Dissolution Behaviors of Trace Muscovite during Pressure Leaching of Hydrothermal Vein Quartz Using H2SO4 and NH4Cl as Leaching Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Effect of Calcination Process on Muscovite Structure
3.2. Effect of Pressure Leaching on Muscovite
3.2.1. Effects of Leaching Conditions on Muscovite
3.2.2. Mechanism Analysis of Muscovite Dissolution
3.3. Removal Efficiency of Trace Muscovite from Vein Quartz
4. Conclusions
- (1)
- Calcination leads to structural damage of the trace muscovite around surface, edge, interior, and cleavage planes. Destroyed sites provide larger specific area and higher chemical activity so as to reduce internal diffusion resistances of leaching agents and chemical reaction resistances. Structural damage of trace muscovite are caused by high-temperature calcination, and further developed during pressure leaching of the quartz sand using H2SO4 and NH4Cl as leaching agents. The trace muscovite is dissolved and separated from quartz sand by coupling effects of calcination and fluorine-free pressure leaching.
- (2)
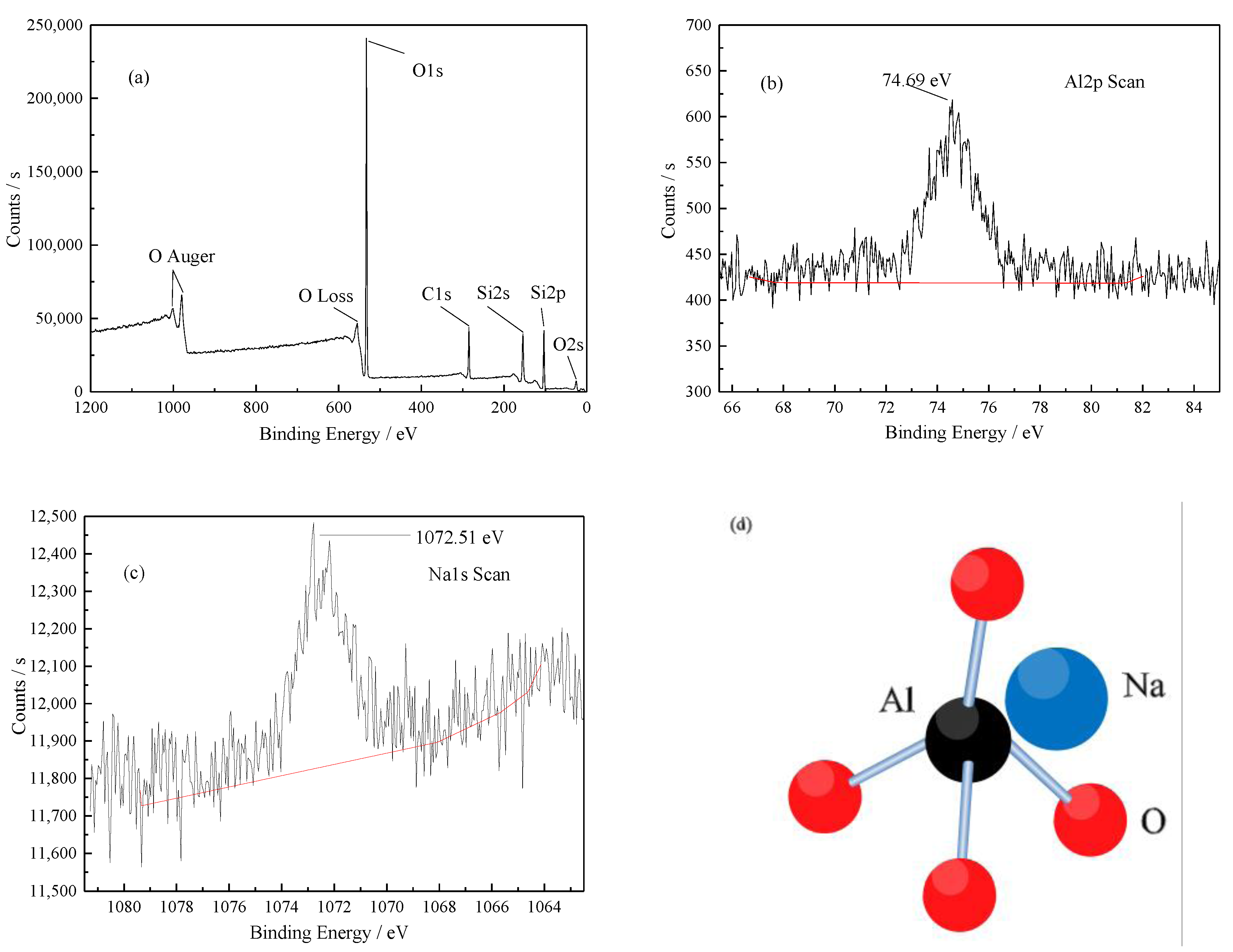
