Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Halloysite Purification
2.3. Characterizations
3. Results and Discussion
3.1. XRD Analysis
3.2. XRF Analysis
3.3. Zeta Potential Analysis
3.4. CEC Analysis
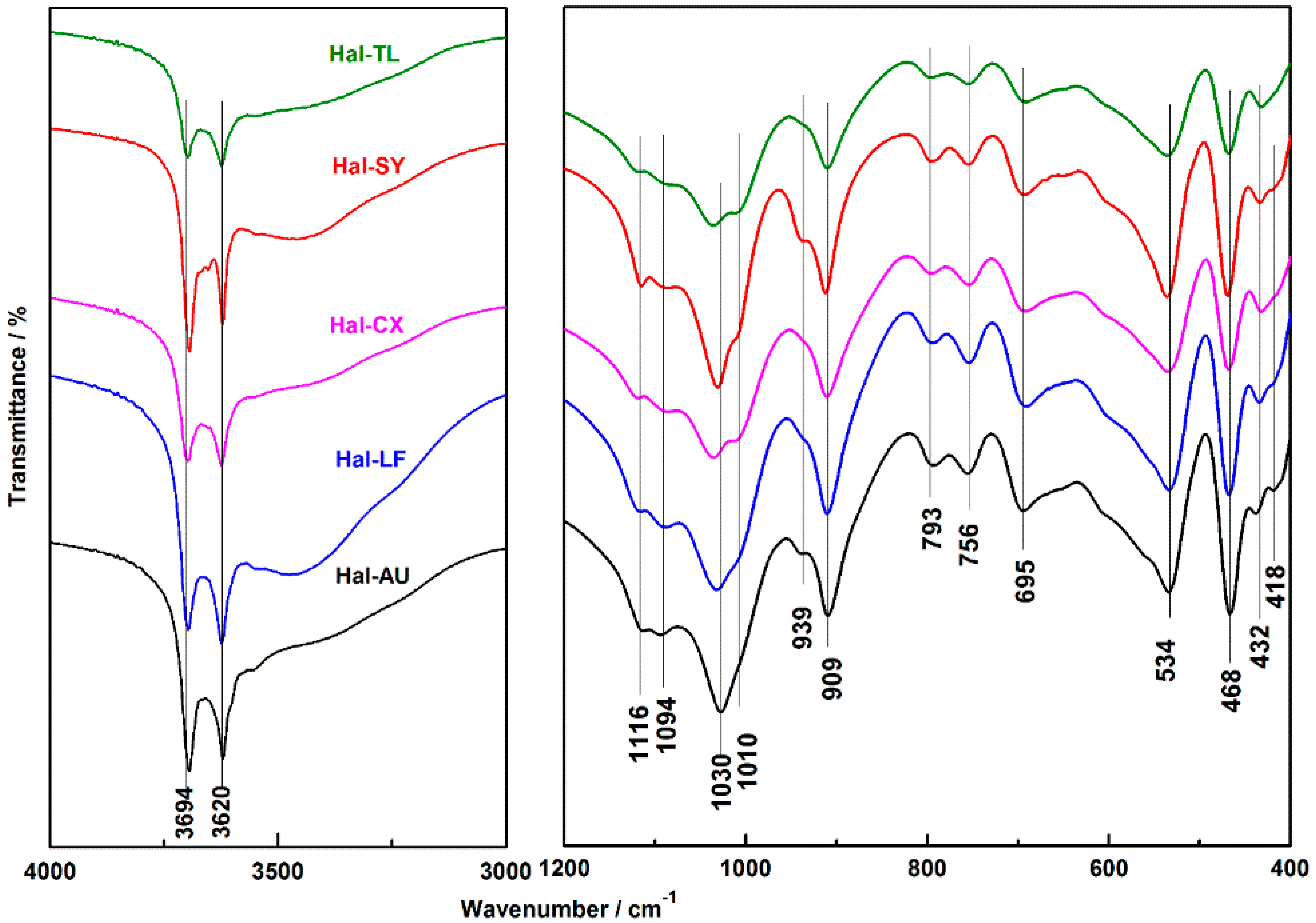
3.5. FTIR Analysis
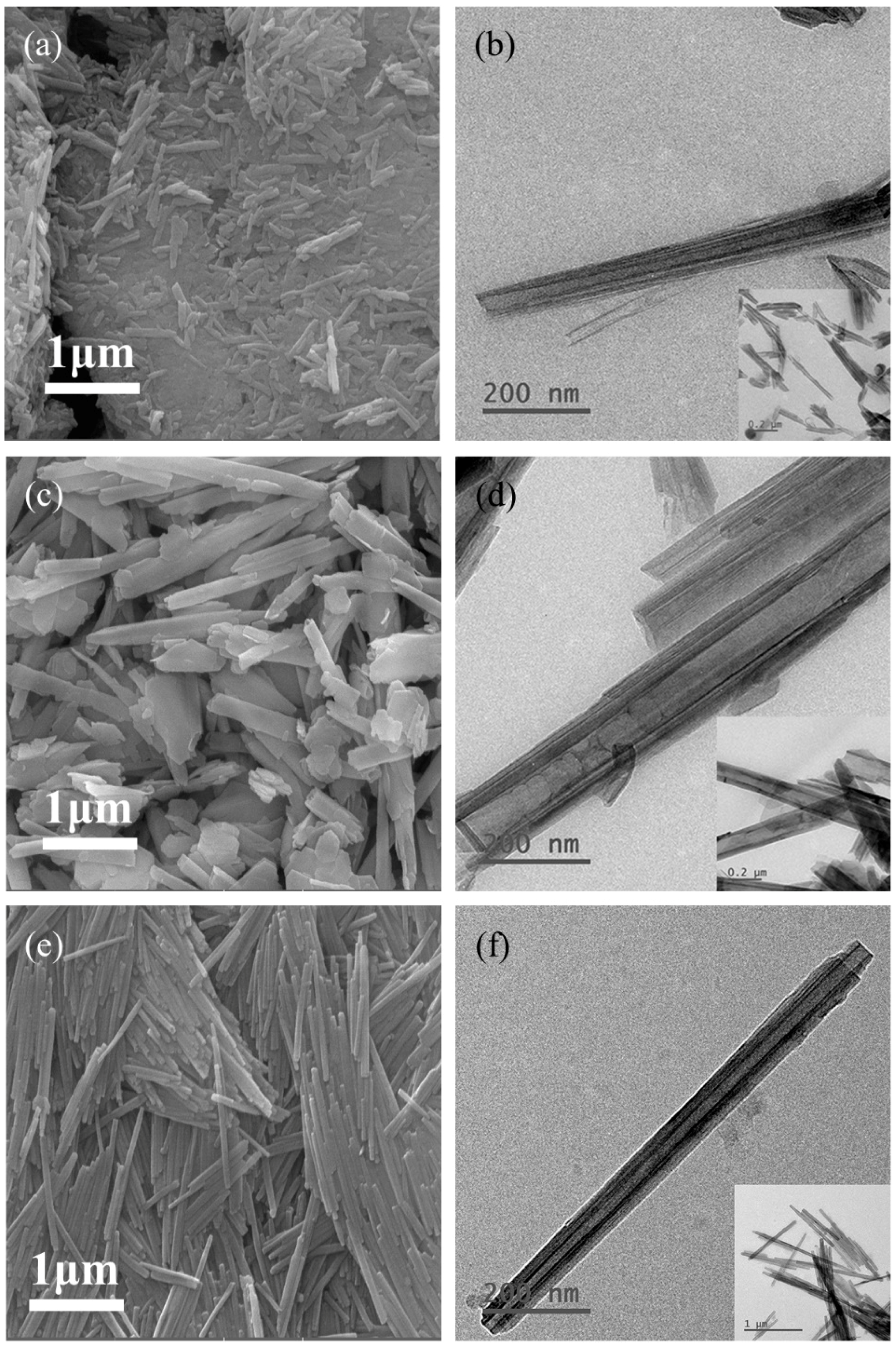
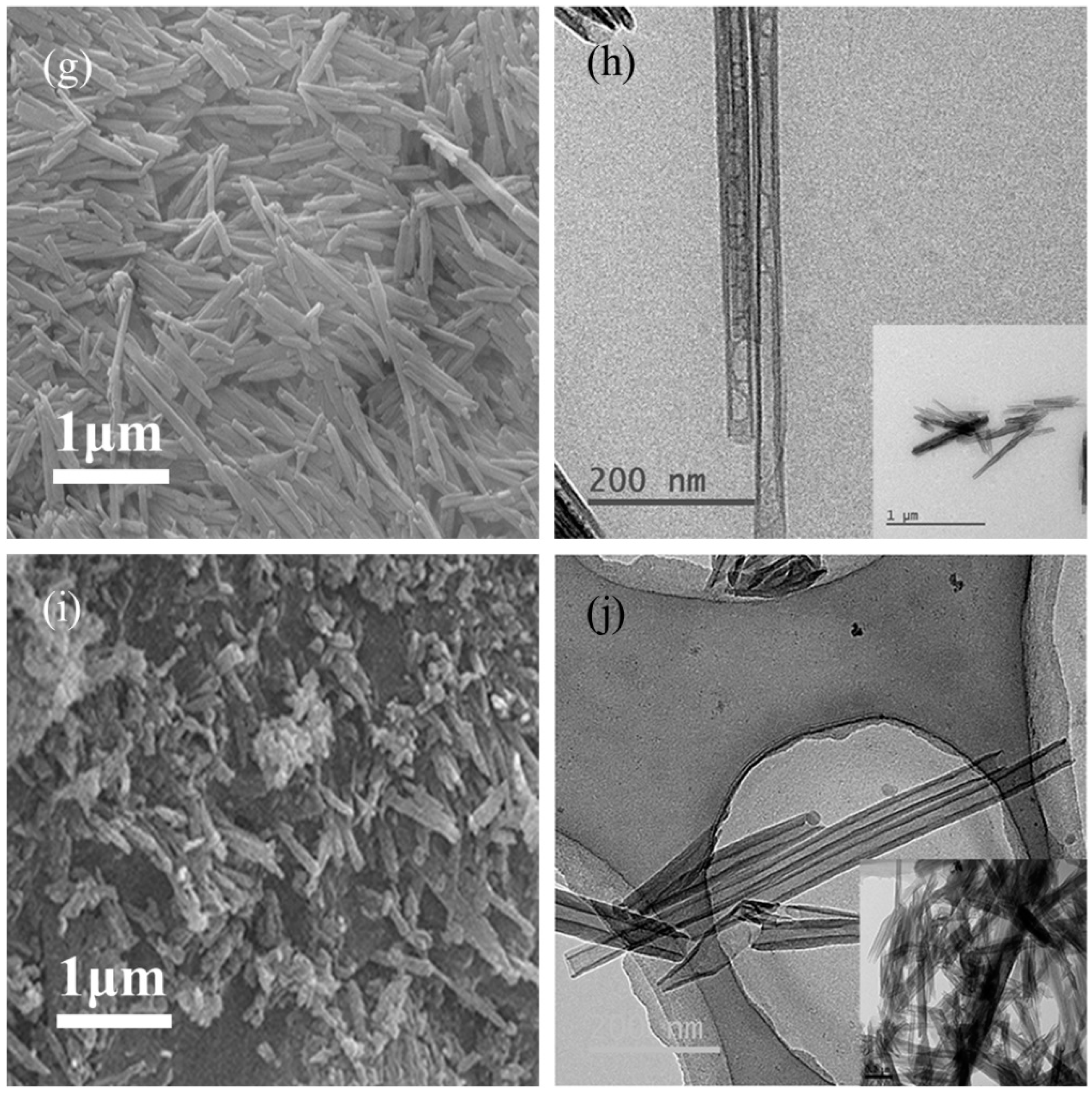
3.6. SEM and TEM Analysis
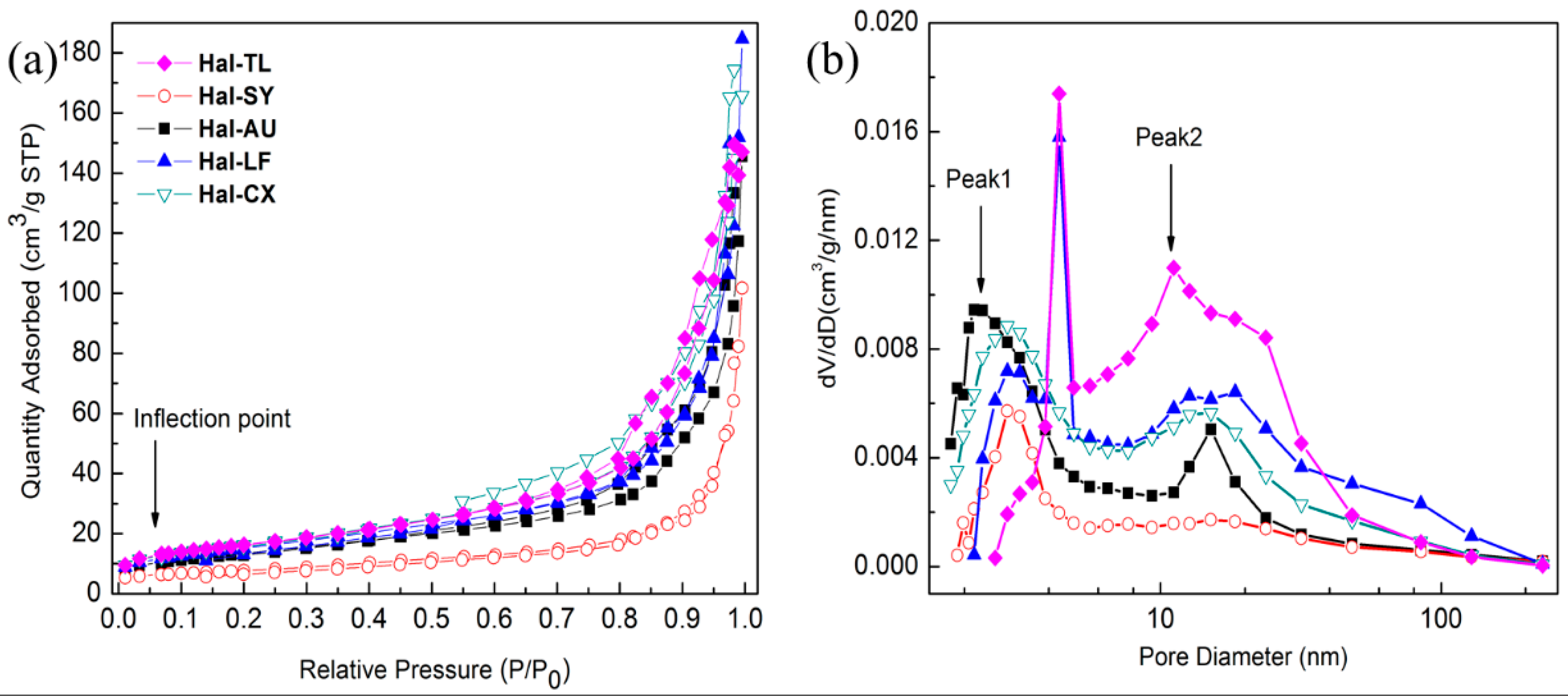
3.7. BET Surface Analysis and Porosity
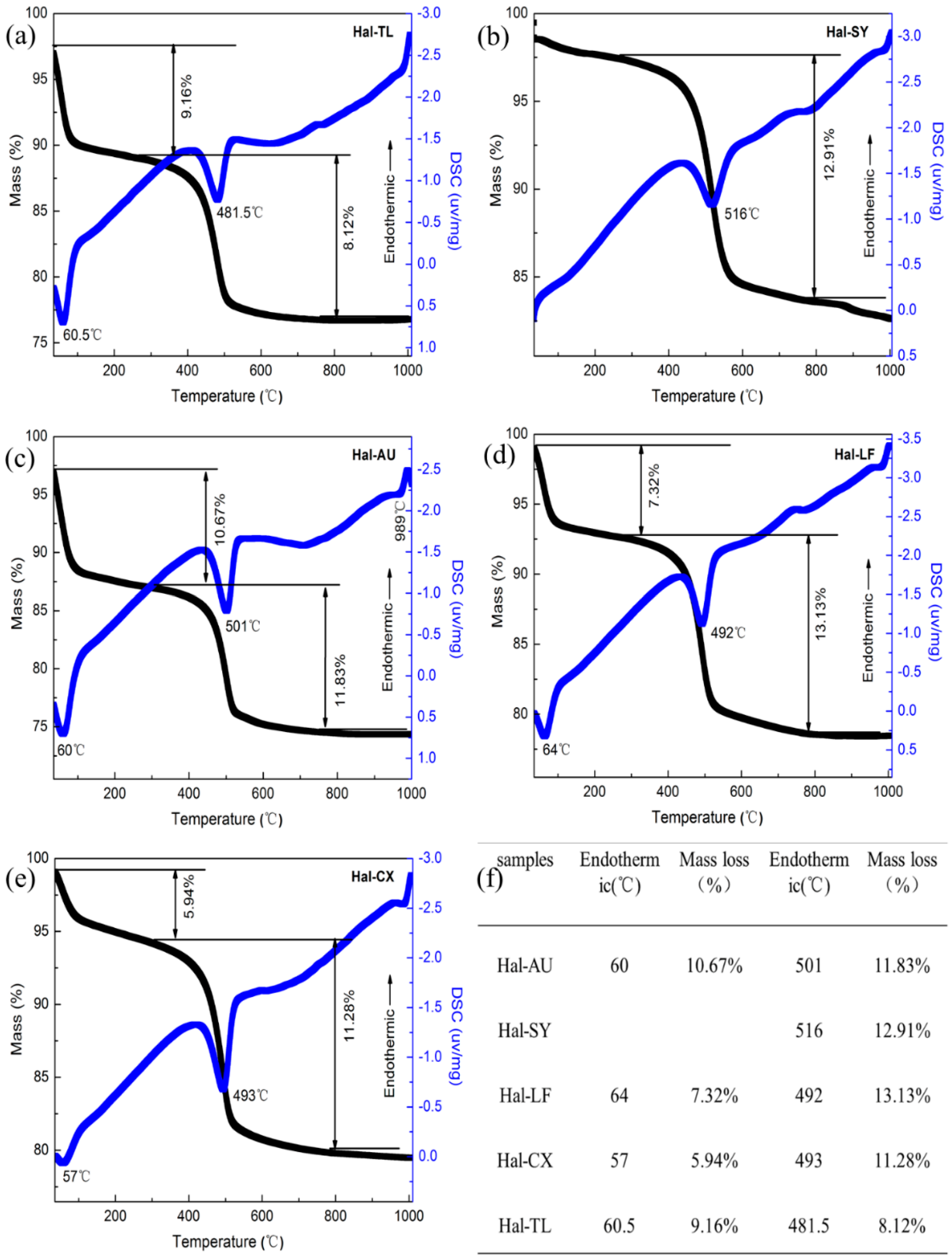
3.8. Thermogravimetric Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Chao, C.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, B.; Zhang, Y.; Xiang, X.; Chen, R. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces 2013, 5, 10559–10564. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U. On the chemistry of clay. Angew. Chem. Int. Ed. 1968, 7, 681–692. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.; Zhang, H.; Liu, J. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Churchman, G.J. The relationship between the hydrated and dehydrated states of an halloysite. Clays Clay Miner. 1972, 20, 241–246. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- García, F.J.; Sergio, G.R.; Andreas, K.; Armin, R. Study of natural halloysite from the Dragon Mine, Utah (USA). Z. Anorg. Allg. Chem. 2010, 635, 790–795. [Google Scholar] [CrossRef]
- Bobos, I. Kaolinite to halloysite-7 Å transformation in the kaolin deposit of São Vicente de Pereira, Portugal. Clays Clay Miner. 2001, 49, 596–607. [Google Scholar] [CrossRef]
- Hillier, S.; Brydson, R.; Delbos, E.; Fraser, T.; Gray, N.; Pendlowski, H.; Phillips, I.; Robertson, J.; Wilson, I. Correlations among the mineralogical and physical properties of halloysite nanotubes (HNTS). Clay Miner. 2016, 51, 325–350. [Google Scholar] [CrossRef]
