Size-Controlled Production of Gold Bionanoparticles Using the Extremely Acidophilic Fe(III)-Reducing Bacterium, Acidocella aromatica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Bioreduction of Au(III) by Ac. aromatica Cell-Suspensions
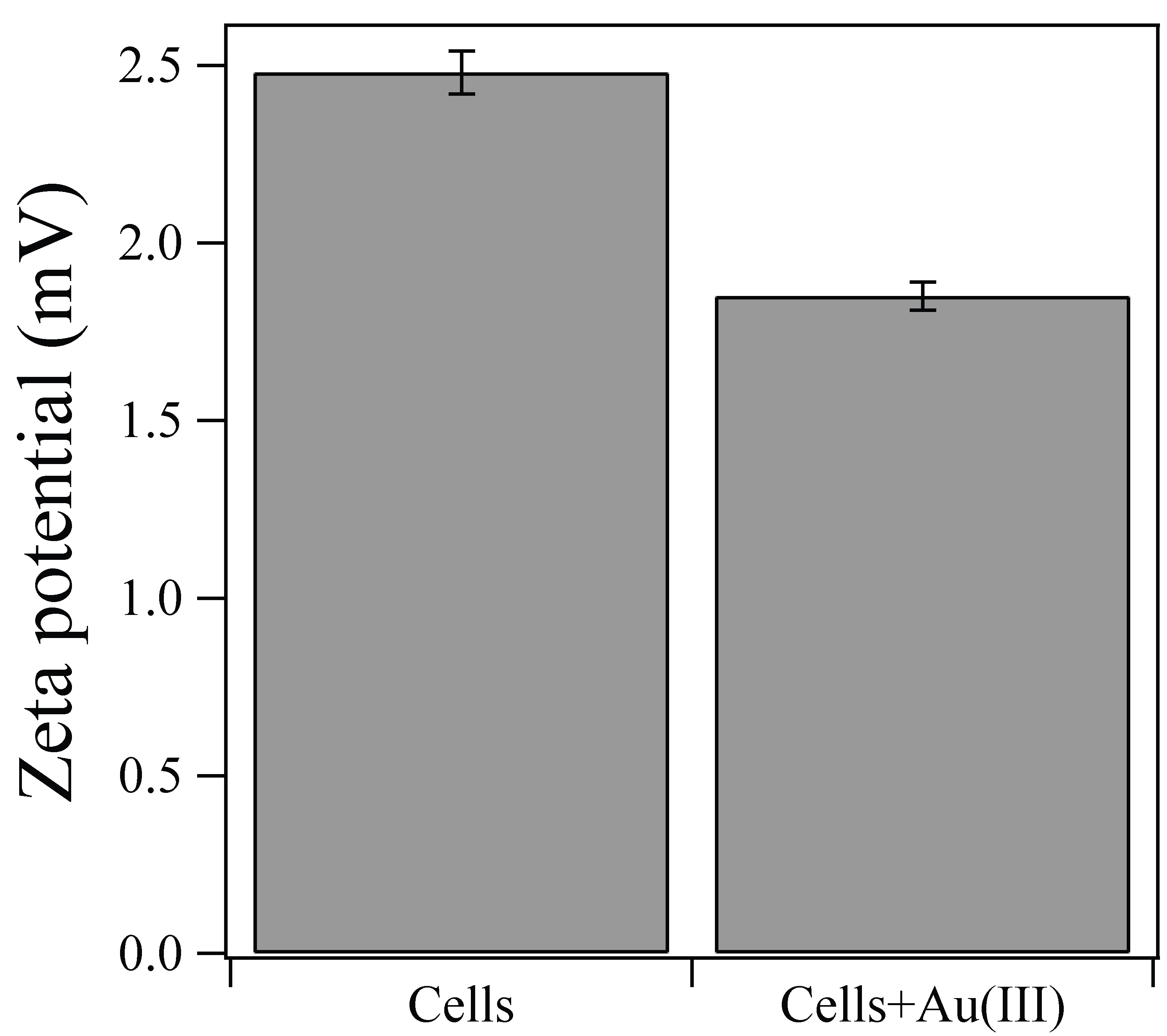
2.3. Zeta-Potential Measurement
2.4. Ultra-Thin Section Transmission Electron Microscopy (TEM) Observation
2.5. Particle Size Analysis Using Image-J