- Si and K within muscovite are preferentially leached before Al during fluorine-free pressure leaching of the hydrothermal vein quartz. S–OH in H2SO4 react with the Si–O–Al structure of calcinated muscovite so as to realize a cation exchange of H+ and Al3+. The remaining active Al2O3 is finally dissolved when Al2(SO4)3 enters into diffusion layer. The reason why the removal rate of Al is limited as 87.5% is that the remaining trace elements Al and Na replace Si in the quartz lattice.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in muscovite-quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Marques, F.O.; Burlini, L.; Burg, J.P. Microstructure and mechanical properties of halite/coarse muscovite synthetic aggregates deformed in torsion. J. Struct. Geol. 2011, 33, 624–632. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Veglió, F.; Passariello, B.; Barbaro, M.; Plescia, P.; Marabini, A.M. Drum leaching tests in iron removal from quartz using oxalic and sulphuric acids. Int. J. Miner. Process. 1998, 54, 183–200. [Google Scholar] [CrossRef]
- Iuga, A.; Cuglesan, I.; Samuila, A.; Blajan, M. Electrostatic separation of muscovite mica from feldspathic pegmatites. IEEE Trans. Ind. Appl. 2004, 40, 422–429. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; Mccarthy, S.; Grammatikopoulos, T.; Waters, K.E. An investigation into the flotation of muscovite with an amine collector and calcium lignin sulfonate depressant. Sep. Purif. Technol. 2015, 149, 216–227. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Grammatikopoulos, T.; Waters, K.E. Understanding the effect of mineralogy on muscovite flotation using QEMSCAN. Int. J. Miner. Process. 2016, 155, 6–12. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Monk, D.J.; Soane, D.S.; Howe, R.T. A review of the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications. Thin Solid Films 1993, 232, 1–12. [Google Scholar] [CrossRef]
- Knotter, D.M. Etching mechanism of vitreous silicon dioxide in HF-based solutions. J. Am. Chem. Soc. 2000, 122, 4345–4351. [Google Scholar] [CrossRef]
- Lei, S.M.; Guo, Z.H. Hazards of fluoride pollution and technical research progress of treating fluoride-containing waste water. Metal Mine 2012, 41, 152–155. [Google Scholar]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J.; Zheng, Q.S. Effects of hydration and hardening of calcium sulfate on muscovite dissolution during pressure acid leaching of black shale. J. Clean. Prod. 2017, 149, 989–998. