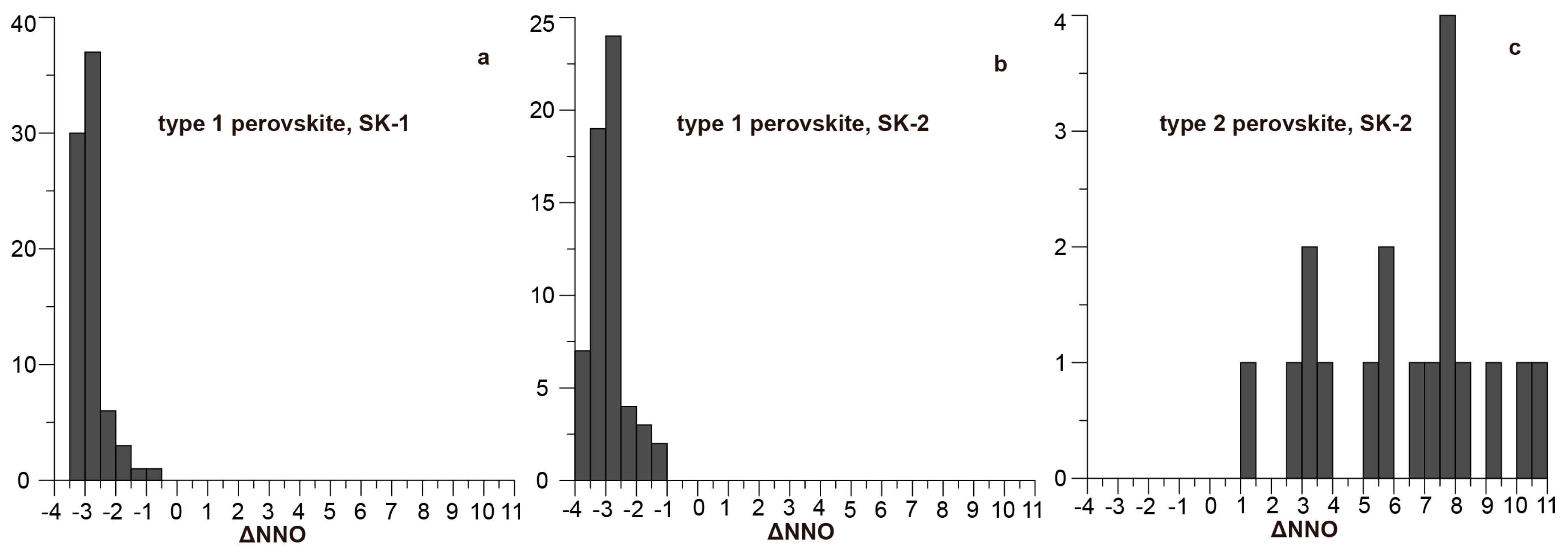
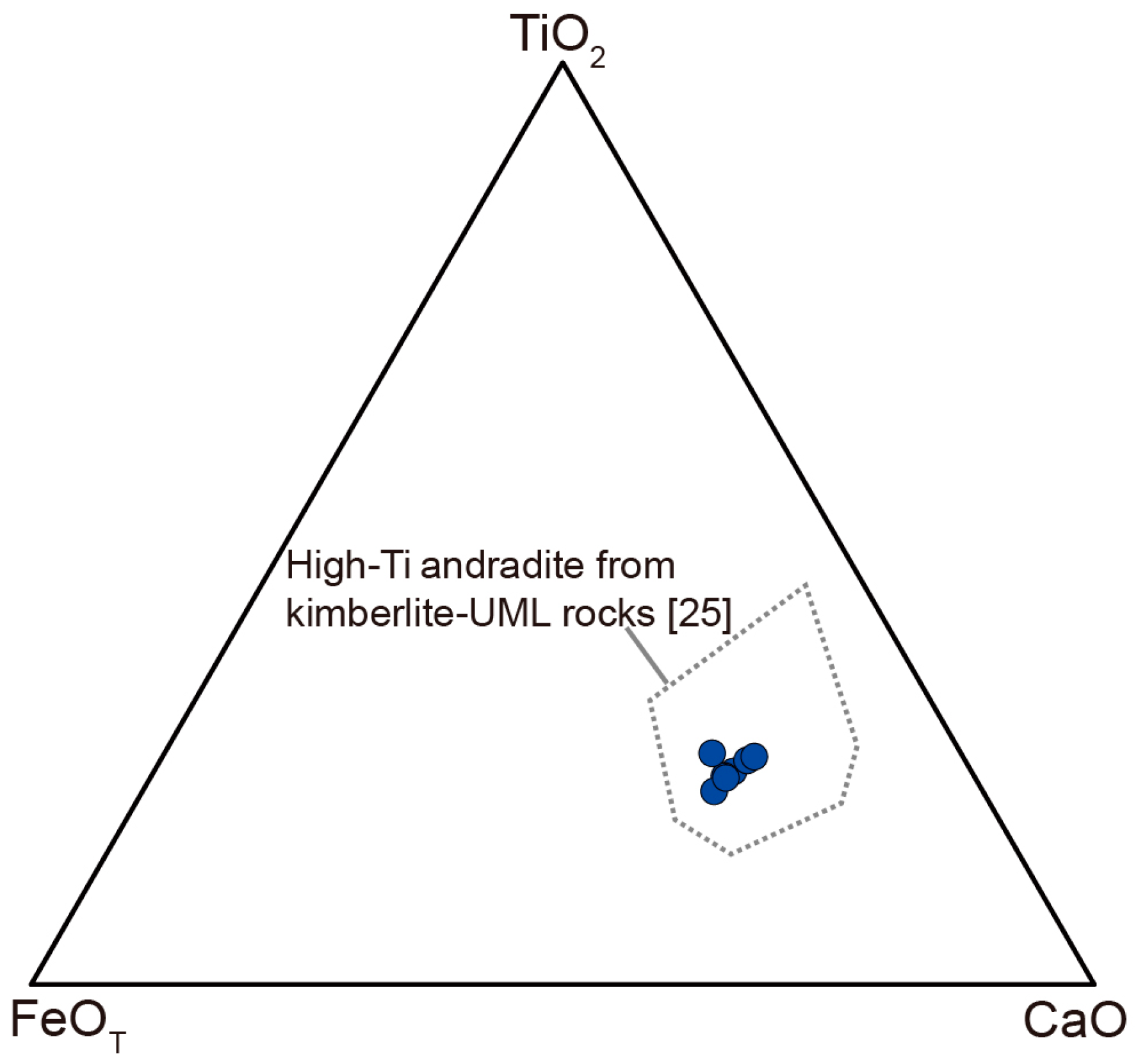
| Mineral | Type 1 Perovskite | Type 2 Perovskite | Aeschynite-(Ce) | Kassite | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SK-1 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | |||||||
| (wt %) | Centre | Centre | Border | Border | Centre | Centre | Border | Border | |||||||
| SrO | 0.34 | 0.18 | 0.23 | 0.25 | 0.20 | 0.19 | 0.20 | 0.24 | bdl | bdl | 0.13 | bdl | bdl | bdl | bdl |
| ZrO2 | 0.10 | 0.21 | 0.23 | 0.38 | 0.08 | 0.14 | 0.19 | 0.28 | 0.09 | 0.46 | 0.74 | 0.25 | 0.43 | 0.40 | 0.08 |
| Nb2O5 | 0.60 | 0.98 | 0.73 | 0.61 | 0.54 | 0.63 | 0.44 | 0.50 | 0.10 | bdl | 3.10 | 0.99 | 0.63 | 0.46 | 0.62 |
| CaO | 34.68 | 34.64 | 37.11 | 37.63 | 35.36 | 35.27 | 38.06 | 38.19 | 39.95 | 38.20 | 7.24 | 8.00 | 22.49 | 22.12 | 21.32 |
| ThO2 | - | - | - | - | - | - | - | - | - | - | 0.37 | bdl | - | - | - |
| Na2O | 0.57 | 0.57 | 0.35 | 0.22 | 36.53 | 0.60 | 0.28 | 0.22 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO | 0.06 | bdl | 0.05 | 0.09 | 0.08 | bdl | 0.05 | 0.05 | 0.14 | 0.08 | 0.04 | 0.05 | 0.26 | 0.05 | bdl |
| Al2O3 | 0.33 | 0.30 | 0.24 | 0.31 | 0.25 | 0.29 | 0.23 | 0.28 | bdl | bdl | bdl | bdl | bdl | 0.03 | bdl |
| SiO2 | bdl | bdl | bdl | 0.04 | bdl | bdl | bdl | bdl | 0.61 | 0.60 | 0.38 | bdl | 0.05 | 0.06 | 0.07 |
| BaO | 0.19 | 0.12 | - | 0.08 | - | 0.13 | 0.18 | 0.12 | - | - | 0.46 | 0.15 | - | - | - |
| TiO2 | 54.36 | 54.26 | 54.13 | 56.30 | 54.99 | 54.75 | 57.10 | 56.67 | 55.49 | 53.48 | 48.61 | 52.20 | 62.42 | 64.74 | 65.26 |
| La2O3 | 0.97 | 1.02 | 0.67 | 0.50 | 0.60 | 0.84 | 0.62 | 0.53 | bdl | bdl | 7.79 | 11.11 | bdl | bdl | 0.15 |
| Ce2O3 | 3.07 | 3.03 | 1.79 | 0.88 | 2.18 | 2.84 | 0.89 | 0.89 | bdl | bdl | 18.34 | 17.99 | 0.23 | 0.48 | 0.58 |
