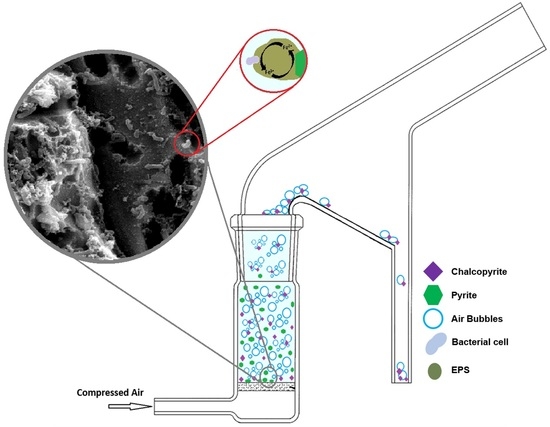
Selective Attachment of Leptospirillum ferrooxidans for Separation of Chalcopyrite and Pyrite through Bio-Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Samples
2.2. Bacterial Culture and Adjustment to Mineral
Free EPS
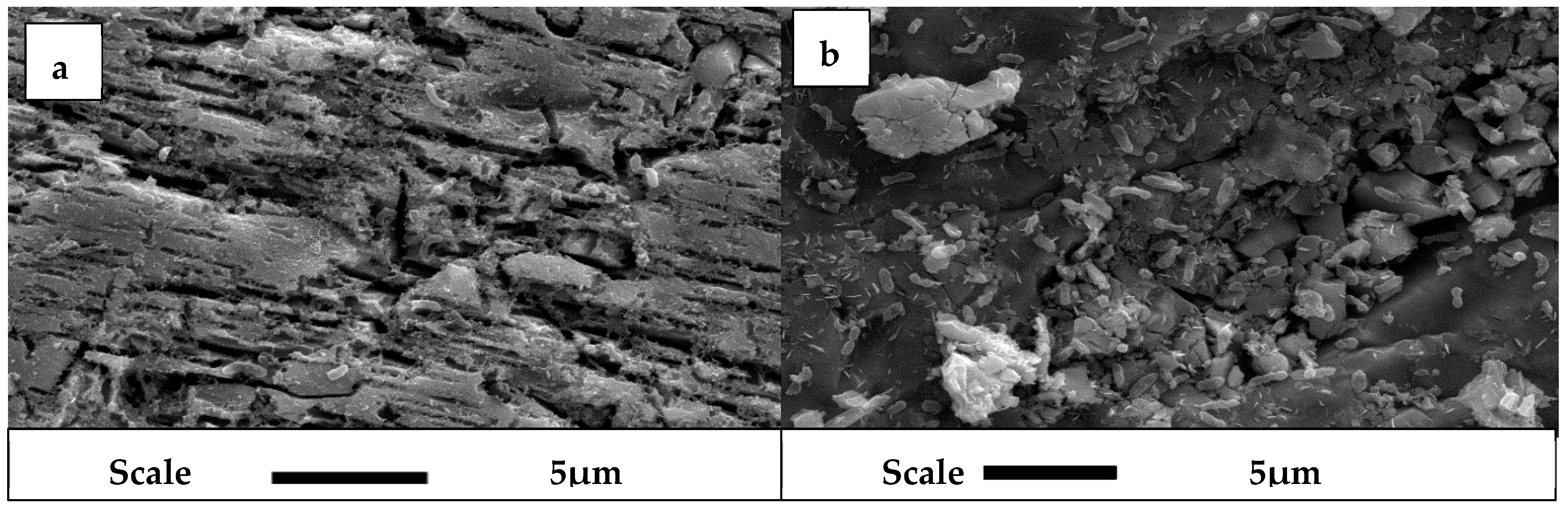
2.3. Scanning Electron Microscopy (SEM)
2.4. Micro Flotation Tests
X-Ray Diffraction (XRD) Analysis
3. Results
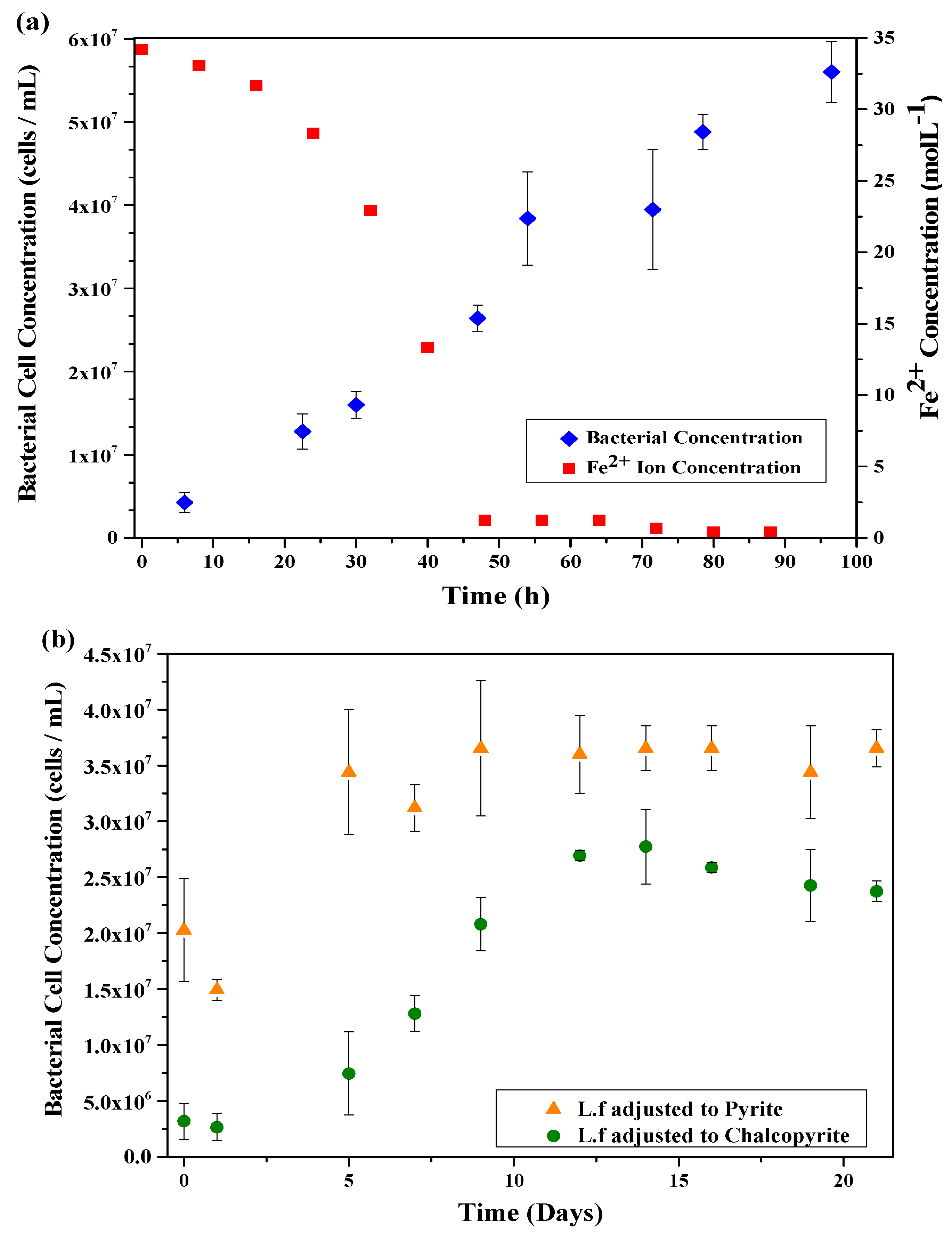
3.1. Leptospirillum Ferrooxidans Growth
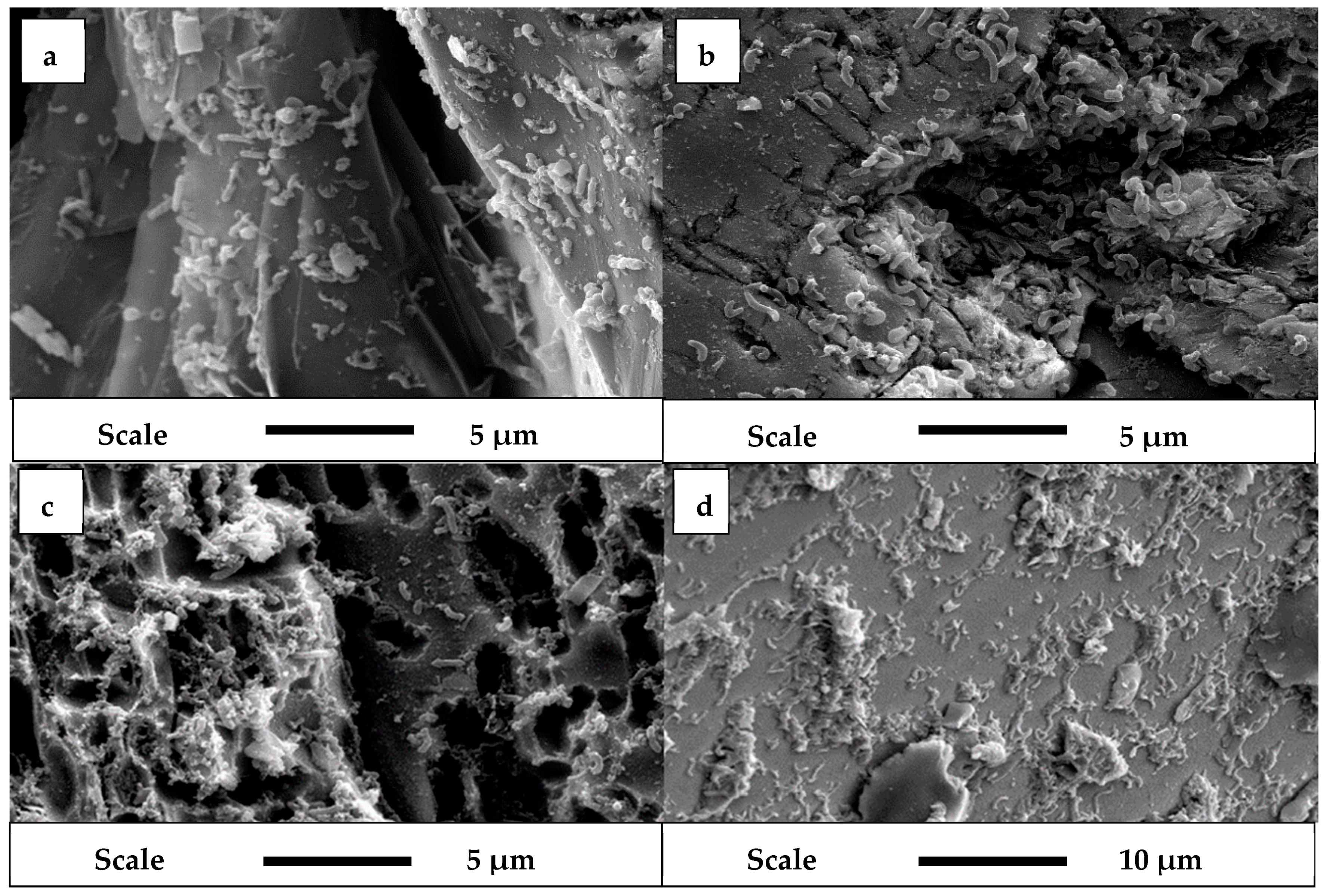
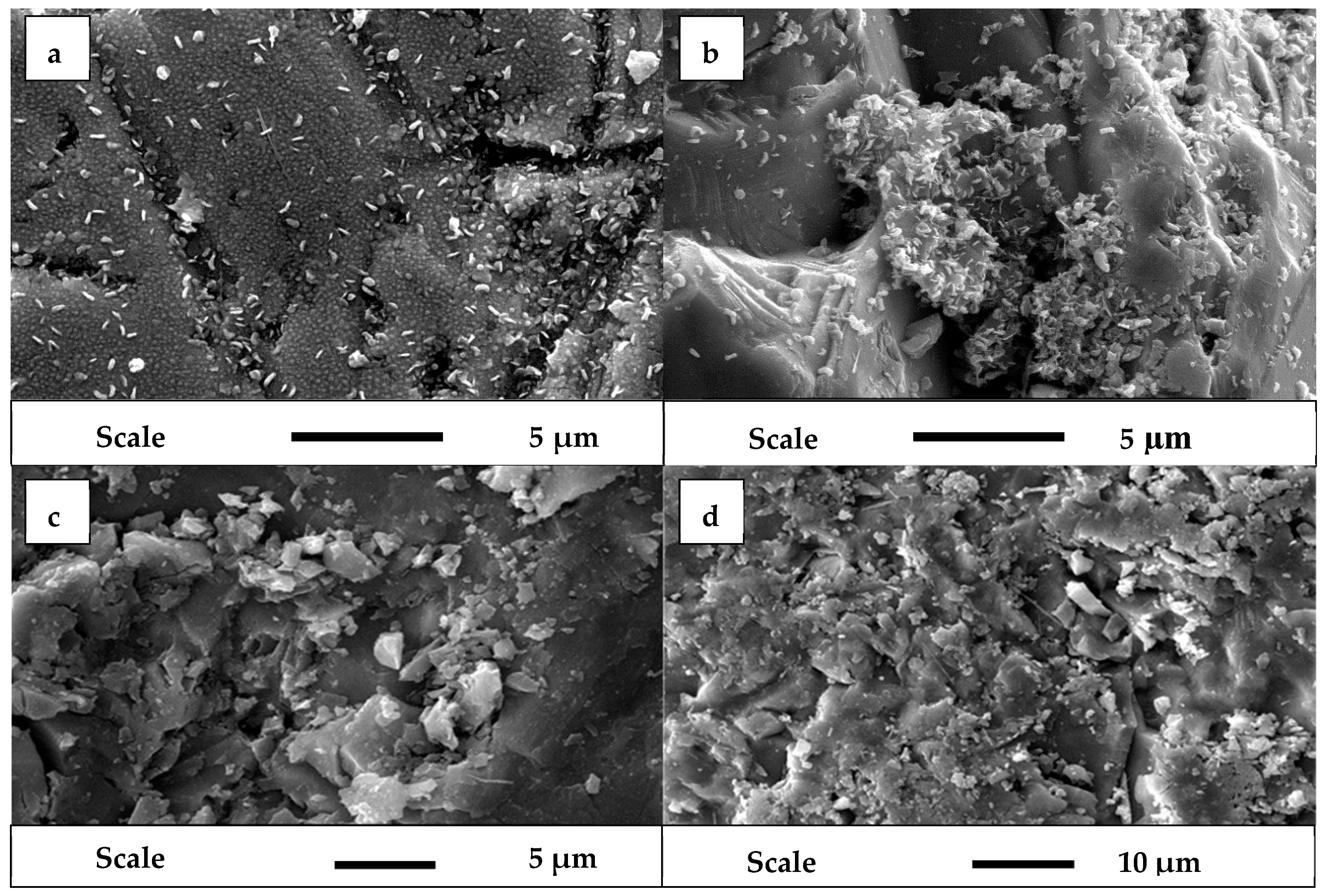
3.2. SEM Analysis
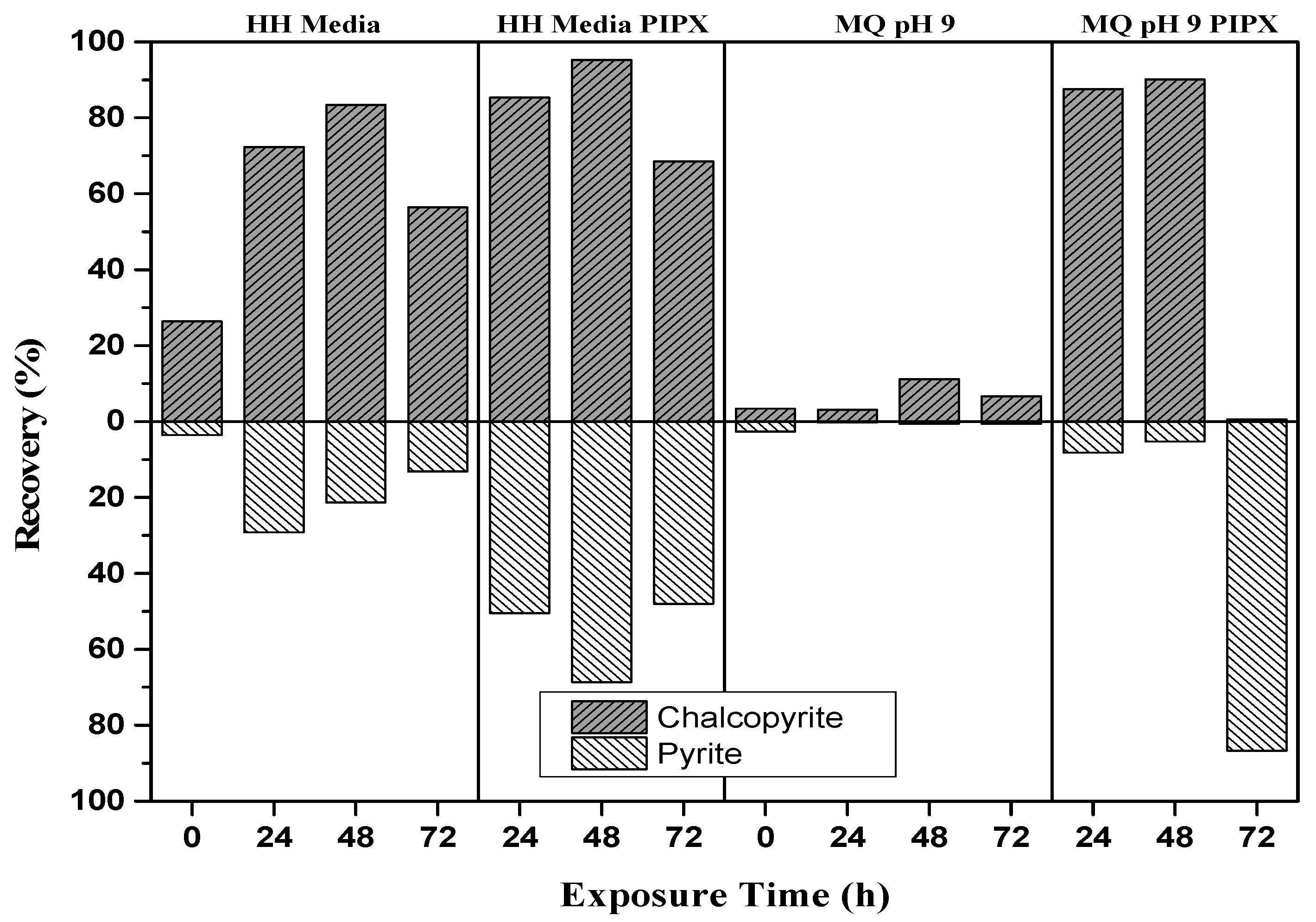
3.3. Microflotation Tests
3.3.1. Baseline Flotation Tests
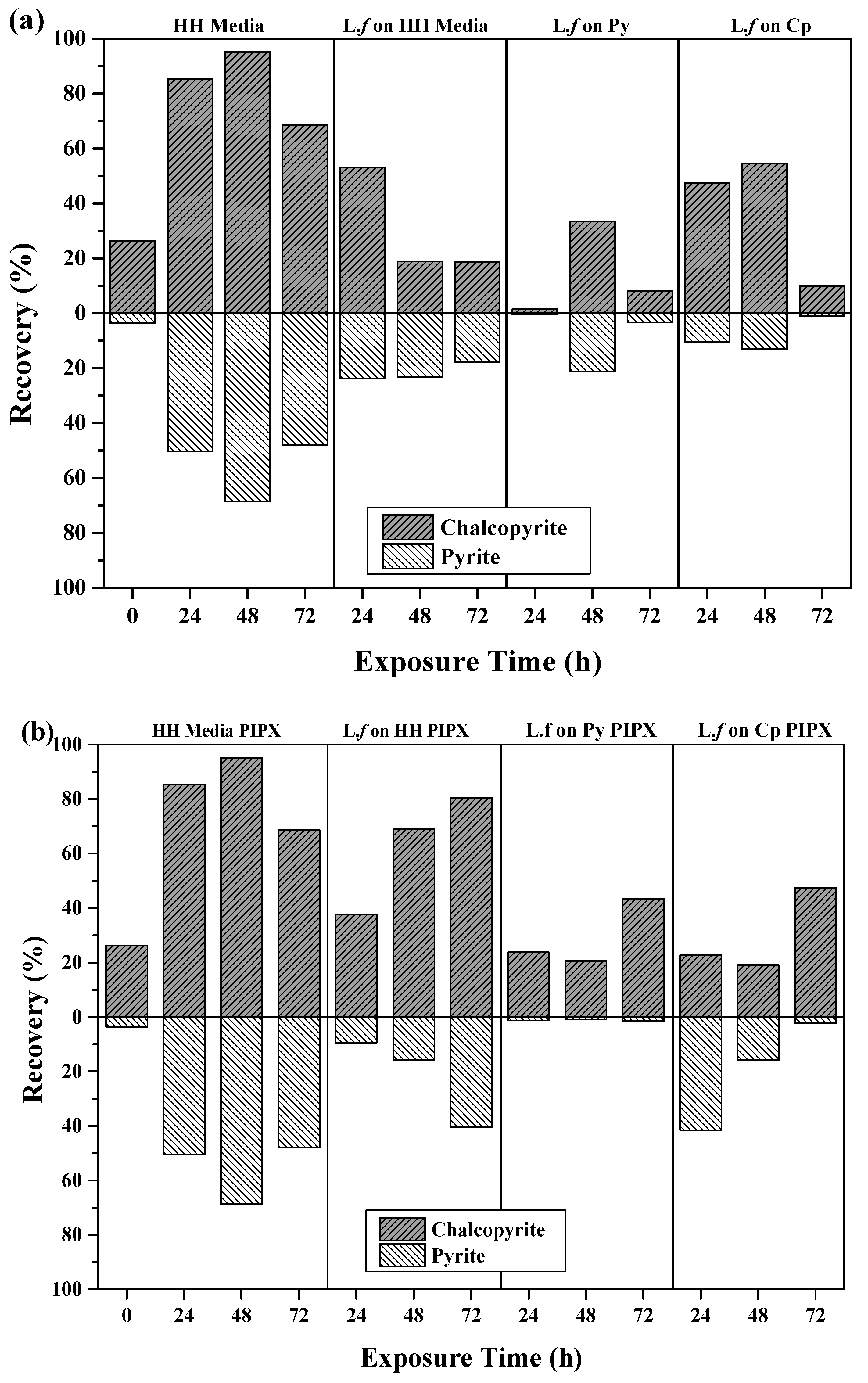
3.3.2. The Effect of L.f Growth Conditions on Recovery
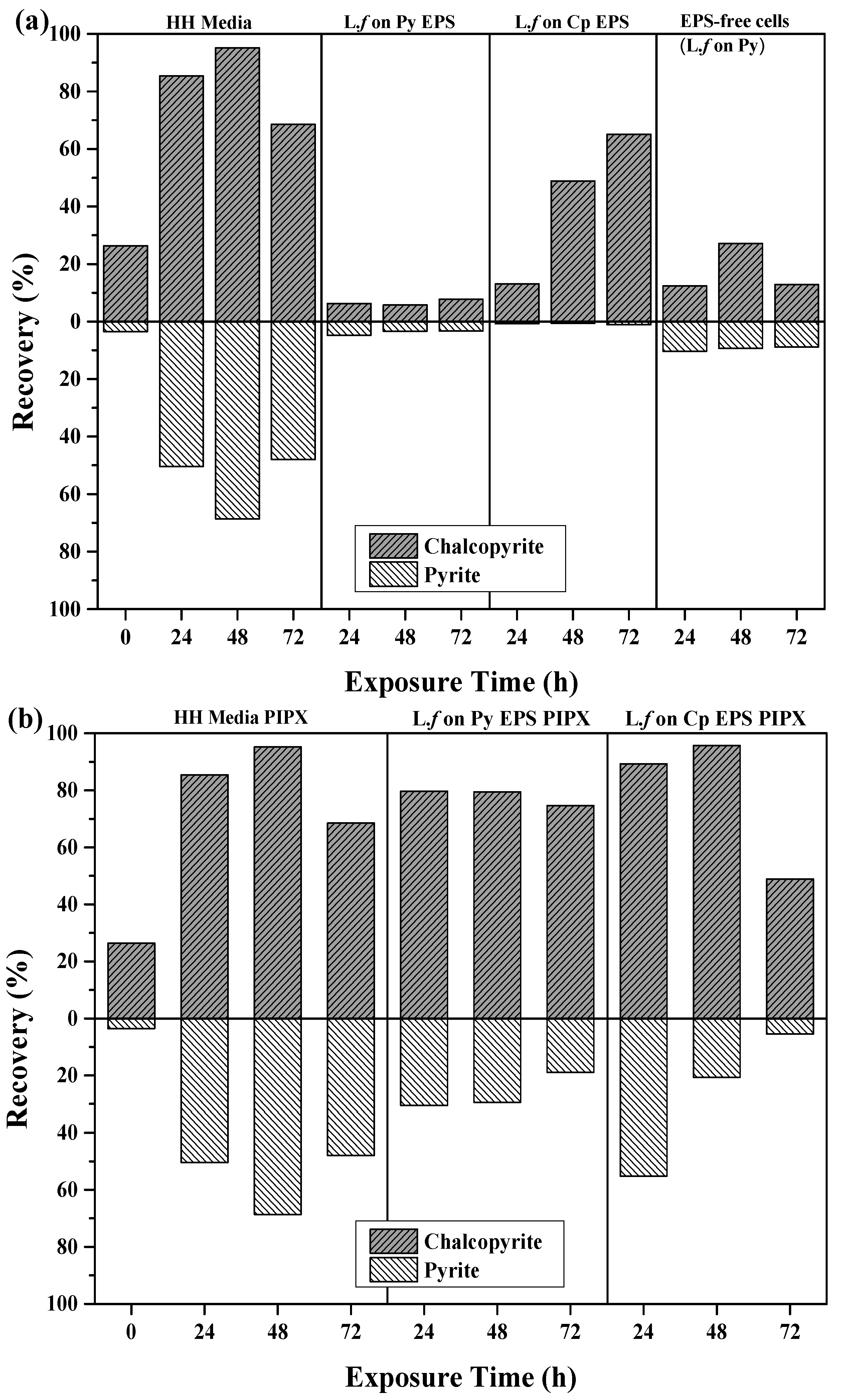
3.3.3. The Effect of EPS on Recovery
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wills, B.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2011. [Google Scholar]
- Chandraprabha, M.; Natarajan, K. Surface chemical and flotation behaviour of chalcopyrite and pyrite in the presence of Acidithiobacillus thiooxidans. Hydrometallurgy 2006, 83, 146–152. [Google Scholar] [CrossRef]