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J. Study of the dissolution behavior of muscovite in stone coal by oxygen pressure acid leaching. Metall. Mater. Trans. B Proc. Metall. Mater. Proc. Sci. 2016, 47, 694–701. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.Y.; Lei, S.M. Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals 2017, 7, 161. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.Y.; Lei, S.M.; Liu, Y.Y.; Xia, Z.J.; Xie, F.X. Trace muscovite dissolution separation from vein quartz by elevated temperature and pressure acid leaching using sulphuric acid and ammonia chloride solutions. Physicochem. Probl. Mineral Process. 2017. [Google Scholar] [CrossRef]
- China Technical Committee for Standardization of Microbeam Analysis. GB/T 15617-2002 Methods for Quantitative Analysis of Silicate Minerals by Electron Probe; Standards Press of China: Beijing, China, 2002. [Google Scholar]
- Crist, B.V. Handbook of Monochromatic XPS Spectra, the Elements and Native Oxides; John Wiley & Sons: New York, NY, USA, 2000; pp. 20–106. [Google Scholar]
- Lin, L.; An, L.Y.; Liu, S.R. Study on extracting potassium from sericite by roasting. Ind. Miner. Process. 2012, 41, 12–15. [Google Scholar]
- He, D.S.; Li, Q.S.; Qang, X.C.; Liu, X.; Qin, F. Dissolution behavior of muscovite in mixed solution of sulfuric acid and hydrofluoric acid. Nonferrous Met. (Extr. Metall.) 2016, 12, 37–39. [Google Scholar]
- Liu, C.; Lin, J. Influence of calcination temperature on dielectric constant and structure of the micro-crystalline muscovite. China Non-Met. Min. Ind. Her. 2008, 5, 38–46. [Google Scholar]
- Lin, M.; Pei, Z.Y.; Liu, Y.Y.; Xia, Z.J.; Xiong, K.; Lei, S.M.; Wang, E.W. High-efficiency trace Na extraction from crystal quartz ore used for fused silica—A pretreatment technology. Int. J. Min. Met. Mater. 2017, 24, 1075–1086. [Google Scholar] [CrossRef]
- Lei, S.M.; Pei, Z.Y.; Zhong, L.L.; Ma, Q.L.; Huang, D.D.; Yang, Y.Y. Study on the technology and mechanism of reverse flotation and hot pressing leaching with vein quartz. Non-Met. Mines 2014, 2, 40–43. [Google Scholar]
- Ranjitham, A.M.; Khangaonkar, P.R. Leaching behaviour of calcinated magnesite with ammonium chloride solutions. Hydrometallurgy 1990, 23, 177–189. [Google Scholar] [CrossRef]