- Churchman, G.J.; Pasbakhsh, P.; Lowe, D.J.; Theng, B.K.G. Unique but diverse: Some observations on the formation, structure and morphology of halloysite. Clay Miner. 2016, 51, 395–416. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent advances on surface modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar]
- Rawtani, D. Multifarious applications of halloysite nano tubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Zhang, Y.; Tang, A.; Yang, H.; Ouyang, J. Applications and interfaces of halloysite nanocomposites. Appl. Clay Sci. 2015, 119, 8–17. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Cao, B.C.; Wu, P.R.; Ma, L.; Liu, Z. Templated synthesis of graphene nanosheets within curling layered nanostructure of halloysite nanotubes. Mater. Lett. 2017, 202, 62–65. [Google Scholar] [CrossRef]
- Mo, J.; Ma, W.; Zhang, W.; Yuan, J. Structure and properties of carbon intercalated halloysite and its organosilicone hybrid film with low dielectric constant. Mater. Des. 2017, 128, 56–63. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.J.; Kim, Y.H. Improvement of interfacial adhesion of incorporated halloysite-nanotubes in fiber-reinforced epoxy-based composites. Appl. Sci. 2017, 7, 441. [Google Scholar] [CrossRef]
- Fu, L.; Yang, H.; Tang, A.; Hu, Y. Engineering a tubular mesoporous silica nanocontainer with well-preserved clay shell from natural halloysite. Nano Res. 2017, 10, 2782–2799. [Google Scholar] [CrossRef]
