Micropore Structure of Cement-Stabilized Gold Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Mercury Intrusion Porosimetry
3. Results and Discussion
4. Conclusions
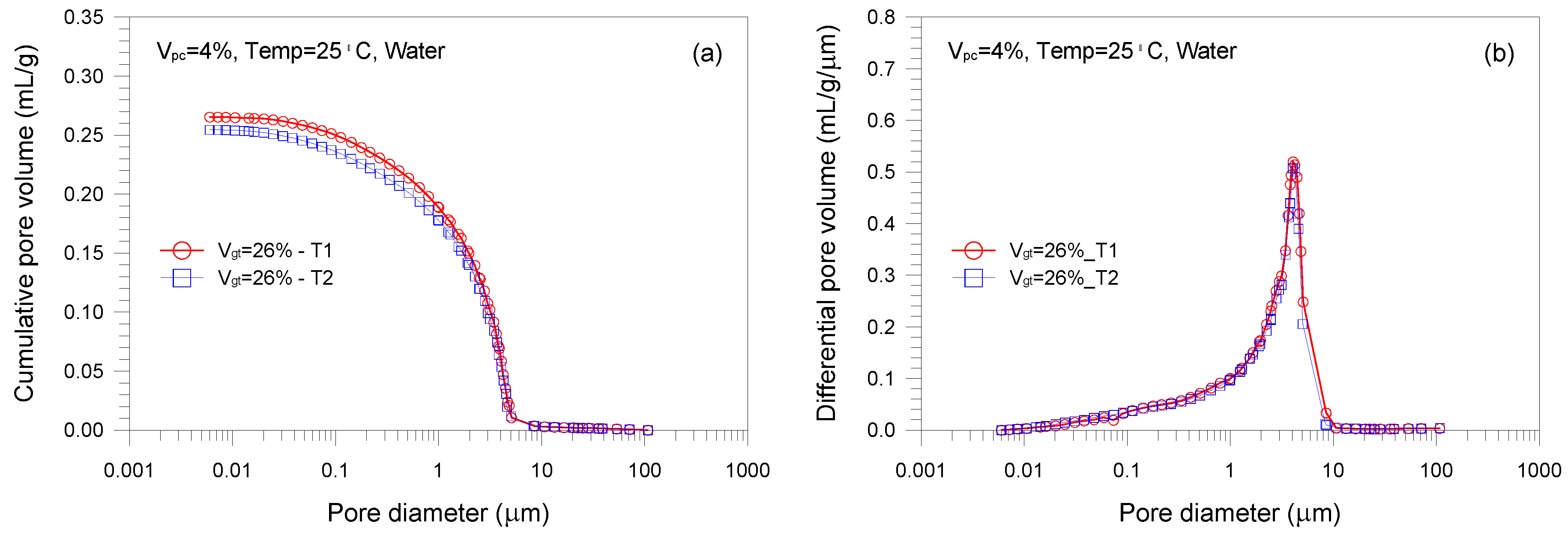
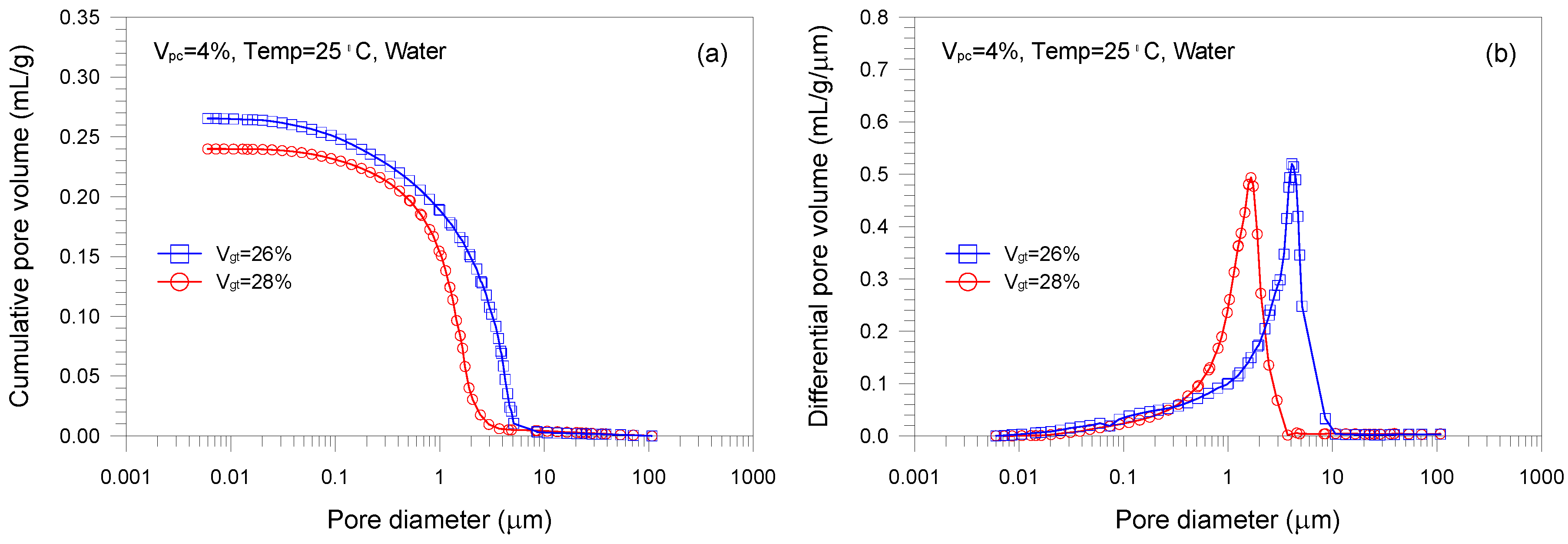
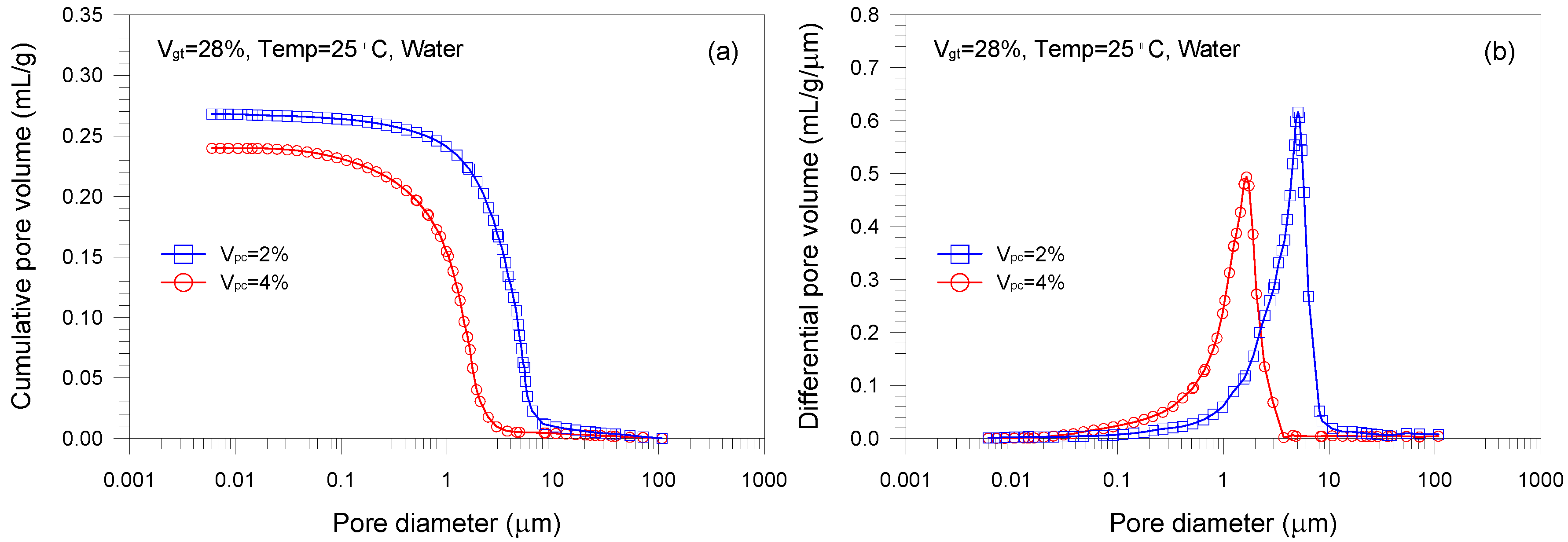
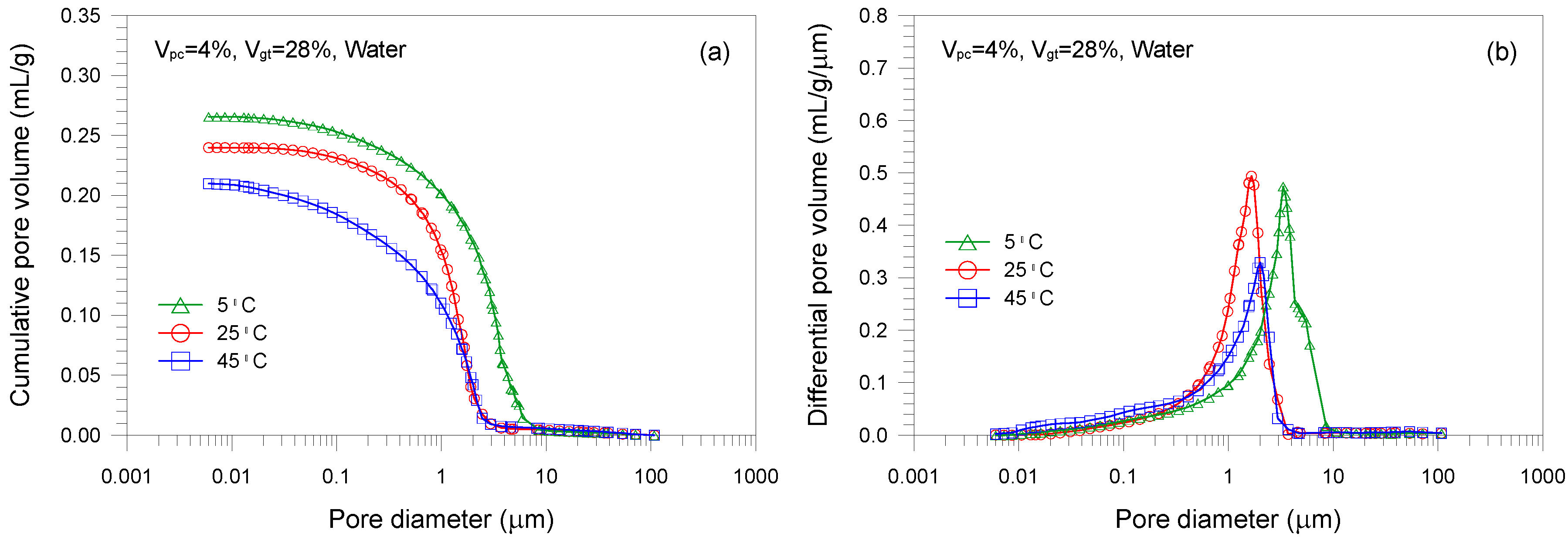
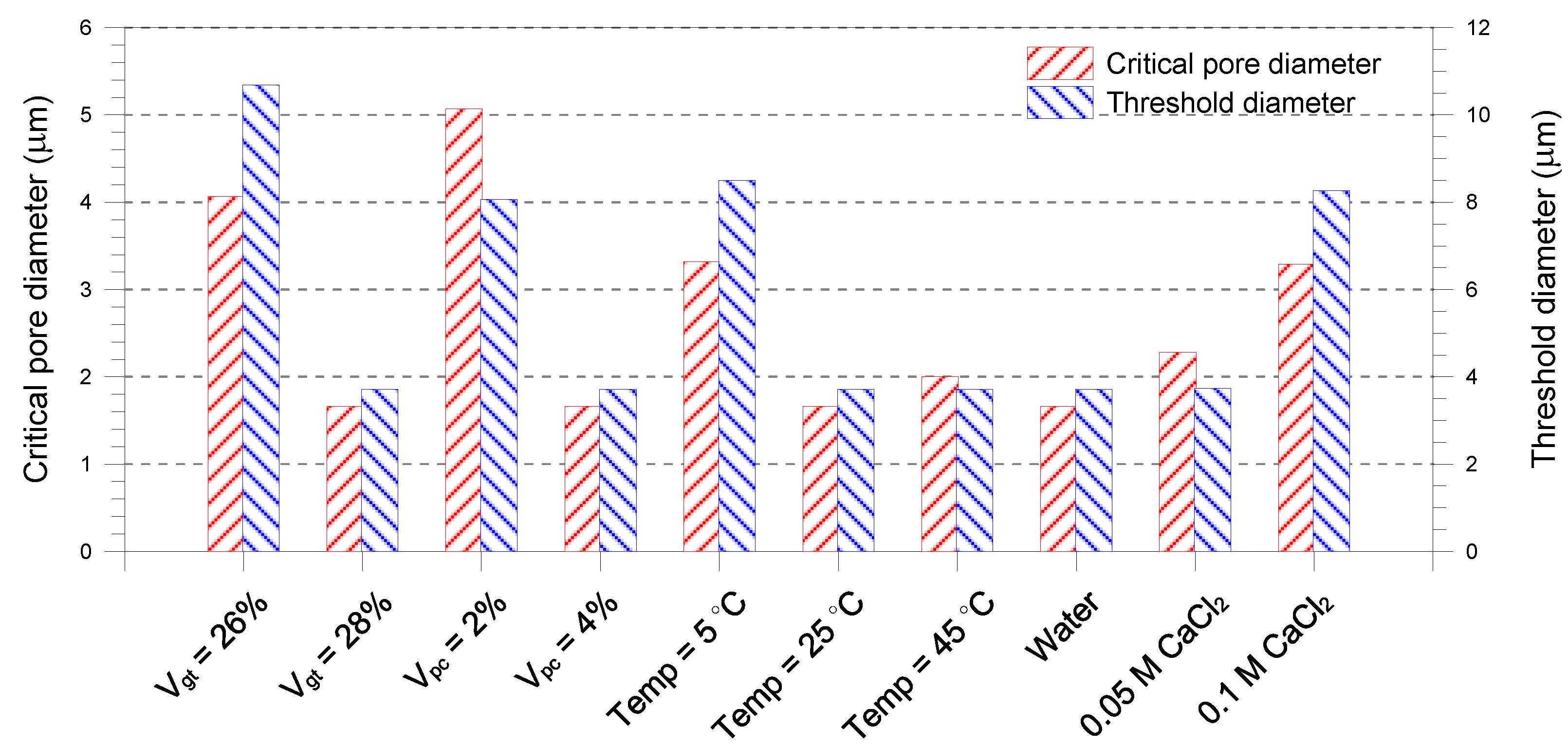
- A single-porosity structure, in which a single-mode pore size distribution is present, is observed in the gold tailings stabilized with Portland cement. The critical and threshold pore diameters are in the ranges of 1.66–5.07 μm and 3.71–10.69 μm, respectively.
- The total pore volume and mean pore diameter of the mixture samples decrease with increasing the volume fractions of the tailings and cement, indicating a denser microstructure with a finer distribution. This is because higher tailings and cement proportion results in a decrease of the average distance of particle assembly of the cement-stabilized tailings.
- An increase in curing temperature of the mixture samples results in a decrease in the total pore volume. This is mainly attributed to the dependence of the formation of hydration products on temperature. The sample cured at 5 °C has a coarser pore structure than those cured at 25 and 45 °C.