| Nd2O3 | 1.56 | 1.50 | 1.08 | 0.35 | 1.18 | 1.50 | 0.34 | 0.41 | bdl | bdl | 6.88 | 3.99 | 0.24 | 0.22 | 0.28 |
| Cr2O3 | - | - | - | - | - | - | - | - | - | - | bdl | 0.05 | - | - | - |
| Pr2O3 | 0.45 | 0.43 | 0.17 | 0.19 | 0.08 | 0.36 | 0.19 | 0.17 | bdl | bdl | 1.97 | 1.45 | 0.12 | 0.08 | bdl |
| MnO | 0.02 | 0.05 | 0.04 | bdl | 0.03 | bdl | 0.02 | 0.05 | bdl | 0.03 | bdl | 0.19 | 0.15 | 0.37 | 1.28 |
| Sm2O3 | - | - | - | - | - | - | - | - | - | - | 0.51 | 0.19 | - | - | - |
| Fe2O3 | 1.28 | 1.36 | 1.74 | 1.31 | 0.99 | 1.29 | 1.16 | 1.01 | 2.00 | 4.03 | 0.59 | 0.66 | 1.29 | 0.93 | 0.51 |
| Gd2O3 | - | - | - | - | - | - | - | - | - | - | 0.24 | 0.13 | - | - | - |
| HfO2 | - | - | - | - | - | - | - | - | - | - | 0.11 | bdl | - | - | - |
| Ta2O5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.07 | bdl | 0.06 | 0.11 | bdl | 0.04 | bdl |
| K2O | 0.04 | 0.03 | 0.02 | 0.00 | 0.03 | 0.03 | 0.02 | 0.02 | 0.00 | 0.02 | - | - | bdl | 0.01 | bdl |
| Total | 98.63 | 98.74 | 98.57 | 99.16 | 98.25 | 98.92 | 99.99 | 99.65 | 98.80 | 97.19 | 97.53 | 97.79 | 88.37 | 90.05 | 90.19 |
| ΣLREE2O3 | 6.04 | 5.98 | 3.70 | 1.93 | 4.04 | 5.54 | 2.04 | 1.99 | 0.14 | 0.15 | 35.49 | 34.73 | 0.59 | 0.81 | 1.03 |
| (apfu) | O = 3 | O = 3 | Σcations = 3 | Σcations = 3 | |||||||||||
| Sr | 0.005 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 |
| Y | - | - | - | - | - | - | - | - | - | - | 0.000 | 0.000 | - | - | - |
| Zr | 0.001 | 0.002 | 0.003 | 0.004 | 0.001 | 0.002 | 0.002 | 0.003 | 0.001 | 0.005 | 0.018 | 0.006 | 0.001 | 0.001 | 0.001 |
| Nb | 0.006 | 0.011 | 0.008 | 0.006 | 0.006 | 0.007 | 0.005 | 0.005 | 0.001 | 0.000 | 0.070 | 0.022 | 0.012 | 0.008 | 0.011 |