3. Results and Discussion
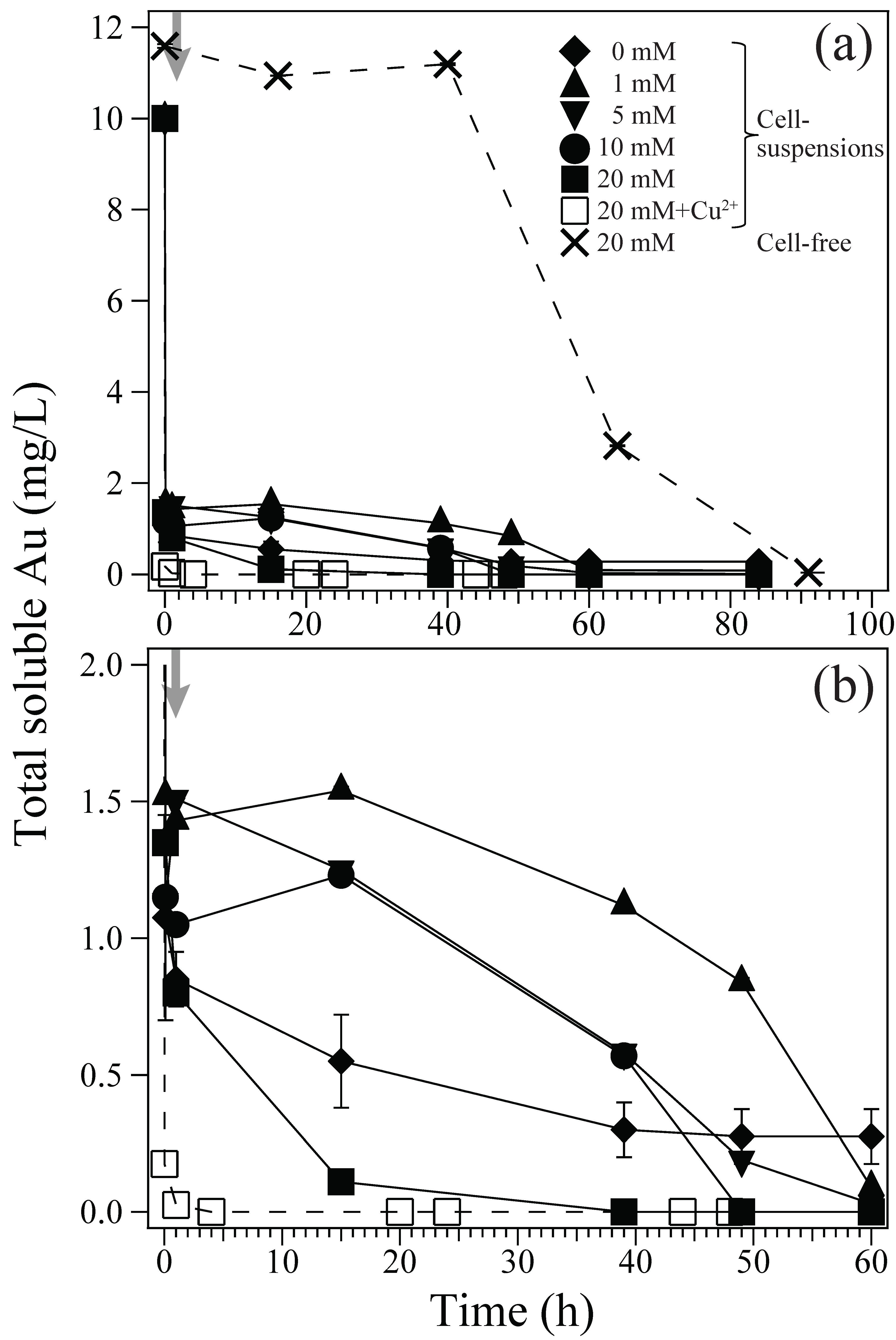
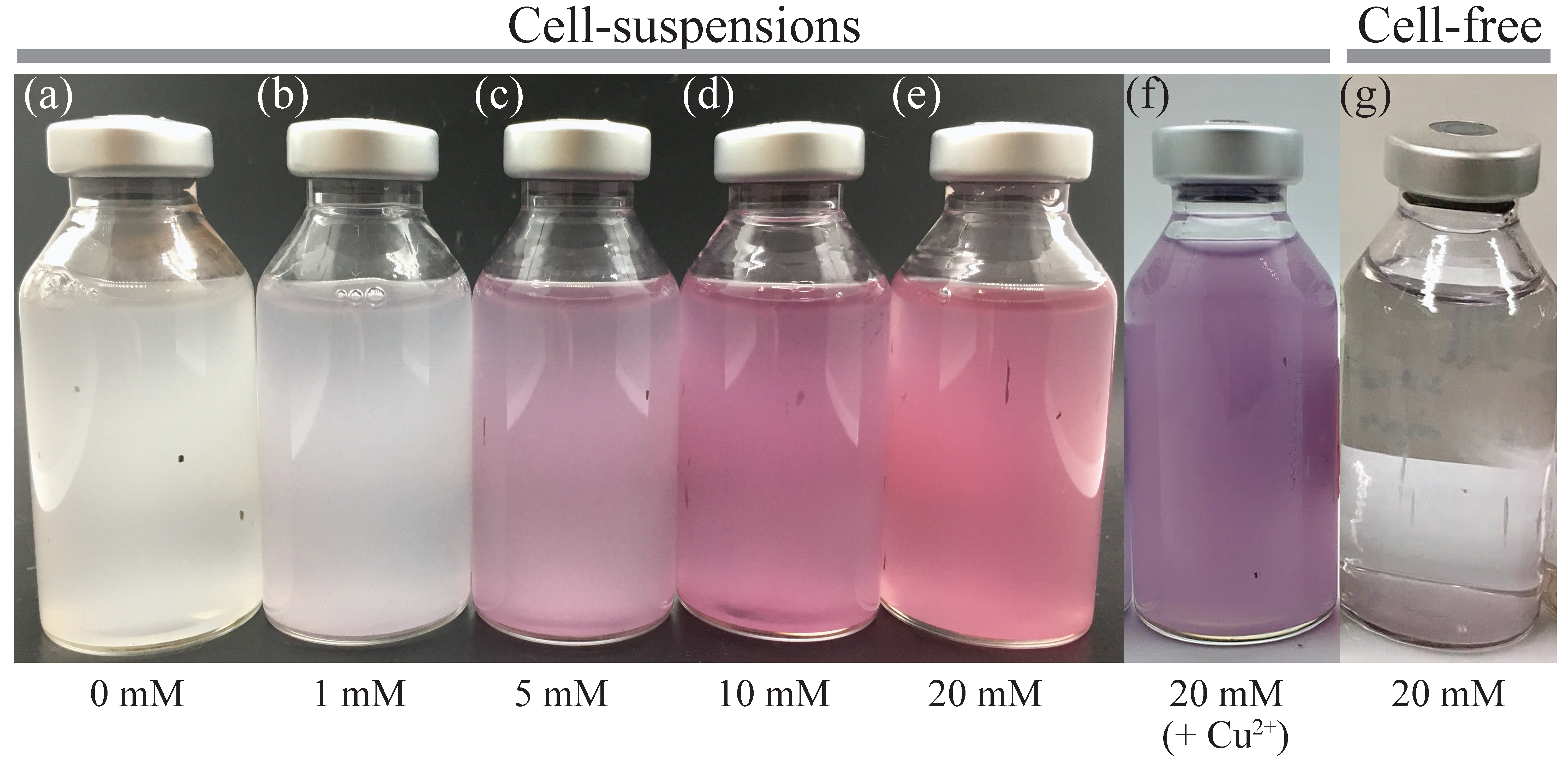
3.1. Au(III) Biosorption and Bioreduction
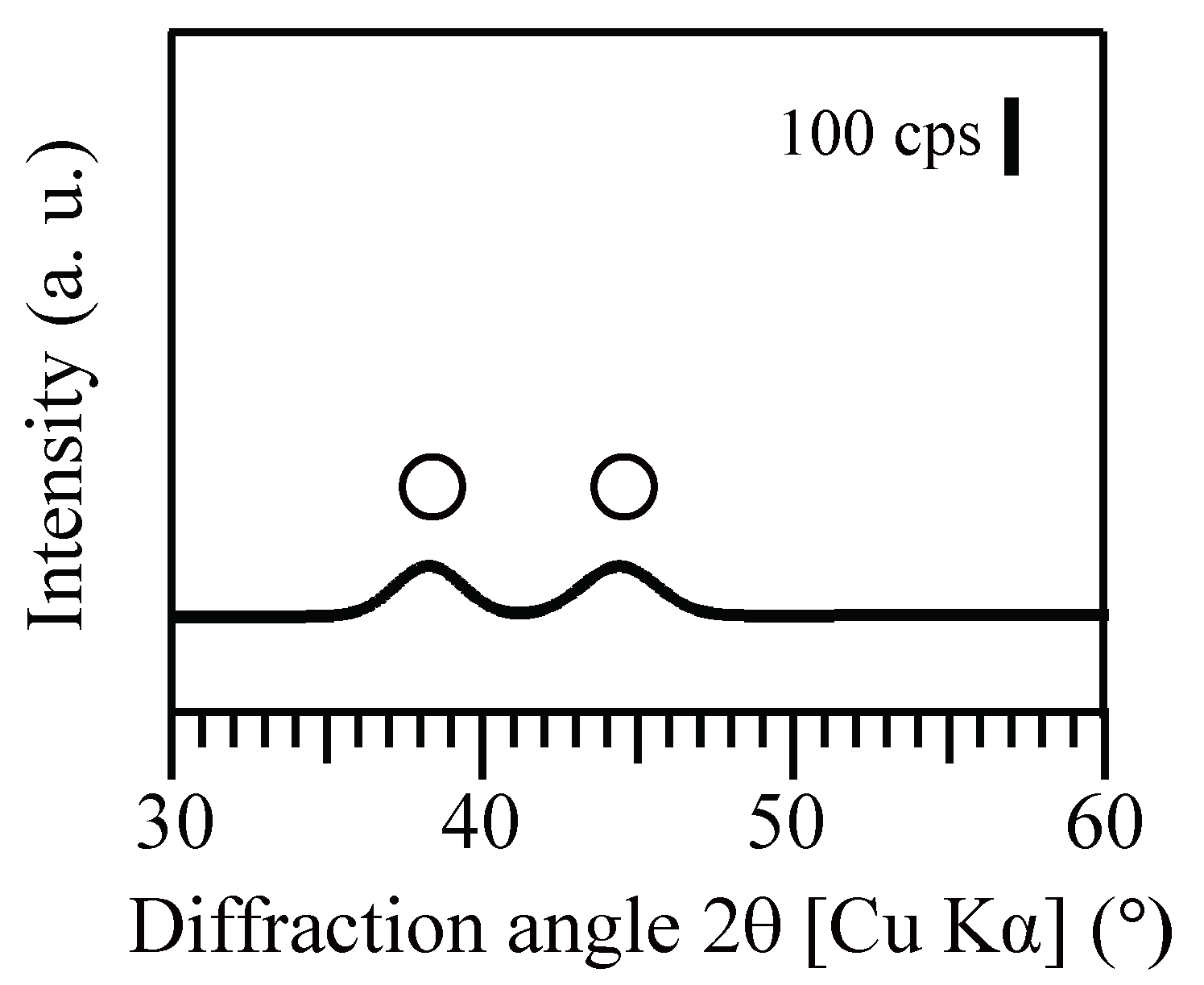