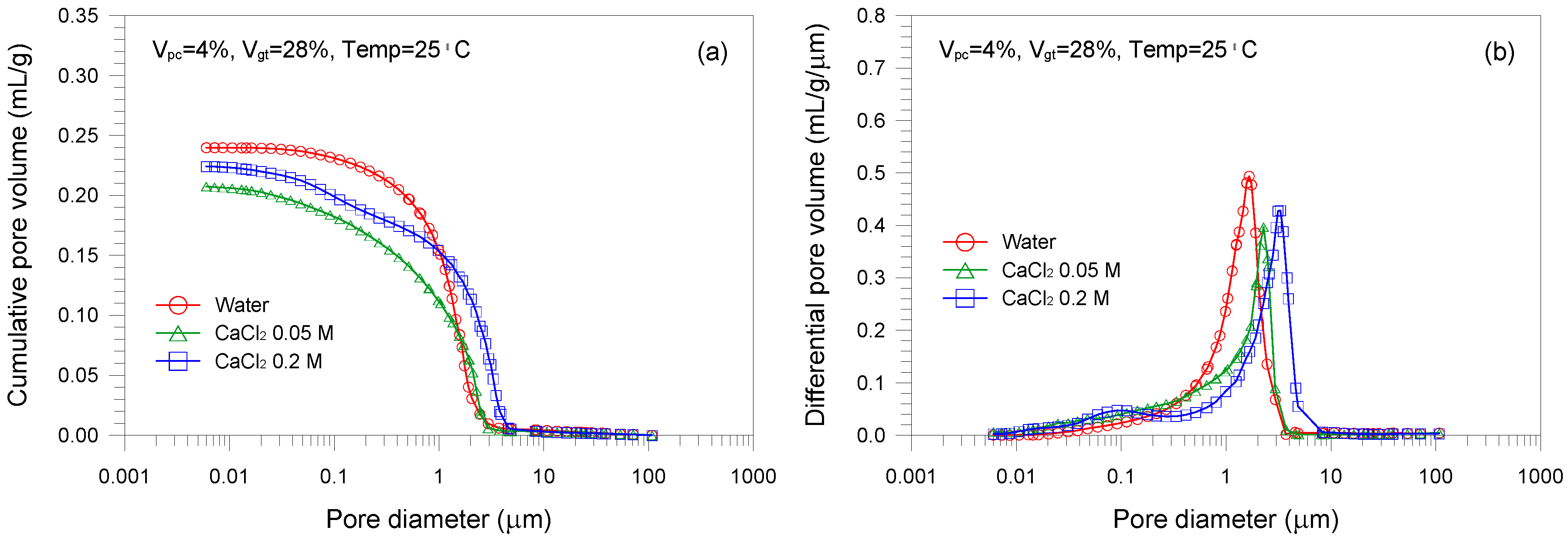
- The mixture sample mixed with water has the lower pore volume than those with CaCl2 solutions. Increasing the CaCl2 concentration leads to a continuous reduction in the mean pore size of the mixed samples. The lower mean pore size is attributed to the formation of aggregated and flocculated structure induced by the change in interparticle forces.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rico, M.; Benito, G.; Salgueiro, A.R.; Diez-Herrero, A.; Pereira, H.G. Reported tailings dam failures: A review of the European incidents in the worldwide context. J. Hazard. Mater. 2008, 152, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Shang, J.Q.; Wang, H.; Zhao, C. In-situ study of beneficial utilization of coal fly ash in reactive mine tailings. J. Environ. Manag. 2014, 135, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.Q.; Wang, H.L.; Kovac, V.; Fyfe, J. Site-specific study on stabilization of acid-generating mine tailings using coal fly ash. J. Mater. Civ. Eng. 2006, 18, 140–151. [Google Scholar] [CrossRef]
- Nehdi, M.; Tariq, A. Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementitious materials: A review. J. Environ. Eng. Sci. 2007, 6, 423–436. [Google Scholar] [CrossRef]
