Near-Field Optical Examination of Potassium n-Butyl Xanthate/Chalcopyrite Flotation Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. ATR FT-IR Measurements
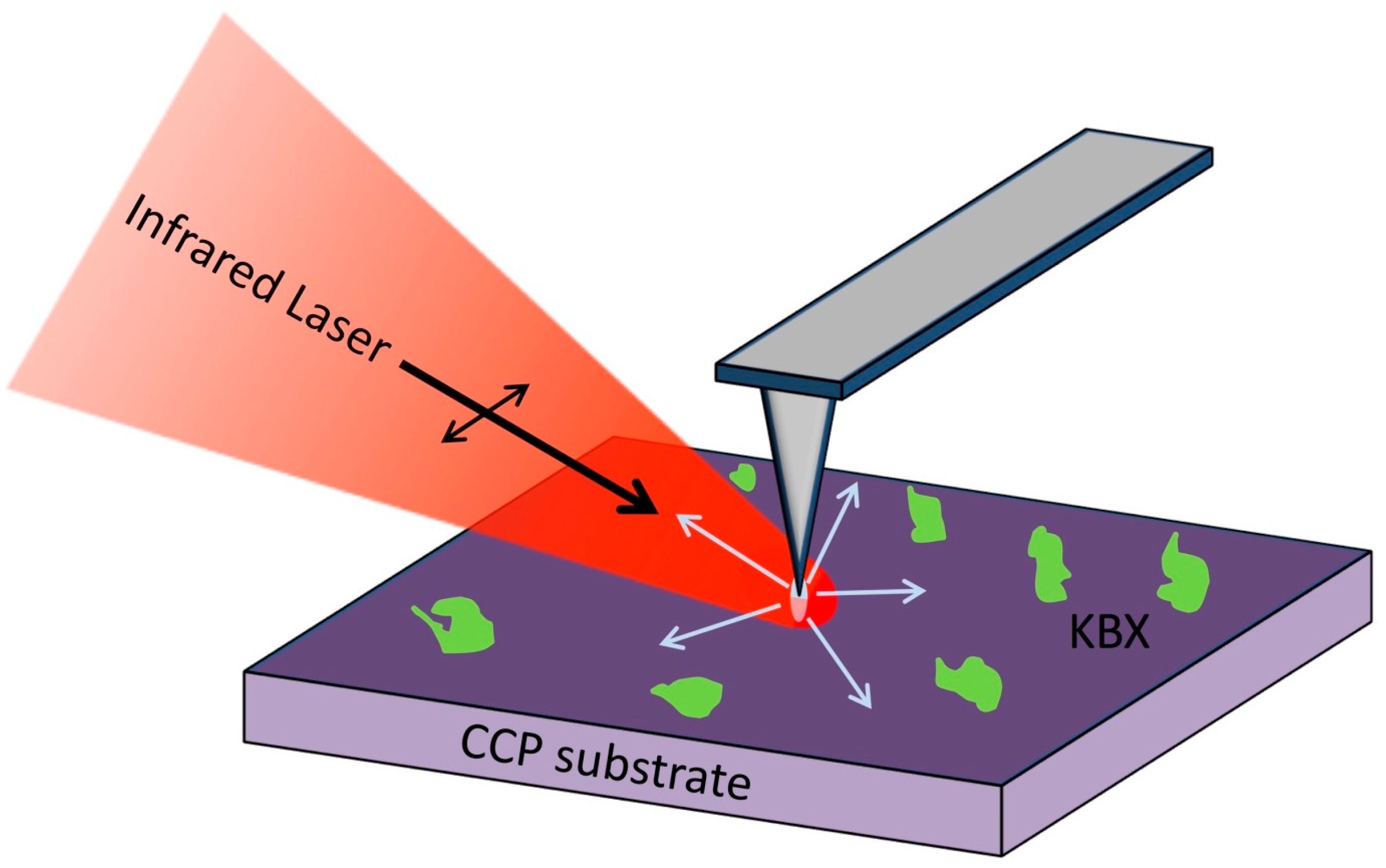
2.3. Near-Field Microscopy Setup
3. Results
3.1. ATR FT-IR Measurements
3.2. s-SNIM of the CCPX-2b Sample Applying the Free-Electron Laser
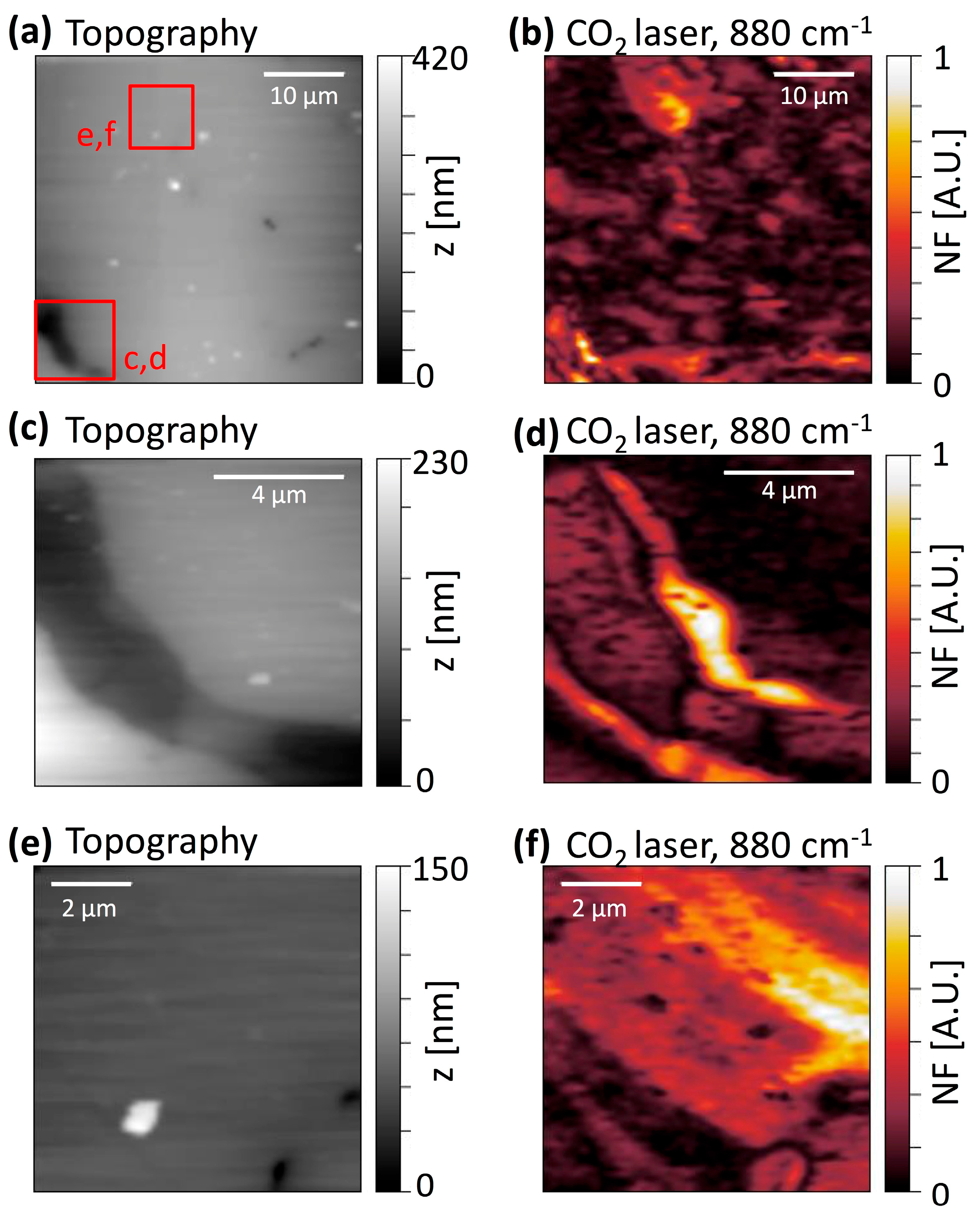
3.3. s-SNIM of CCPX-3b and CCPX-4b Samples with the CO2-Laser
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leppinen, J.O.; Basilio, C.I.; Yoon, R.H. In-situ FTIR study of ethyl xanthate on sulfide minerals under controlled potential. Int. J. Miner. Process. 1989, 26, 259–274. [Google Scholar] [CrossRef]
- Chernyshova, I.V. Anodic processes on a galena (PbS) electrode in the presence of n-butyl xanthate studied FTIR-spectroelectrochemically. J. Phys. Chem. B 2001, 105, 8185–8191. [Google Scholar] [CrossRef]
- Chernyshova, I.V. In situ FTIR−spectroelectrochemical study of the anodic processes on a galena (PbS) electrode under open-air conditions in the absence and presence of n-butyl xanthate. Langmuir 2002, 18, 6962–6968. [Google Scholar] [CrossRef]
- Fredriksson, A.; Homgren, A.; Forsling, W. Kinetics of collector adsorption on mineral surfaces. Miner. Eng. 2006, 19, 784–789. [Google Scholar] [CrossRef]
- Fredriksson, A.; Hellström, P.; Öberg, S.; Holmgren, A. Comparison between in situ total internal reflection vibrational spectroscopy of an adsorbed collector and spectra calculated by ab initio density functional theory methods. J. Phys. Chem. C 2007, 111, 9299–9304. [Google Scholar] [CrossRef]
- Fredriksson, A.; Holmgren, A. An in-situ ATR-FTIR investigation of adsorption and orientation of heptyl xanthate at the lead sulphide/aqueous solution interface. Miner. Eng. 2008, 12, 1000–1004. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Z.; Sun, C. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces. J. Mol. Struct. 2013, 1048, 434–440. [Google Scholar] [CrossRef]
- Pillai, K.C.; Young, V.Y.; Bockris, J.O.M. X-ray photoelectron spectroscopy studies of xanthate adsorption on pyrite mineral surfaces. J. Colloid Interface Sci. 1985, 103, 145–153. [Google Scholar] [CrossRef]