3.2. Size, Density and Localization of Bio-AuNPs
3.2.1. Size and Density
3.2.2. Localization
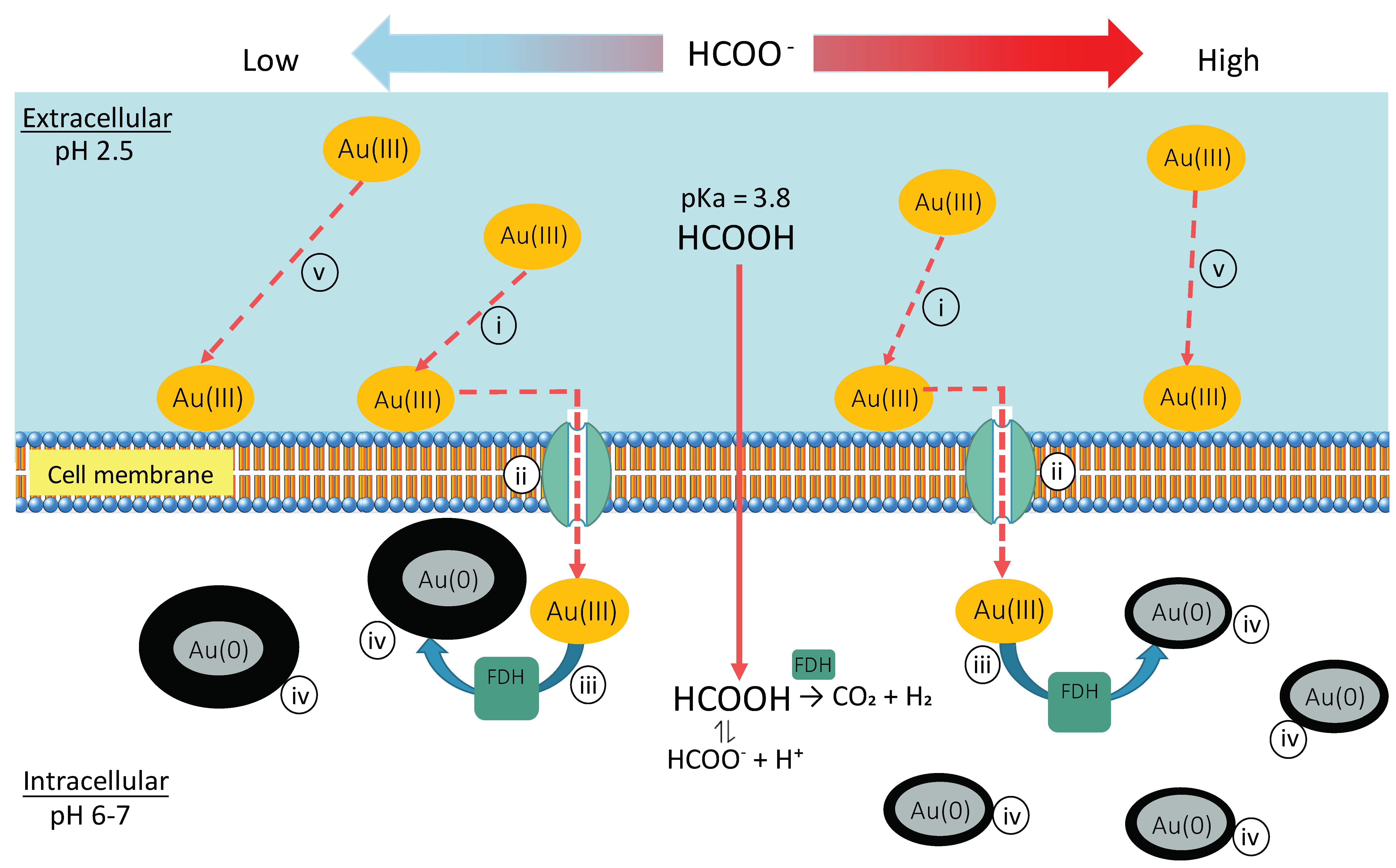
3.3. Schematic Summary of Bio-AuNPs Production by Ac. aromatica
4. Conclusions
- The acidophilic Fe(III)-reducing heterotrophic bacterium, Acidocella aromatica PFBC was successfully used for intracellular gold recovery as bio-AuNPs from highly acidic Au(III) solutions via a simple one-step reaction.