- Yeheyis, M.B.; Shang, J.Q.; Yanful, E.K. Long-term evaluation of coal fly ash and mine tailings co-placement: A site-specific study. J. Environ. Manag. 2009, 91, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Hills, C.D.; Sweeney, R.E.H.; Buenfeld, N.R. Mircrostructural study of carbonated cement-solidified synthetic heavy metal waste. Waste Manag. 1999, 19, 325–331. [Google Scholar] [CrossRef]
- Belem, T.; Bussiere, B.; Benzaazoua, M. The Effect of Microstructural Evolution on the Physical Properties of Paste Backfill. Proceedings of Tailings and Mine Waste’01, Fort Collins, CO, USA, 16–19 January 2002; pp. 365–374. [Google Scholar]
- Rahman, M.; Siddique, A.; Uddin, K. Microstructure and chemical properties of cement treated soft Bangladesh clays. Soils Found. 2010, 50, 1–7. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Yan, H. Quantitative evaluation of microstructure characteristics of cement consolidated soil. Bull. Eng. Geol. Environ. 2013, 72, 233–236. [Google Scholar] [CrossRef]
- Wang, K.; Mishulovich, A.; Shah, S.P. Activations and properties of cementitious materials made with cement-kiln dust and class F fly ash. J. Mater. Civ. Eng. 2007, 19, 112–119. [Google Scholar] [CrossRef]
- Sajedi, F.; Razak, H.A. ComParison of different methods for activation of ordinary Portland cement-slag motars. Constr. Build. Mater. 2011, 25, 30–38. [Google Scholar] [CrossRef]