- Chandraprabha, M.; Natarajan, K.; Modak, J.M. Selective separation of pyrite and chalcopyrite by biomodulation. Colloids Surf. B Biointerfaces 2004, 37, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Partha, P.; Natarajan, K. Microbially enhanced removal of pyrite and chalcopyrite from oxide gangue minerals with reference to desulfurization of tailings. Miner. Metall. Process. 2004, 21, 169–178. [Google Scholar]
- Dwyer, R.; Bruckard, W.; Rea, S.; Holmes, R. Bioflotation and bioflocculation review: Microorganisms relevant for mineral beneficiation. Miner. Process. Extr. Metall. 2012, 121, 65–71. [Google Scholar] [CrossRef]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Govender, Y.; Gericke, M. Extracellular polymeric substances (EPS) from bioleaching systems and its application in bioflotation. Miner. Eng. 2011, 24, 1122–1127. [Google Scholar] [CrossRef]
- Deo, N.; Natarajan, K. Interaction of Bacillus polymyxa with some oxide minerals with reference to mineral beneficiation and environmental control. Miner. Eng. 1997, 10, 1339–1354. [Google Scholar] [CrossRef]
- Chandraprabha, M.; Natarajan, K.; Somasundaran, P. Selective separation of arsenopyrite from pyrite by biomodulation in the presence of Acidithiobacillus ferrooxidans. J. Colloid Interface Sci. 2004, 276, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Santhiya, D.; Natarajan, K. Surface modification studies on sulphide minerals using bioreagents. Int. J. Miner. Process. 2003, 72, 175–188. [Google Scholar] [CrossRef]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.