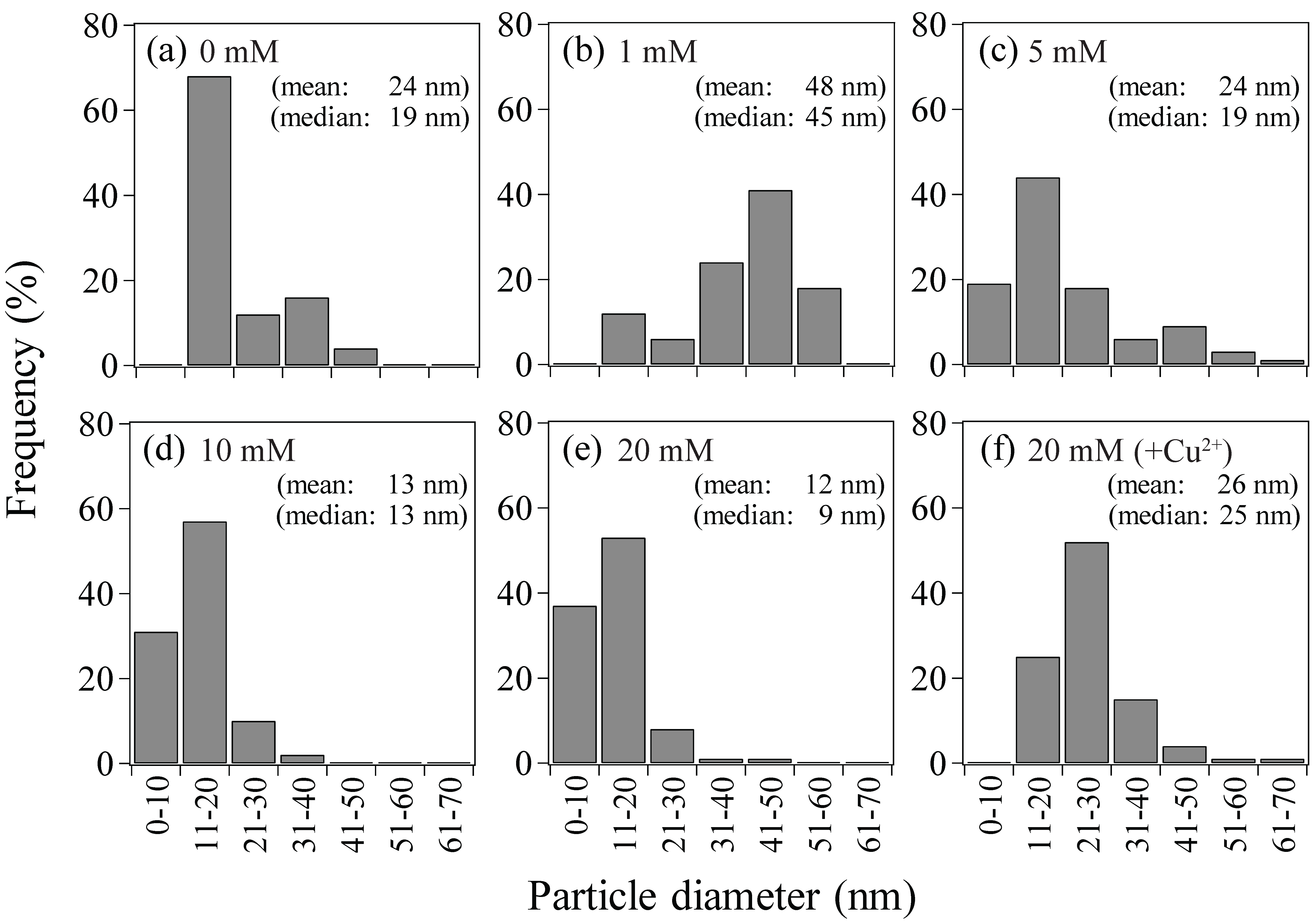
- Use of increasing concentration of formate (as e-donor) corresponded to a greater number of Au(0) nucleation sites with less crystal growth: At 1, 5, 10, or 20 mM formate, bio-AuNPs of 48, 24, 13, or 12 nm, with 2.3, 17, 62, or 97 particles/cell, respectively, were produced.