- Shi, C. An overview on the activation of reactivity of natural pozzolans. Can. Geotech. J. 2001, 28, 778–786. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Feldman, R.F. Time-dependent and intrinsic characteristics of Portland cement hydrated in the presence of calcium chloride. Il Cemento. 1978, 75, 311–322. [Google Scholar]
- Juenger, M.C.G.; Monteiro, P.J.M.; Gartner, E.M.; Denbeaux, G.P. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cem. Concr. Res. 2005, 35, 19–25. [Google Scholar] [CrossRef]
- Daniels, J.L.; Janardhanam, R. Cold-weather subgrade stabilization. In Soil Improvement; Geotechnical Special Publication; Schaefer, V.R., Filz, G.M., Sehn, A.L., Wissmann, K.J., Eds.; ASCE Press: Denver, CO, USA, 2007; pp. 1–10. [Google Scholar]
- Bowers, B.F.; Daniels, J.L.; Anderson, J.B. Field considerations for calcium chloride modification of soil-cement. J. Mater. Civ. Eng. 2014, 26, 65–70. [Google Scholar] [CrossRef]
- Washburn, E.W. Note on a method of determining the distribution of pore sizes in a porous material. Proc. Natl. Acad. Sci. USA. 1921, 7, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Shang, J.Q.; Jeong, S. Thermo-mechanical properties and microfabric of fly ash-stabilized gold tailings. J. Hazard. Mater. 2014, 276, 323–331. [Google Scholar] [CrossRef] [PubMed]