- Xu, L.J.; Qu, G.; Zhou, Z.G. A process for recovery leaching of strontium from strontium waste residues using ammonia chloride. J. Chongqin Univ. (Nat. Sci. Ed.) 2008, 31, 1174–1177. [Google Scholar]
- El-Salmawy, M.S.; Nakahiro, Y.; Wakamatsu, T. The role of alkaline earth cations in flotation separation of quartz from feldspar. Min. Eng. 1993, 6, 1231–1243. [Google Scholar] [CrossRef]
- Gotze, J. Classification, mineralogy and industrial potential of SiO2, minerals and rocks. In Quartz: Deposits, Mineralogy and Analytics, 1st ed.; Möckel, R., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Beall, G.H. Industrial applications of silica. Silica Phys. Behav. Geochem. Mater. Appl. 1994, 29, 469–505. [Google Scholar]
- Gotze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Shea, J.J. Handbook of monochromatic XPS spectra—The elements and native oxides [book review]. IEEE Electr. Insul. Mag. 2003, 19, 73. [Google Scholar] [CrossRef]
- Zang, F.F.; Lei, S.M.; Zhong, L.L.; Pei, Z.Y.; Yang, Y.Y.; Xiong, K. Purification of vein quartz by mixed acid thermal pressure leaching and it’s mechanism. China Min. Mag. 2016, 25, 106–110. [Google Scholar]