- Zheng, T.; Ni, X. Loading the polyol carbonization agent into clay nanotubes for the preparation of environmentally stable UV-cured epoxy materials. J. Appl. Polym. Sci. 2017, 134, 45045. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite nanotubes: Controlled access and release by smart gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Du, Y.; Ma, X. Selective fabrication of iron oxide particles in halloysite lumen. Mater. Chem. Phys. 2015, 151, 14–17. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; González, A.J.; Wang, D. Bioinspired polydopamine-induced assembly of ultrafine Fe(OH)3 nanoparticles on halloysite toward highly efficient fire retardancy of epoxy resin via an action of interfacial catalysis. Polym. Chem. 2017, 8, 3926–3936. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhao, Z.; Zhang, Y.; Yang, H. Textual properties and catalytic performances of halloysite hybrid CeO2-ZrO2 nanoparticles. J. Colloid Interface Sci. 2017, 505, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Ouyang, J.; Yang, H. Palladium nanoparticles deposited on silanized halloysite nanotubes: Synthesis, characterization and enhanced catalytic property. Sci. Rep. 2013, 3, 2948. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, Q.; Wang, H.; Yang, Y. Catalytically active silver nanoparticles loaded in the lumen of halloysite nanotubes via electrostatic interactions. J. Mater. Sci. 2017, 52, 8391–8400. [Google Scholar] [CrossRef]
- Wang, P.; Lv, A.; Hu, J.; Xu, J.A.; Lu, G. In situ synthesis of SAPO-34 grown onto fully calcined kaolin microspheres and its catalytic properties for the MTO reaction. Ind. Eng. Chem. Res. 2011, 50, 12741–12749. [Google Scholar] [CrossRef]
- Riela, S.; Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar]