- HorpibulsUK, S.; Rachan, R.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Bulid. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirement; Taylor and Francis: New York, NY, USA, 2006. [Google Scholar]
- Kamruzzaman, A.H.M.; Chew, S.H.; Lee, F.H. Microstructure of cement-treated Singapore marine clay. Proc. Inst. Civ. Eng. Ground Improv. 2006, 10, 113–123. [Google Scholar] [CrossRef]
- Cook, R.A.; Hover, K.C. Mercury porosimetry of hardened cement pastes. Cem. Concr. Res. 1999, 29, 933–943. [Google Scholar] [CrossRef]
- Luo, L.; Ye, G.; Schutter, G.D. Effect of corrosion inhibitors on cement paste pore structure. Proc. Inst. Civ. Eng. Constr. Mater. 2008, 161, 73–84. [Google Scholar] [CrossRef]
- Lemaire, K.; Deneele, D.; Bonnet, S.; Legret, M. Effects of lime and cement treatment on the physicochemical, microstructural and mechanical characteristics of a plastic silt. Eng. Geol. 2013, 166, 255–261. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metal. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Xing, H.; Yang, X.; Xu, C.; Ye, G. Strength characteristics and mechanics of salt-rich soil-cement. Eng. Geol. 2009, 130, 33–38. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry: For Clay Technologists, Geologists and Soil Scientists; Reprint Version; John Wiley and Sons: New York, NY, USA, 1991. [Google Scholar]