-H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Jiao, W.; Jiang, H.; Sand, W.; Xia, J.; Liu, X.; Qin, W.; Qiu, G.; Hu, Y. Adhesion forces between cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans or Leptospirillum ferrooxidans and chalcopyrite. Colloids Surf. B Biointerfaces 2012, 94, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, L.; Giaveno, A.; Pogliani, C.; Donati, E. Bioleaching of a polymetallic sulphide mineral by native strains of Leptospirillum ferrooxidans from Patagonia Argentina. Process Biochem. 2008, 43, 445–450. [Google Scholar] [CrossRef]
- Kim, G.; Park, K.; Choi, J.; Gomez-Flores, A.; Han, Y.; Choi, S.Q.; Kim, H. Bioflotation of malachite using different growth phases of Rhodococcus opacus: Effect of bacterial shape on detachment by shear flow. Int. J. Miner. Process. 2015, 143, 98–104. [Google Scholar] [CrossRef]
- Kim, H.N.; Hong, Y.; Lee, I.; Bradford, S.A.; Walker, S.L. Surface characteristics and adhesion behavior of Escherichia coli O157:H7: Role of extracellular macromolecules. Biomacromolecules 2009, 10, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.H.; Vilinska, A.; Chernyshova, I. Minerals bioprocessing: R & D needs in mineral biobeneficiation. Hydrometallurgy 2010, 104, 465–470. [Google Scholar]
- Sand, W.; Gerke, T.; Hallmann, R.; Schippers, A. Sulfur chemistry, biofilm, and the (in) direct attack mechanism—A critical evaluation of bacterial leaching. Appl. Microbiol. Biotechnol. 1995, 43, 961–966. [Google Scholar] [CrossRef]