- Leporatti, S. Halloysite clay nanotubes as nano-bazookas for drug delivery. Polym. Int. 2017, 66, 1111–1118. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite nanotubes supported Ag and ZnO nanoparticles with synergistically enhanced antibacterial activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Wang, X.; Weiner, M.L. Hydrogen Storage Apparatus Comprised of Halloysite. U.S. Patent 7,425,232 B2, 16 September 2008. [Google Scholar]
- Jin, J.; Zhang, Y.; Ouyang, J.; Yang, H. Halloysite nanotubes as hydrogen storage materials. Phys. Chem. Miner. 2014, 41, 323–331. [Google Scholar] [CrossRef]
- Jin, J.; Fu, L.; Yang, H.; Ouyang, J. Carbon hybridized halloysite nanotubes for high-performance hydrogen storage capacities. Sci. Rep. 2015, 5, 12429. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Lin, Y.; Pi, P. Durable underwater superoleophobic PDDA/halloysite nanotubes decorated stainless steel mesh for efficient oil-water separation. Appl. Surf. Sci. 2017, 416, 344–352. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Chowdhury, A.D.; Doong, R. New avenue for appendage of graphene quantum dots on halloysite nanotubes as anode materials for high performance supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4930–4940. [Google Scholar] [CrossRef]
- Zhang, H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017, 6, 573–581. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Burzo, E. Halloysite and Pyrosmalite Groups of Silicates; Springer: Heidelberg, Germany, 2009; pp. 235–352. [Google Scholar]