- Page, P.W.; Hazell, L.B. X-ray photoelectron spectroscopy (XPS) studies of potassium amyl xanthate (KAX) adsorption on precipitated PbS related to galena flotation. Int. J. Miner. Process. 1989, 25, 97–100. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. X-ray photoelectron spectroscopic and electrochemical studies of the interaction of xanthate with galena in relation to the mechanism proposed by Page and Hazell. Int. J. Miner. Process. 1990, 28, 301–311. [Google Scholar] [CrossRef]
- Laajalehto, K.; Nowak, P.; Pomianowski, A.; Suoninen, E. Xanthate adsorption at PbS/aqueous interfaces: Comparison of XPS, infrared and electrochemical results. Colloids Surf. 1991, 57, 319–333. [Google Scholar] [CrossRef]
- Laajalehto, K.; Nowak, P.; Suoninen, E. On the XPS and IR identification of the products of xanthate sorption at the surface of galena. Int. J. Miner. Process. 1993, 37, 123–147. [Google Scholar] [CrossRef]
- Laajehto, K.; Smart, R.S.C.; Ralston, J.; Suoninen, E. STM and XPS investigation of reaction of galena in air. Appl. Surf. Sci. 1993, 64, 29–39. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Kravets, I.M.; Buckley, A.N.; Woods, R. Submonolayer adsorption of alkyl xanthates on galena. Int. J. Miner. Process. 1994, 41, 99–114. [Google Scholar] [CrossRef]
- Buckley, A.N.; Goh, S.W.; Lamb, R.N.; Woods, R. Interaction of thiol collectors with pre-oxidised sulfide minerals. Int. J. Miner. Process. 1994, 72, 163–174. [Google Scholar] [CrossRef]
- Szargan, R.; Uhlig, I.; Wittstock, G.; Roßbach, P. New methods in flotation research—Application of synchrotron radiation to investigation of adsorbates on modified galena surfaces. Int. J. Miner. Process. 1997, 51, 151–161. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Lamb, R.N.; Fan, L.J.; Yang, Y. XPS, Static SIMS and NEXAFS spectroscopic investigation of thiol adsorption on metals and metal sulfides. ECS Trans. 2006, 2, 107–119. [Google Scholar] [CrossRef]
- O`Dea, A.R.; Prince, K.E.; Smart, R.S.C.; Gerson, A.R. Secondary ion mass spectrometry investigation of the interaction of xanthate with galena. Int. J. Miner. Process. 2001, 61, 121–143. [Google Scholar] [CrossRef]
- Piantadosi, C.; Smart, R.S.C. Statistical comparison of hydrophobic species on galena and pyrite particles in flotation concentrates and tails from TOF-SIMS evidence. Int. J. Miner. Process. 2002, 64, 43–54. [Google Scholar] [CrossRef]