- He, Z.-G.; Yang, Y.-P.; Shan, Z.; Hu, Y.-H.; Zhong, H. Effect of pyrite, elemental sulfur and ferrous ions on eps production by metal sulfide bioleaching microbes. Trans. Nonferrous Met. Soc. China 2014, 24, 1171–1178. [Google Scholar] [CrossRef]
- Hosseini, T.; Kolahdoozan, M.; Tabatabaei, Y.; Oliazadeh, M.; Noaparast, M.; Eslami, A.; Manafi, Z.; Alfantazi, A. Bioflotation of sarcheshmeh copper ore using Thiobacillus ferrooxidans bacteria. Miner. Eng. 2005, 18, 371–374. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(iii) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “House of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xia, J.-L.; Hong, F.-F.; Tao, X.-X.; Leng, Y.-W.; Zhao, Y.-D. Analysis of sulfur speciation on chalcopyrite surface bioleached with Acidithiobacillus ferrooxidans. Miner. Eng. 2012, 27, 60–64. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Rohwerder, T.; Sand, W. Mechanisms and Biochemical Fundamentals of Bacterial Metal Sulfide Oxidation. In Microbial Processing of Metal Sulfides; Springer: Berlin, Germany, 2007; pp. 35–58. [Google Scholar]
- Sharma, P.; Das, A.; Rao, K.H.; Forssberg, K. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions. Hydrometallurgy 2003, 71, 285–292. [Google Scholar] [CrossRef]