- Schroeder, P.A. Ti-bearing phases in the huber formation, an east georgia kaolin deposit. Clays Clay Miner. 2000, 48, 151–158. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H. Insight into the physicochemical aspects of kaolins with different morphologies. Appl. Clay Sci. 2013, 74, 58–65. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite Tubule Nanoreactors for Industrial and Agricultural Applications; Apple Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Levis, S.R.; Deasy, P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef]
- Alberola, J.A.; Mondragón, R.; Juliá, J.E.; Hernández, L.; Cabedo, L. Characterization of halloysite-water nanofluid for heat transfer applications. Appl. Clay Sci. 2014, 99, 54–61. [Google Scholar] [CrossRef]
- Shahriari, F.; Higashi, T.; Tamura, K. Effects of clay addition on soil protease activities in andosols in the presence of cadmium. Soil Sci. Plant Nutr. 2010, 56, 560–569. [Google Scholar] [CrossRef]
- Takahashi, T.; Dahlgren, R.A.; Theng, B.K.G.; Whitton, J.S.; Soma, M. Potassium-selective, halloysite-rich soils formed in volcanic materials from Northern California. Soil Sci. Soc. Am. J. 2001, 65, 516–526. [Google Scholar] [CrossRef]
- Bailey, S.W. Halloysite—A critical assessment. Sci. Geol. Mem. 1990, 86, 89–98. [Google Scholar]
- Newman, R.H.; Childs, C.W.; Churchman, G.J. Aluminium coordination and structural disorder in halloysite and kaolinite by 27Al NMR spectroscopy. Clay Miner. 1994, 29, 305–312. [Google Scholar] [CrossRef]
- Cheng, H.; Frost, R.L.; Yang, J.; Liu, Q.; He, J. Infrared and infrared emission spectroscopic study of typical chinese kaolinite and halloysite. Spectrochim. Acta Part A 2010, 77, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Guo, B.; Fu, L.; Yang, H.; Hu, Y.; Tang, A.; Long, H.; Jin, Y.; Chen, J.; Jiang, J. Radical guided selective loading of silver nanoparticles at interior lumen and out surface of halloysite nanotubes. Mater. Des. 2016, 110, 169–178. [Google Scholar] [CrossRef]