- Goh, W.S.; Buckley, A.N.; Lamb, R.N.; Woods, R. The ability of static ion mass spectrometry to discriminate submonolayer from multilayer adsorption of thiol collectors. Miner. Eng. 2006, 19, 571–581. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B. TOF-SIMS studies of surface chemistry of minerals subjected to flotation separation—A review. Miner. Eng. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Beaussart, A.; Parkinson, L.; Mierczynska-Vasilev, A.; Ralston, J.; Beattie, D.A. Effect of adsorbed polymers on bubble-particle attachement. Langmuir 2009, 25, 13290–13294. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Beattie, D.A. In situ atomic force microscopy of modified dextrin adsorption on hydrophobic and hydrophilic layered silicate minerals. J. Colloid Interface Sci. 2010, 344, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of tailored carboxymethyl cellulose polymers on talc and chalcopyrite: Correlation between coverage, wettability, and flotation. Miner. Eng. 2010, 23, 11–13. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. An Atomic Force Microscopy Study of the Adsorption of Collectors on Chalcopyrite. In Microscopy: Advances in Scientific Research and Education; Formatex Research Center: Badajoz, Spain, 2014; Volume 2, pp. 967–973. ISBN 978-84-942134-4-1. [Google Scholar]
- Sedeva, I.G.; Fetzer, R.; Fornasiero, D.; Ralston, J.; Beattie, D.A. Adsorption of modified dextrins to a hydrophobic surface: QCM-D studies, AFM imaging, and dynamic contact angle measurements. J. Colloid Interface Sci. 2010, 345, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Knoll, B.; Keilmann, F. Enhanced dielectric contrast in scattering-type scanning near-field optical microscopy. Opt. Commun. 2000, 182, 321–328. [Google Scholar] [CrossRef]
- Hillenbrand, R.; Knoll, B.; Keilmann, F. Pure optical contrast in scattering-type scanning near-field microscopy. J. Microsc. 2001, 202, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.; Taubner, T.; Hillenbrand, R.; Keilmann, F. Infrared spectroscopic mapping of single nanoparticles and viruses at nanoscale resolution. Nano Lett. 2006, 6, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, R.; Keilmann, F. Material-specific mapping of metal/semiconductor/dielectric nanosystems at 10 nm resolution by backscattering near field optical microscopy. Appl. Phys. Lett. 2002, 80, 25–27. [Google Scholar] [CrossRef]