- Kim, H.N.; Walker, S.L.; Bradford, S.A. Macromolecule mediated transport and retention of Escherichia coli O157:H7 in saturated porous media. Water Res. 2010, 44, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Laskowski, J.S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–245. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [PubMed]
- Olson, G.J. Rate of pyrite bioleaching by Thiobacillus ferrooxidans: Results of an interlaboratory comparison. Appl. Environ. Microbiol. 1991, 57, 642–644. [Google Scholar] [PubMed]
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of Leptospirillum ferrooxidans for leaching. Appl. Environ. Microbiol. 1992, 58, 85–92. [Google Scholar] [PubMed]
- Ohmura, N.; Kitamura, K.; Saiki, H. Mechanism of microbial flotation using Thiobacillus ferrooxidans for pyrite suppression. Biotechnol. Bioeng. 1993, 41, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Pecina, E.; Rodriguez, M.; Castillo, P.; Diaz, V.; Orrantia, E. Effect of Leptospirillum ferrooxidans on the flotation kinetics of sulphide ores. Miner. Eng. 2009, 22, 462–468. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Lamb, R.N.; Rosenberg, R.A.; Moran, D. The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air. Geochim. Cosmochim. Acta 2006, 70, 2210–2228. [Google Scholar] [CrossRef]
- Pearce, C.I.; Pattrick, R.A.D.; Vaughan, D.J.; Henderson, C.M.B.; van der Laan, G. Copper oxidation state in chalcopyrite: Mixed cu d9 and d10 characteristics. Geochim. Cosmochim. Acta 2006, 70, 4635–4642. [Google Scholar] [CrossRef]