- Jia, Z.; Luo, Y.; Guo, B.; Yang, B.; Du, M.; Jia, D. Reinforcing and flame-retardant effects of halloysite nanotubes on LLDPE. Polym. Plast. Technol. Eng. 2009, 48, 607–613. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P.; Wilson, M.J. Tubular and spheroidal halloysite in pyroclastic deposits in the area of the Roccamonfina volcano (Southern Italy). Geoderma 2001, 99, 295–316. [Google Scholar] [CrossRef]
- Wayne, R.P.; Barnes, I.; Biggs, P.; Burrows, J.P.; Canosa-Mas, C.E.; Hjorth, J.; Bras, G.L.; Moortgat, G.K.; Perner, D.; Poulet, G. The nitrate radical: Physics, chemistry, and the atmosphere. Atmos. Environ. 1991, 25, 1–203. [Google Scholar] [CrossRef]
- Gates, W.P.; Kloprogge, J.T.; Madejová, J.; Bergaya, F. Infrared and Raman Spectroscopies of Clay Minerals; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, D.; He, H. Changes in structure, morphology, porosity, and surface activity of mesoporous halloysite nanotubes under heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Kohyama, N.; Fukushima, K.; Fukami, A. Observation of the hydrated form of tubular halloysite by an electron microscope equipped with an environmental cell. Clays Clay Miner. 1978, 26, 25–40. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, Z.; Zhang, Y.; Yang, H. High morphological stability and structural transition of halloysite (Hunan, China) in heat treatment. Appl. Clay Sci. 2014, 101, 16–22. [Google Scholar] [CrossRef]