| Ca | 0.885 | 0.883 | 0.938 | 0.932 | 0.924 | 0.894 | 0.936 | 0.942 | 0.985 | 0.960 | 0.387 | 0.417 | 0.986 | 0.957 | 0.926 |
| Th | - | - | - | - | - | - | - | - | - | - | 0.004 | 0.000 | - | - | - |
| Na | 0.026 | 0.026 | 0.016 | 0.010 | 0.022 | 0.027 | 0.012 | 0.010 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 0.002 | 0.001 | 0.002 | 0.003 | 0.003 | 0.000 | 0.002 | 0.002 | 0.005 | 0.003 | 0.003 | 0.004 | 0.016 | 0.003 | 0.000 |
| Al | 0.009 | 0.008 | 0.007 | 0.008 | 0.007 | 0.008 | 0.006 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Si | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.014 | 0.019 | 0.000 | 0.002 | 0.002 | 0.003 |
| Ba | 0.002 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.002 | 0.001 | - | - | 0.009 | 0.003 | - | - | - |
| Ti | 0.974 | 0.970 | 0.960 | 0.979 | 0.977 | 0.974 | 0.985 | 0.981 | 0.960 | 0.943 | 1.823 | 1.908 | 1.922 | 1.967 | 1.990 |
| La | 0.008 | 0.008 | 0.005 | 0.004 | 0.005 | 0.007 | 0.005 | 0.004 | 0.000 | 0.000 | 0.132 | 0.184 | 0.000 | 0.000 | 0.002 |
| Ce | 0.027 | 0.026 | 0.015 | 0.007 | 0.019 | 0.025 | 0.007 | 0.007 | 0.000 | 0.000 | 0.335 | 0.320 | 0.004 | 0.007 | 0.009 |
| Nd | 0.013 | 0.013 | 0.009 | 0.003 | 0.010 | 0.013 | 0.003 | 0.003 | 0.000 | 0.000 | 0.123 | 0.069 | 0.003 | 0.003 | 0.004 |
| Cr | - | - | - | - | - | - | - | - | - | - | 0.000 | 0.002 | - | - | - |
| Pr | 0.004 | 0.004 | 0.001 | 0.002 | 0.001 | 0.003 | 0.002 | 0.001 | 0.000 | 0.000 | 0.036 | 0.026 | 0.002 | 0.001 | 0.000 |
| Mn | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.006 | 0.004 | 0.010 | 0.036 |
| Sm | - | - | - | - | - | - | - | - | - | - | 0.009 | 0.003 | - | - | - |
| Fe | 0.023 | 0.024 | 0.031 | 0.023 | 0.018 | 0.023 | 0.020 | 0.017 | 0.035 | 0.071 | 0.022 | 0.024 | 0.040 | 0.028 | 0.016 |
| Gd | - | - | - | - | - | - | - | - | - | - | 0.004 | 0.002 | - | - | - |