- Moon, K.; Park, H.; Kim, J.; Do, Y.; Lee, S.; Lee, G.; Kang, H.; Han, H. Subsurfae Nanoimaging by Broadband Terahertz Pulse Near-Field Microscopy. Nano Lett. 2015, 15, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kuschewski, F.; von Ribbeck, H.-G.; Döring, J.; Winnerl, S.; Eng, L.M.; Kehr, S.C. Narrow-band near-field nanoscopy in the spectral range from 1.3 to 8.5 THz. Appl. Phys. Lett. 2016, 108, 113102. [Google Scholar] [CrossRef]
- Stinson, H.T.; Sternbach, A.; Najera, O.; Jing, R.; Mcleod, A.S.; Slusar, T.V.; Mueller, A.; Anderegg, L.; Kim, H.T.; Rozenberg, M. Imaging the nanoscale phase separation in vanadium dioxide thin films at terahertz frequencies. Arxiv, 2017; arXiv:1711.05242. [Google Scholar]
- Hillenbrand, R.; Taubner, T.; Keilmann, F. Phonon-enhanced light–matter interaction at the nanometre scale. Nature 2002, 418, 159. [Google Scholar] [CrossRef] [PubMed]
- Döring, J.; von Ribbeck, H.-G.; Fehrenbacher, M.; Kehr, S.C.; Eng, L.M. Near-field resonance shifts of ferroelectric barium titanate domains upon low-temperature phase transition. Appl. Phys. Lett. 2014, 105, 053109. [Google Scholar] [CrossRef]
- Kehr, S.C.; Cebula, M.; Mieth, O.; Härtling, T.; Seidel, J.; Grafström, S.; Eng, L.M.; Winnerl, S.; Stehr, D.; Helm, M. Anisotropy contrast in phonon-enhanced apertureless near-field microscopy using a free-electron laser. Phys. Rev. Lett. 2008, 100, 256403. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Winnerl, S.; Schneider, H.; Helm, M.; Wenzel, M.T.; Ribbeck, H.-G.; Eng, L.M.; Kehr, S.C. Quantitative determination of the charge carrier concentration of ion implanted silicon by IR-near-field spectroscopy. Opt. Express 2010, 18, 26206. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.J.; Keilmann, F.; Wittborn, J.; Aizpurua, J.; Hillenbrand, R. Terahertz Near-Field Nanoscopy of Mobile Carriers in Single Semiconductor Nanodevices. Nano Lett. 2008, 8, 3766–3770. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.J.; Ziegler, A.; Köck, T.; Hillenbrand, R. Infrared spectroscopy of strained semiconductors. Nat. Nanotechnol. 2009, 4, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Pollard, B.; Muller, E.A.; Hinrichs, K.; Raschke, M.B. Vibrational nano-spectroscopic imaging correlating structure with intermolecular coupling and dynamics. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, M.P.; Kehr, S.C.; Park, T.H.; Milde, P.; Zerwerck, U.; Loppacher, C.; Eng, L.M.; Therien, M.J.; Engheta, N.; Bonnell, D. Probing polarization and dielectric function of molecules with higher order harmonics in scattering-near-field scanning optical microscopy. J. Appl. Phys. 2009, 106, 114307. [Google Scholar] [CrossRef]
- Kehr, S.C.; Liu, Y.M.; Martin, L.W.; Yu, P.; Yang, C.-H.; Wenzel, M.T.; Jacob, R.; von Ribbeck, H.-G.; Helm, M.; Zhang, X. Near-field examination of perovskite-based superlenses and superlens-enhanced probe-object coupling. Nat. Commun. 2011, 2, 249. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, M.; Winnerl, S.; Schneider, H.; Döring, J.; Kehr, S.C.; Eng, L.M.; Huo, Y.; Schmidt, O.G.; Yao, K.; Liu, Y.; et al. Plasmonic Superlensing in Doped GaAs. Nano Lett. 2015, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Nguyen, T.A.H.; Taran, E.; Mahler, S.; Nguyen, A.V. Differences in adhesion of A. thiooxidans and A. ferrooxidans on chalcopyrite as revealed by atomic force microscopy with bacterial probes. Miner. Eng. 2014, 64, 9–15. [Google Scholar] [CrossRef]
- Babel, B.; Rudolph, M. Characterizing mineral wettabilities on a microscale by colloidal probe atomic force microscopy. Miner. Eng. 2018, in press. [Google Scholar]
- Fei, Z.; Rodin, A.S.; Andreev, G.O.; Bao, W.; McLeod, A.S.; Wagner, M.; Zhang, L.M.; Zhao, Z.; Thiemens, M.; Dominguez, G.; Fogler, M.M. Gate-tuning of graphene plasmons revealed by infrared nano-imaging. Nature 2012, 487, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, G.; Bachelot, R.; Royer, P. A reflection-mode apertureless scanning near-field optical microscope developed from a commercial scanning probe microscope. Rev. Sci. Instrum. 1998, 69, 1735–1743. [Google Scholar] [CrossRef]
- Esslinger, M.; Dorfmüller, J.; Khunsin, E.; Vogelgesang, R.; Kern, K. Background-free imaging of plasmonic structures with cross-polarized apertureless scanning near-field optical microscopy. Rev. Sci. Instrum. 2012, 83, 033704. [Google Scholar] [CrossRef] [PubMed]
- Little, L.H.; Poling, G.W.; Leja, J. Infrared Spectra of Xanthate Compounds, II. Assignment of Vibrational Frequencies. Can. J. Chem. 1961, 39, 745–754. [Google Scholar] [CrossRef]