- Liu, Y.F.; Huang, Z.L.; Yang, N.; Sun, J.J. The research of preparation of ultra-pure quartz sand. Non-Met. Mines 2016, 39, 84–86. [Google Scholar]
- Lei, S.M.; Lin, M.; Pei, Z.Y.; Wang, E.W.; Zang, F.F.; Xiong, K. Occurrence and removal of mineral impurities in quartz. China Min. Mag. 2016, 25, 79–83. [Google Scholar]
- Pankrath, R.; Florke, O.W. Kinetics of Al-Si exchange in low and high quartz: Calculation of al diffusion coefficients. Eur. J. Mineral. 1994, 6, 435–457. [Google Scholar] [CrossRef]
- Botis, S.M.; Pan, Y. Theoretical calculations of [AlO4/M+]0 defects in quartz and crystal-chemical controls on the uptake of Al. Mineral. Mag. 2009, 73, 537–550. [Google Scholar] [CrossRef]

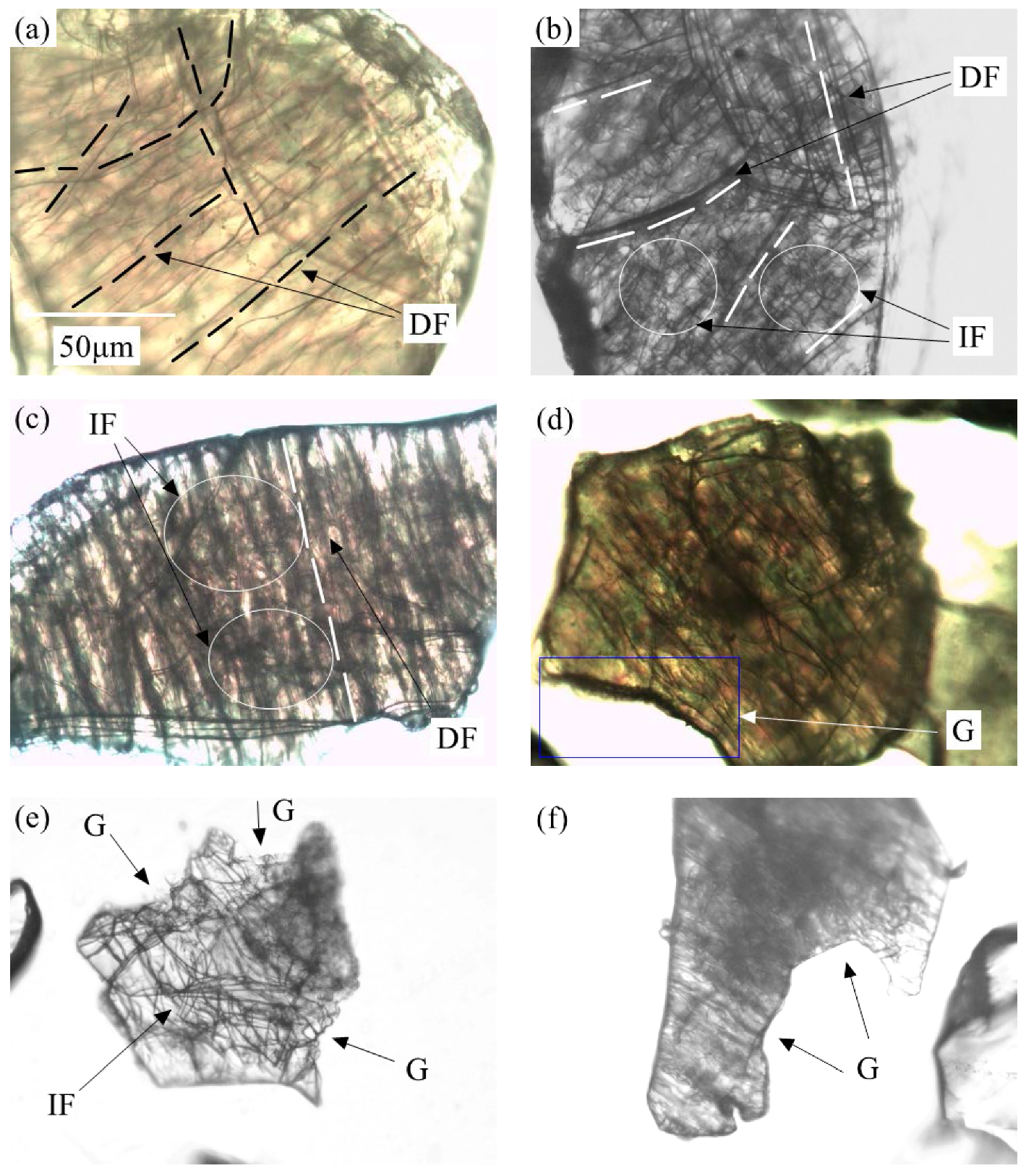
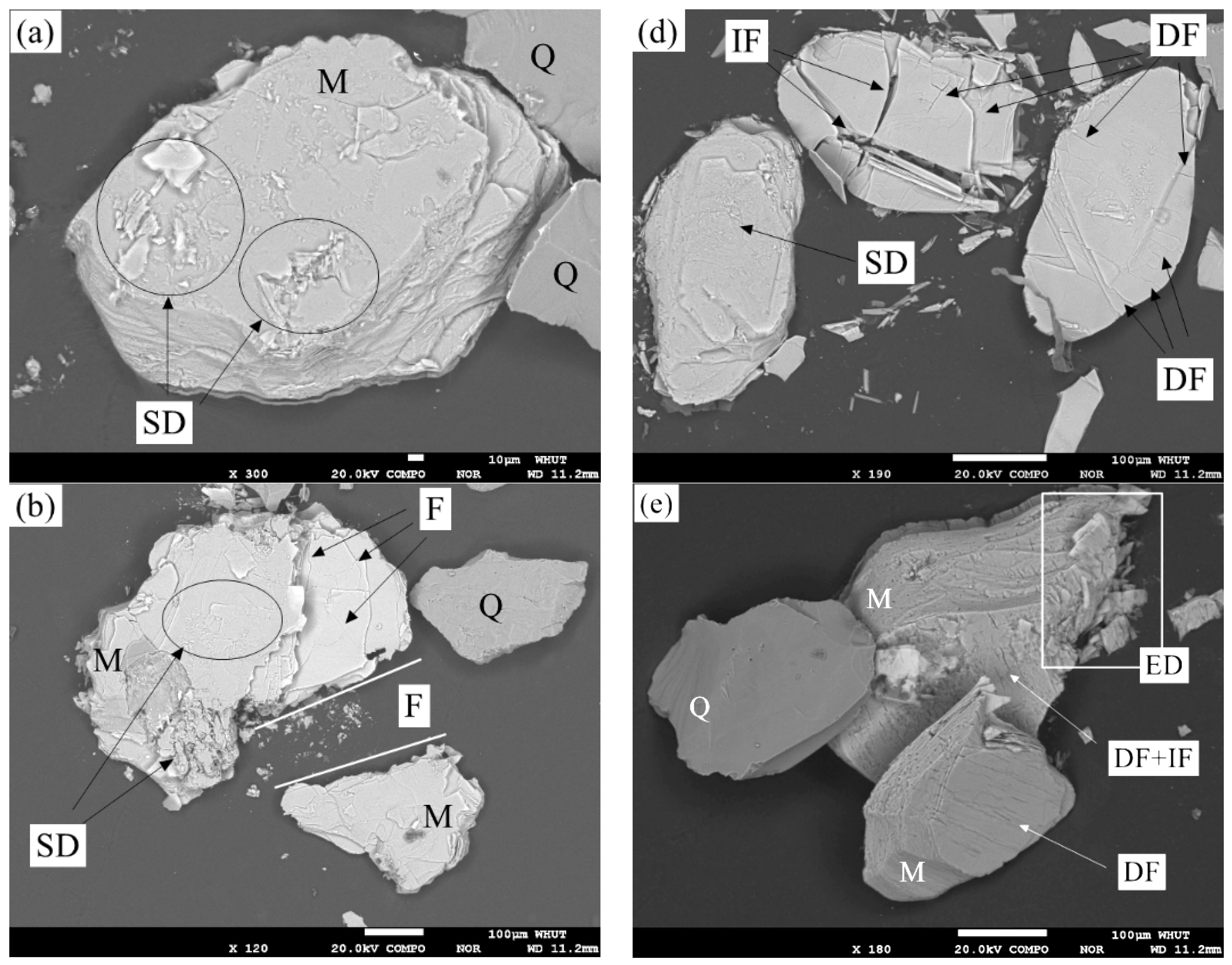
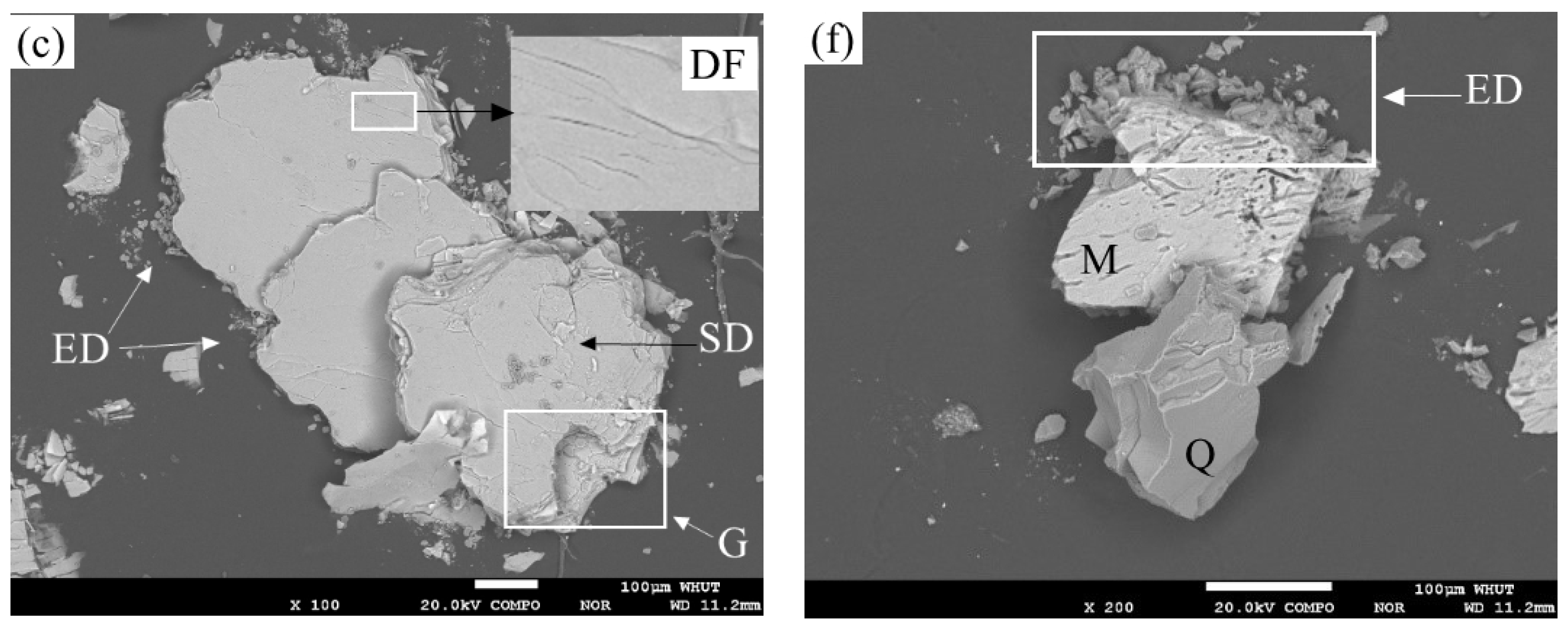
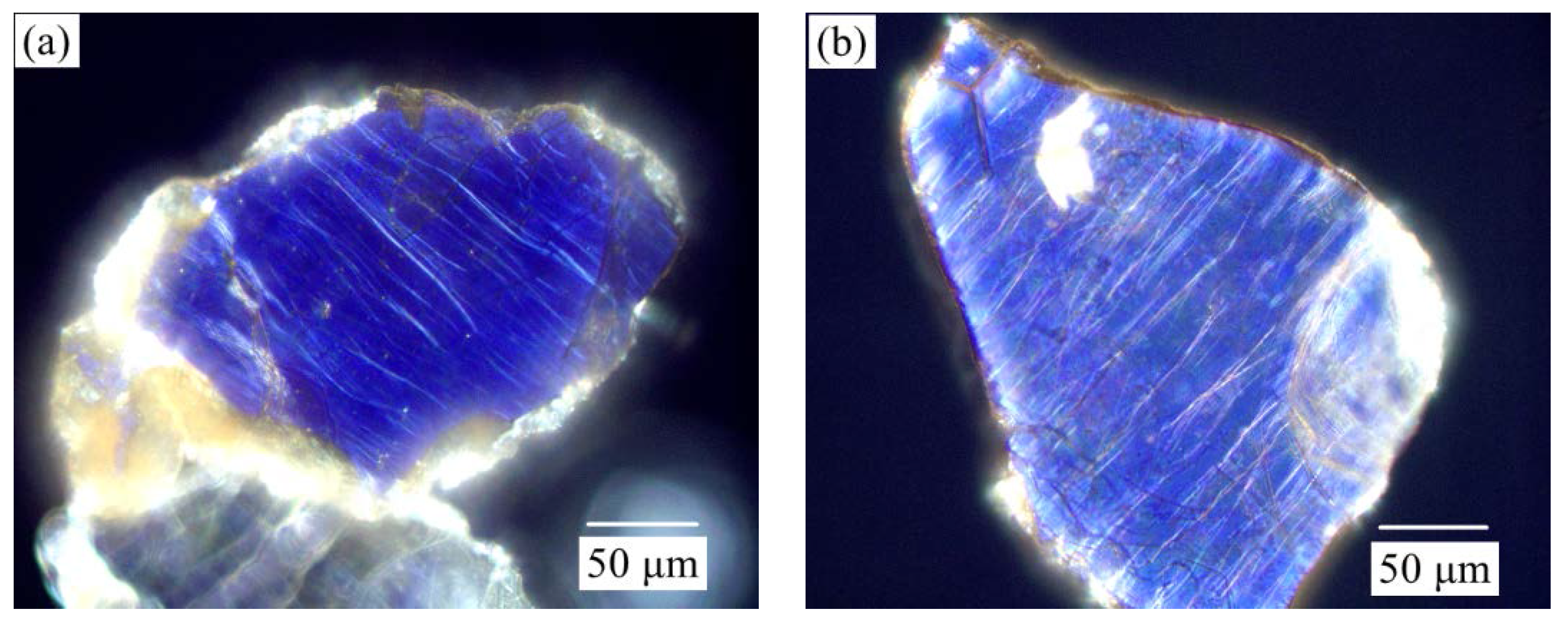




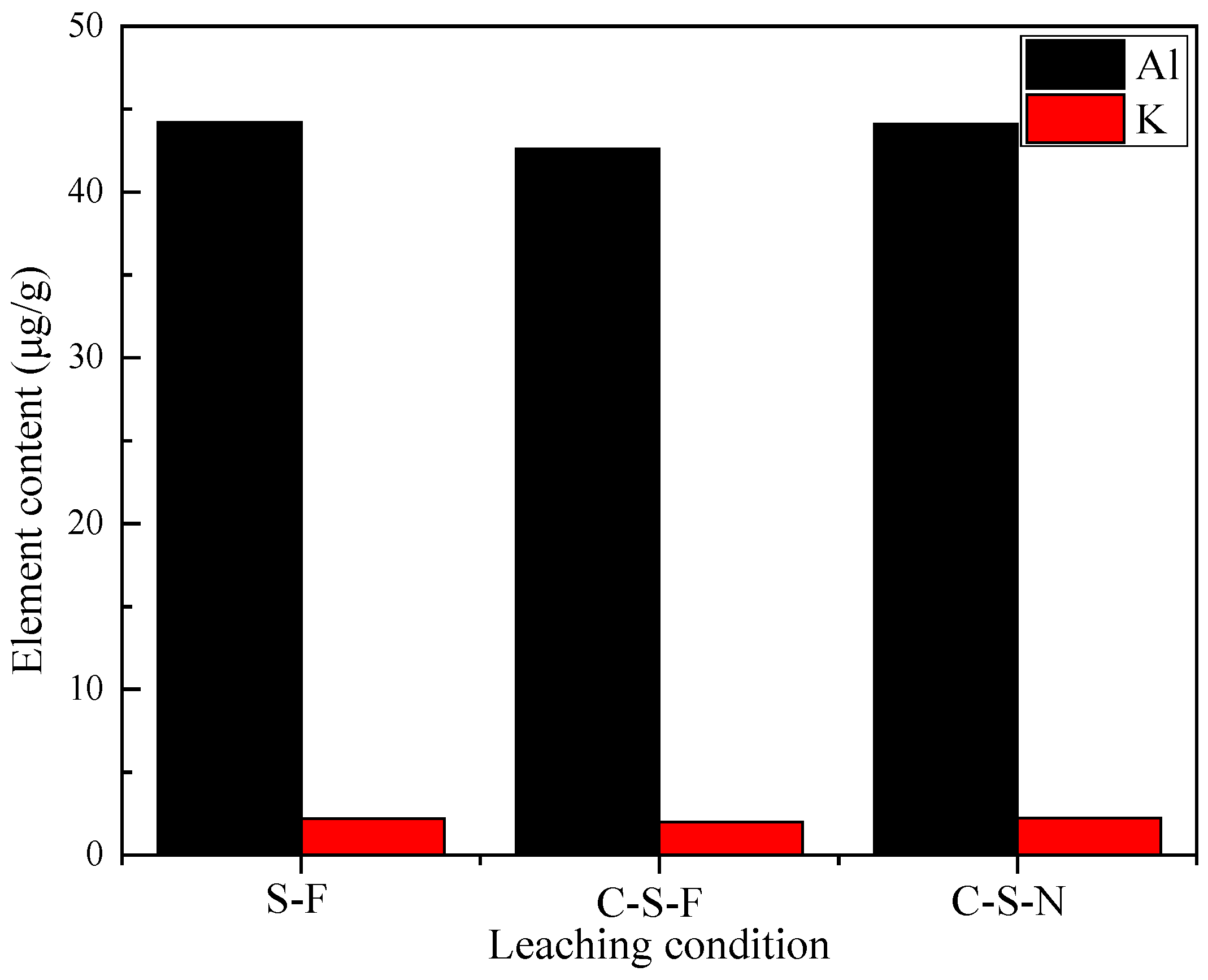
| Element | Fe | Li | Mg | Ni | Ti | Ca | K | Na | Al | Zr | Others | In Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ore | 61.2 | 2.20 | 11.8 | 1.01 | 8.34 | 8.05 | 118 | 13.5 | 353 | 6.46 | 35.1 | 619 |