| Hf | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.000 | - | - | - |
| Ta | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 |
| K | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | - | - | 0.000 | 0.000 | 0.000 |
| ΣLREE | 0.052 | 0.051 | 0.031 | 0.016 | 0.034 | 0.047 | 0.017 | 0.016 | 0.001 | 0.001 | 0.512 | 0.533 | 0.009 | 0.012 | 0.015 |
| (wt %) | #1 | #2 | #4 | #5 | #15 | #17 | #19 | #20 | #21 |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 20.20 | 25.44 | 21.62 | 21.86 | 21.51 | 23.18 | 20.69 | 21.24 | 20.00 |
| Al2O3 | 0.63 | 0.69 | 0.98 | 0.98 | 0.85 | 0.89 | 0.85 | 0.84 | 0.92 |
| Cr2O3 | 0.32 | 0.83 | 1.23 | 0.77 | 2.81 | 0.37 | 2.63 | 1.25 | 2.22 |
| TiO2 | 26.33 | 23.39 | 14.80 | 14.60 | 15.50 | 13.30 | 15.64 | 14.46 | 16.02 |
| MgO | 0.80 | 1.46 | 0.08 | 0.20 | 0.17 | 0.47 | 0.14 | 0.10 | 0.68 |
| Na2O | bdl | 0.04 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| BaO | 0.10 | 0.11 | bdl | bdl | bdl | bdl | bdl | 0.10 | 0.11 |
| MnO | 0.13 | 0.09 | 0.07 | 0.09 | 0.06 | 0.08 | 0.10 | 0.08 | 0.22 |
| FeO | 13.62 | 11.88 | 14.34 | 15.16 | 13.07 | 16.04 | 12.42 | 15.13 | 14.95 |
| SrO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| ZrO2 | 0.79 | 0.77 | 0.20 | 0.28 | 0.18 | 0.30 | 0.54 | 0.40 | 0.35 |
| K2O | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | bdl | bdl |
| CaO | 30.61 | 30.24 | 34.92 | 34.82 | 35.19 | 34.12 | 35.24 | 34.94 | 32.91 |
| Total | 93.86 | 95.22 | 88.26 | 88.77 | 89.35 | 88.77 | 88.25 | 88.53 | 88.39 |
| Recalculated Analyses | |||||||||
| Fe2O3 | 15.14 | 13.20 | 15.94 | 16.85 | 14.52 | 17.83 | 13.80 | 16.81 | 16.61 |
| FeO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 95.12 | 96.28 | 89.88 | 90.47 | 90.81 | 90.55 | 89.66 | 90.26 | 90.06 |