- Zhong, X.H.; Liu, Y.; Xu, T.; Liu, W.Y. Studies on the thermal behavior and decomposition mechanism of dickite-potassium acetate complexes. J. Therm. Anal. Calorim. 2017, 129, 1095–1102. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Raghdi, A. Mechanism and kinetic parameters of the thermal decomposition of gibbsite Al(OH)3 by thermogravimetric analysis. Acta Phys. Pol. A 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Ndayiragije, S.; Delvaux, B. Coexistence of allophane, gibbsite, kaolinite and hydroxy-Al-interlayered 2:1 clay minerals in a perudic Andosol. Geoderma 2003, 117, 203–214. [Google Scholar] [CrossRef]

| Composition (%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | TiO2 | P2O5 | SO3 | Cl | CEC (cmol/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hal-SY | 41.90 | 37.67 | 0.32 | 0.06 | 1.83 | 0.18 | 0 | 0.02 | 2.60 | 1.33 | 0.01 | 40 |
| Hal-TL | 44.98 | 36.28 | 0.10 | 0.04 | 0.04 | 0.24 | 0 | 0.03 | 0.12 | 0.25 | 0.03 | 20 |
| Hal-AU | 45.03 | 35.78 | 1.01 | 0.03 | 0.01 | 0.23 | 0.07 | 0.05 | 0 | 0.16 | 0.17 | 8 |
| Hal-LF | 53.15 | 44.66 | 0.19 | 0.13 | 0.15 | 0.24 | 0.09 | 0 | 0 | 1.29 | 0.06 | 24 |
| Hal-CX | 44.33 | 37.81 | 0.20 | 0 | 0.12 | 0.02 | 0 | 0 | 0.05 | 0.30 | 0.05 | 32 |
| Suggested Assignments | Wavenumbers (cm−1) | ||||
|---|---|---|---|---|---|
| Hal-AU | Hal-SY | Hal-LF | Hal-CX | Hal-TL | |
| stretching of perpendicular surface –O–H | 3694 | 3694 | 3697 | 3697 | 3697 |
| –O–H stretching of inner hydroxyls | 3620 | 3620 | 3623 | 3623 | 3623 |
| the bending of –OH in adsorbed water | 1642 | 1649 | 1642 | 1650 | 1650 |
| (Broad band) Si–O stretching | 1027 | 1030 | 1032 | 1035 | 1035 |
| –O–H deformation of inner hydroxyls | 910 | 913 | 910 | 911 | 910 |
| Symmetric stretching of Si–O | 793 | 796 | 794 | 796 | 796 |
| the perpendicular Si–O stretching | 756 | 755 | 754 | 754 | 754 |