- Vilinska, A.; Rao, K.H. Leptosririllum ferrooxidans-sulfide mineral interactions with reference to bioflotation nad bioflocculation. Trans. Nonferrous Met. Soc. China 2008, 18, 1403–1409. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.-L.; Wei, D.-H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Bastías, M.; Gentina, J. Variables affecting the growth and ferrous oxidation capacity of L. Ferrooxidans in continuous culture. Hydrometallurgy 2010, 104, 351–355. [Google Scholar]
- Khmeleva, T.N.; Beattie, D.A.; Georgiev, T.V.; Skinner, W.M. Surface study of the effect of sulphite ions on copper-activated pyrite pre-treated with xanthate. Miner. Eng. 2003, 16, 601–608. [Google Scholar] [CrossRef]
- Houot, R.; Duhamet, D. Floatability of chalcopyrite in the presence of dialkyl-thionocarbamate and sodium sulfite. Int. J. Miner. Process. 1993, 37, 273–282. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS Academic: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2007. [Google Scholar]
- Orell, A.; Navarro, C.A.; Arancibia, R.; Mobarec, J.C.; Jerez, C.A. Life in blue: Copper resistance mechanisms of bacteria and archaea used in industrial biomining of minerals. Biotechnol. Adv. 2010, 28, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Usher, K.; Shaw, J.; Kaksonen, A.; Saunders, M. Elemental analysis of extracellular polymeric substances and granules in chalcopyrite bioleaching microbes. Hydrometallurgy 2010, 104, 376–381. [Google Scholar] [CrossRef]