| Concentrate 1 | 1.12 | 2.04 | 7.15 | - | 5.38 | 4.40 | 2.24 | 12.2 | 44.1 | 6.45 | 14.16 | 99.2 |
| Leaching Condition 1 | 0.025 mol/L H2SO4 0.050 mol/L NH4Cl | 0.050 mol/L H2SO4 0.100 mol/L NH4Cl | 0.100 mol/L H2SO4 0.200 mol/L NH4Cl |
|---|---|---|---|
| 150 °C | Figures 2a and 3a | Figures 2d and 3d | |
| 200 °C | Figures 2b and 3b | Figures 2e and 3e | Figure 4b |
| 250 °C | Figures 2c, 3c and 6 | Figures 2f and 3f |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Z.; Lin, M.; Liu, Y.; Lei, S. Dissolution Behaviors of Trace Muscovite during Pressure Leaching of Hydrothermal Vein Quartz Using H2SO4 and NH4Cl as Leaching Agents. Minerals 2018, 8, 60. https://doi.org/10.3390/min8020060
Pei Z, Lin M, Liu Y, Lei S. Dissolution Behaviors of Trace Muscovite during Pressure Leaching of Hydrothermal Vein Quartz Using H2SO4 and NH4Cl as Leaching Agents. Minerals. 2018; 8(2):60. https://doi.org/10.3390/min8020060
Chicago/Turabian StylePei, Zhenyu, Min Lin, Yuanyuan Liu, and Shaomin Lei. 2018. "Dissolution Behaviors of Trace Muscovite during Pressure Leaching of Hydrothermal Vein Quartz Using H2SO4 and NH4Cl as Leaching Agents" Minerals 8, no. 2: 60. https://doi.org/10.3390/min8020060