| the perpendicular Si–O stretching | 694 | 693 | 691 | 692 | 692 |
| the bending deformation of Al–O–Si | 534 | 536 | 534 | 535 | 535 |
| the bending deformation of Si–O–Si | 466 | 469 | 468 | 468 | 468 |
| Stretching of [SiO4] tetrahedron | 434 | 433 | 432 | 432 | 432 |
| Deformation of [SiO4] tetrahedron | 418 | 416 | 417 | - | - |
| Halloysite | Dominant Particle Shapes | Length (nm) | Inner Diam. (nm) | Outer Diam. (nm) | Pore Shape | Length/Diameter Ratio |
|---|---|---|---|---|---|---|
| TL | Short and stubby, tubular and plate-like | 200~1000 | 10~25 | 40~90 | Cylindrical | 6.79 |
| SY | Tubular and plate-like | 300~3000 | 20~150 | 50~300 | Prismatic | 8.13 |
| AU | Long and thin tubular | 400~1600 | 10~25 | 40~90 | Cylindrical | 15.31 |
| LF | Long and thin tubular | 300~1300 | 10~30 | 30~80 | Cylindrical | 10.76 |
| CX | Long tubular | 500~1500 | 10~30 | 40~60 | Cylindrical | 9.63 |
| Samples | Surface Area (m²/g) | Pore Volume (cm³/g) | Average Pore Size (nm) |
|---|---|---|---|
| Hal-SY | 27.0 | 0.2 | 22.0 |
| Hal-TL | 58.2 | 0.2 | 14.7 |
| Hal-AU | 48.7 | 0.2 | 16.4 |
| Hal-LF | 55.4 | 0.2 | 17.7 |
| Hal-CX | 58.7 | 0.3 | 16.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Mu, D.; Zhang, Y.; Yang, H. Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals. Minerals 2018, 8, 108. https://doi.org/10.3390/min8030108
Ouyang J, Mu D, Zhang Y, Yang H. Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals. Minerals. 2018; 8(3):108. https://doi.org/10.3390/min8030108
Chicago/Turabian StyleOuyang, Jing, Dawei Mu, Yi Zhang, and Huaming Yang. 2018. "Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals" Minerals 8, no. 3: 108. https://doi.org/10.3390/min8030108