- Rojas-Chapana, J.A.; Tributsch, H. Interfacial activity and leaching patterns of Leptospirillum ferrooxidans on pyrite. FEMS Microbiol. Ecol. 2004, 47, 19–29. [Google Scholar] [CrossRef]
- Crundwell, F. How do bacteria interact with minerals? Hydrometallurgy 2003, 71, 75–81. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Lahti-Tommila, H.; Garcia, O., Jr.; Puhakka, J.A.; Tuovinen, O.H. Bacterial and chemical leaching of chalcopyrite concentrates as affected by the redox potential and ferric/ferrous iron ratio at 22 °C. Int. J. Miner. Process. 2014, 132, 1–7. [Google Scholar] [CrossRef]
| Conditions | |
|---|---|
| Baseline | M.Q Water adjusted to pH9 with NaOH |
| HH Media | |
| Food Source | L.f grown in HH Media (Fe2+) |
| L.f grown on Py | |
| L.f grown on Cp | |
| EPS | EPS supernatant extracted from L.f grown on Py |
| EPS supernatant extracted from L.f grown on Cp | |
| EPS-Free Cells (L.f grown on Py) | |
| Exposure Time (h) | Pyrite (cells/mm2) | Chalcopyrite (cells/mm2) |
|---|---|---|
| 2 | 1.5 × 106 ± 0.04 × 106 | 0.0 |
| 24 | 3.8 × 106 ± 0.13 × 106 | 0.0 |
| 48 | 3.9 × 106 ± 0.19 × 106 | 0.0 |
| 72 | 4.0 × 106 ± 0.16 × 106 | 0.0 |
| 168 | 1.3 × 107 ± 0.65 × 106 | 1.6 × 106 ± 0.03 |
| Exposure Condition | Exposure Time (h) | Cp (%) | Py (%) |
|---|---|---|---|
| HH Media | 0 | 26.4 | 3.55 |
| 24 | 72.3 | 29.2 | |
| 48 | 83.5 | 21.4 | |