- Basařová, P.; Bartovská, L.; Kořínek, K.; Horn, D. The influence of flotation agent concentration on the wettability and flotability of polystyrene. J. Colloid Interface Sci. 2005, 286, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Feng, B.; Wong, C.; Miao, J.; Ma, B.; Zhou, H. The critical importance of pulp concentration on the flotation of galena from a low grade lead-zinc ore. J. Mater. Res. Technol. 2016, 5, 131–135. [Google Scholar] [CrossRef]
- Kursun, H. The influence of frother types and concentrations on fine particles’ entrainment using column flotation. Sep. Sci. Technol. 2016, 52, 722–731. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Cases, J.M.; Alnot, M.; Ehrhardt, J.J. XPS Characterization of Chalcopyrite, Tetrahedrite, and Tennantite Surface Products after Different Conditioning. 1. Aqueous Solution at pH 10. Langmuir 1996, 12, 2519–2530. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Asanov, I.P.; Okotrub, A.V.; Vamek, V.A.; Vyalikh, D.V. Spectroscopic and electrochemical characterization of the surface layers of chalcopyrite (CuFeS2) reacted in acidic solutions. Appl. Surface Sci. 2004, 225, 395–409. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Karacharov, A.A.; Likhatski, M.N. Effect of adsorption of butyl xanthate on galena, PbS, and HOPG surfaces as studied by atomic force microscopy and spectroscopy and XPS. Int. J. Miner. Process. 2015, 144, 81–89. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firkala, T.; Kuschewski, F.; Nörenberg, T.; Klopf, J.M.; Pashkin, A.; Foerstendorf, H.; Rudolph, M.; Kehr, S.C.; Eng, L.M. Near-Field Optical Examination of Potassium n-Butyl Xanthate/Chalcopyrite Flotation Products. Minerals 2018, 8, 118. https://doi.org/10.3390/min8030118
Firkala T, Kuschewski F, Nörenberg T, Klopf JM, Pashkin A, Foerstendorf H, Rudolph M, Kehr SC, Eng LM. Near-Field Optical Examination of Potassium n-Butyl Xanthate/Chalcopyrite Flotation Products. Minerals. 2018; 8(3):118. https://doi.org/10.3390/min8030118
Chicago/Turabian StyleFirkala, Tamás, Frederik Kuschewski, Tobias Nörenberg, J. Michael Klopf, Alexej Pashkin, Harald Foerstendorf, Martin Rudolph, Susanne C. Kehr, and Lukas M. Eng. 2018. "Near-Field Optical Examination of Potassium n-Butyl Xanthate/Chalcopyrite Flotation Products" Minerals 8, no. 3: 118. https://doi.org/10.3390/min8030118