| (apfu) | Cation for 3(Ca + K + Na + Sr + Ba + Mn + Mg) | ||||||||
| Si | 1.7730 | 2.1941 | 1.7241 | 1.7392 | 1.6971 | 1.8626 | 1.6309 | 1.6883 | 1.6432 |
| Al | 0.0655 | 0.0704 | 0.0921 | 0.0920 | 0.0793 | 0.0842 | 0.0786 | 0.0788 | 0.0895 |
| Cr | 0.0222 | 0.0563 | 0.0778 | 0.0485 | 0.1753 | 0.0238 | 0.1639 | 0.0784 | 0.1442 |
| Ti | 1.7385 | 1.5175 | 0.8878 | 0.8738 | 0.9199 | 0.8039 | 0.9273 | 0.8646 | 0.9901 |
| Mg | 0.1049 | 0.1878 | 0.0097 | 0.0240 | 0.0201 | 0.0562 | 0.0159 | 0.0119 | 0.0838 |
| Na | 0.0000 | 0.0063 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Ba | 0.0035 | 0.0037 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0030 | 0.0036 |
| Mn | 0.0097 | 0.0063 | 0.0048 | 0.0060 | 0.0042 | 0.0057 | 0.0066 | 0.0052 | 0.0151 |
| Fe3+ | 0.9996 | 0.8567 | 0.9562 | 1.0085 | 0.8622 | 1.0777 | 0.8186 | 1.0056 | 1.0271 |
| Sr | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Zr | 0.0340 | 0.0325 | 0.0077 | 0.0109 | 0.0068 | 0.0118 | 0.0208 | 0.0156 | 0.0141 |
| K | 0.0011 | 0.0013 | 0.0015 | 0.0010 | 0.0009 | 0.0008 | 0.0009 | 0.0000 | 0.0000 |
| Ca | 2.8784 | 2.7942 | 2.9833 | 2.9679 | 2.9744 | 2.9373 | 2.9759 | 2.9754 | 2.8968 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Melgarejo, J.C.; Castillo-Oliver, M. Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks. Minerals 2018, 8, 51. https://doi.org/10.3390/min8020051
Xu J, Melgarejo JC, Castillo-Oliver M. Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks. Minerals. 2018; 8(2):51. https://doi.org/10.3390/min8020051
Chicago/Turabian StyleXu, Jingyao, Joan Carles Melgarejo, and Montgarri Castillo-Oliver. 2018. "Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks" Minerals 8, no. 2: 51. https://doi.org/10.3390/min8020051