- The presence of Cu2+ was inhibitory to intracellular enzymatic reaction, resulting in significantly fewer Au(0) nucleation, but in enhanced crystal growth.
- It was possible to control the size of bio-AuNPs by modifying the e-donor concentration combined with a use of enzyme inhibitor: bio-AuNPs of 48 nm, 26 nm, or 13 nm (nearly within normal distribution) was produced by use of 1 mM formate, 20 mM formate (+Cu2+), or 10 mM formate, respectively.
- The knowledge obtained from this fundamental study is expected to be applied to the actual acidic Au leachate from “urban mine” resources such as Au-bearing printed circuit boards (PCBs).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bernstein, P.L.; Volcker, P.A. The Power of Gold: The History of an Obsession; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Mwakwari, S.C.; Oyelere, A.K. Gold nanoparticles: From nanomedicine to nanosensing. Nanotechnol. Sci. Appl. 2008, 1, 45. [Google Scholar] [PubMed]
- Das, S.K.; Das, A.R.; Guha, A.K. Gold nanoparticles: Microbial synthesis and application in water hygiene management. Langmuir 2009, 25, 8192–8199. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for co oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L. Nanobiotechnology advances in enzymatic biosensors for the agri-food industry. Environ. Chem. Lett. 2017, 15, 555–560. [Google Scholar] [CrossRef]
- Verma, M.L. Enzymatic nanobiosensors in the agricultural and food industry. In Nanoscience in Food and Agriculture 4; Wiley: Hoboken, NJ, USA, 2017; pp. 229–245. [Google Scholar]
- Lee, J.-S.; Cho, J.; Lee, C.; Kim, I.; Park, J.; Kim, Y.-M.; Shin, H.; Lee, J.; Caruso, F. Layer-by-layer assembled charge-trap memory devices with adjustable electronic properties. Nat. Nanotechnol. 2007, 2, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. CRC 675—Current trends in phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. 2008, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Okibe, N.; Nakayama, D.; Matsumoto, T. Palladium bionanoparticles production from acidic Pd(II) solutions and spent catalyst leachate using acidophilic Fe(III)-reducing bacteria. Extremophiles 2017, 21, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L.; Newman, D.K.; Kappler, A. Ehrlich’s Geomicrobiology, 6th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Korobushkina, E.; Korobushkin, I. The interaction of gold with bacteria and the formation of new gold. Doklady Akademii Nauk SSSR 1986, 287, 978. [Google Scholar]
- Southam, G.; Beveridge, T.J. The in vitro formation of placer gold by bacteria. Geochim. Cosmochim. Acta 1994, 58, 4527–4530. [Google Scholar] [CrossRef]
- Southam, G.; Beveridge, T.J. The occurrence of sulfur and phosphorus within bacterially derived crystalline and pseudocrystalline octahedral gold formed in vitro. Geochim. Cosmochim. Acta 1996, 60, 4369–4376. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Gurunathan, S. Biological synthesis of gold nanocubes from bacillus licheniformis. Bioresour. Technol. 2009, 100, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lin, Z.; Gu, P.; Zhou, J.; Yao, B.; Chen, G.; Fu, J. Extracellular biosynthesis of monodispersed gold nanoparticles by a SAM capping route. J. Nanopart. Res. 2009, 11, 279–288. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, Q.; Fan, X.; Gao, J.; Luo, Y. Rapid biosynthesis of gold nanoparticles by the extracellular secretion of bacillus niabensis 45: Characterization and antibiofilm activity. J. Chem. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Markus, J.; Mathiyalagan, R.; Kim, Y.-J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Husseiny, M.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Deplanche, K.; Macaskie, L. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 2008, 99, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, K.; Tor, J.M.; Nevin, K.P.; Lovley, D.R. Reductive precipitation of gold by dissimilatory Fe(III)-reducing Bacteria and Archaea. Appl. Environ. Microbiol. 2001, 67, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Tsukiyama, T.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S. Intracellular recovery of gold by microbial reduction of AuCl4− ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 2006, 81, 24–29. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, X.; Wang, Y.; Wang, Y.; Hua, J. Diversity of aurum bioreduction by Rhodobacter capsulatus. Mater. Lett. 2008, 62, 4299–4302. [Google Scholar] [CrossRef]
- Nangia, Y.; Wangoo, N.; Sharma, S.; Wu, J.; Dravid, V.; Shekhawat, G.S.; Suri, C.R. Facile biosynthesis of phosphate capped gold nanoparticles by a bacterial isolate Stenotrophomonas maltophilia. Appl. Phys. Lett. 2009, 94, 233901-1–233901-3. [Google Scholar] [CrossRef]
- Reith, F.; Rogers, S.L.; McPhail, D.; Webb, D. Biomineralization of gold: Biofilms on bacterioform gold. Science 2006, 313, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.; Southam, G. Bioaccumulation of gold by sulfate-reducing bacteria cultured in the presence of gold(I)-thiosulfate complex. Geochim. Cosmochim. Acta 2006, 70, 3646–3661. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Ma, X.; Tian, B.; Li, T.; Yu, J.; Dai, S.; Weng, Y.; Hua, Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, 11, 5931–5944. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Southam, G. The effect of thiosulfate-oxidizing bacteria on the stability of the gold-thiosulfate complex. Geochim. Cosmochim. Acta 2005, 69, 3759–3772. [Google Scholar] [CrossRef]
- Jones, R.M.; Hedrich, S.; Johnson, D.B. Acidocella aromatica sp. nov.: An acidophilic heterotrophic alphaproteobacterium with unusual phenotypic traits. Extremophiles 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Nanometals: Formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.P.; Winterbottom, J.M. The conversion of polysaccharides to hydrogen gas. Part 1: The palladium catalysed decomposition of formic acid/sodium formate solutions. J. Technol. Biotechnol. 1988, 41, 121–133. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizki, I.N.; Okibe, N. Size-Controlled Production of Gold Bionanoparticles Using the Extremely Acidophilic Fe(III)-Reducing Bacterium, Acidocella aromatica. Minerals 2018, 8, 81. https://doi.org/10.3390/min8030081
Rizki IN, Okibe N. Size-Controlled Production of Gold Bionanoparticles Using the Extremely Acidophilic Fe(III)-Reducing Bacterium, Acidocella aromatica. Minerals. 2018; 8(3):81. https://doi.org/10.3390/min8030081
Chicago/Turabian StyleRizki, Intan Nurul, and Naoko Okibe. 2018. "Size-Controlled Production of Gold Bionanoparticles Using the Extremely Acidophilic Fe(III)-Reducing Bacterium, Acidocella aromatica" Minerals 8, no. 3: 81. https://doi.org/10.3390/min8030081