| 72 | 56.4 | 13.2 | |
| HH Media PIPX | 24 | 85.3 | 50.4 |
| 48 | 95.2 | 68.7 | |
| 72 | 68.5 | 48.0 | |
| M.Q pH 9 PIPX | 24 | 87.6 | 8.2 |
| 48 | 90.2 | 5.3 | |
| 72 | 0.56 | 86.7 | |
| L.f grown in HH Media (Fe2+) PIPX | 24 | 37.7 | 9.4 |
| 48 | 69.0 | 15.6 | |
| 72 | 80.4 | 40.5 | |
| L.f grown on Py PIPX | 24 | 23.8 | 1.2 |
| 48 | 20.7 | 0.9 | |
| 72 | 43.4 | 1.5 | |
| L.f grown on Cp PIPX | 24 | 22.8 | 41.6 |
| 48 | 19.1 | 15.9 | |
| 72 | 47.4 | 2.3 | |
| EPS supernatant extracted from L.f grown on Py PIPX | 24 | 79.7 | 30.5 |
| 48 | 79.4 | 29.4 | |
| 72 | 74.7 | 18.9 | |
| EPS supernatant extracted from L.f grown on Cp PIPX | 24 | 89.3 | 55.2 |
| 48 | 95.8 | 20.7 | |
| 72 | 48.9 | 5.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleeze, B.; Zhao, J.; Harmer, S.L. Selective Attachment of Leptospirillum ferrooxidans for Separation of Chalcopyrite and Pyrite through Bio-Flotation. Minerals 2018, 8, 86. https://doi.org/10.3390/min8030086
Bleeze B, Zhao J, Harmer SL. Selective Attachment of Leptospirillum ferrooxidans for Separation of Chalcopyrite and Pyrite through Bio-Flotation. Minerals. 2018; 8(3):86. https://doi.org/10.3390/min8030086
Chicago/Turabian StyleBleeze, Belinda, Jing Zhao, and Sarah L. Harmer. 2018. "Selective Attachment of Leptospirillum ferrooxidans for Separation of Chalcopyrite and Pyrite through Bio-Flotation" Minerals 8, no. 3: 86. https://doi.org/10.3390/min8030086