- Wang, Y.H.; Siu, W.K. Structure characteristics and mechanical properties of kaolinite soils. I. Surface charges and structural characterizations. Can. Geotech. J. 2006, 43, 587–600. [Google Scholar] [CrossRef]

| Chemical Composition | Gold Tailings (%) | Portland Cement (%) |
|---|---|---|
| Silicon dioxide (SiO2) | 50.82 | 19.48 |
| Ferric oxide (Fe2O3) | 28.97 | 3.05 |
| Aluminium oxide (Al2O3) | 8.87 | 5.14 |
| Magnesium oxide (MgO) | 3.39 | 2.21 |
| Calcium oxide (CaO) | 3.19 | 64.72 |
| Potassium oxide (K2O) | 0.84 | 0.44 |
| Titanium dioxide (TiO2) | 0.45 | ND |
| Magnesium oxide (MnO) | 0.36 | ND |
| Phosphorus pentoxide (P2O5) | 0.15 | ND |
| Sodium oxide (Na2O) | 0.02 | 0.17 |
| Chromium oxide (Cr2O3) | 0.02 | ND |
| Sulphur trioxide (SO3) | 0.05 | 3.36 |
| Loss of ignition (LOI) | 0.05 | 0.96 |
| Sample ID | Vgt | Vpc | Temperature (°C) | CaCl2 Conc. (M) | Total Pore Volume (mL/g) |
|---|---|---|---|---|---|
| Effect of tailings volume faction | |||||
| A1 | 26 | 4 | 25 | 0 | 0.265 |
| A2 | 28 | 4 | 25 | 0 | 0.240 |
| Effect of cement volume faction | |||||
| B1 | 28 | 2 | 25 | 0 | 0.268 |
| B2 (=A2) | 28 | 4 | 25 | 0 | 0.240 |
| Effect of curing temperature | |||||
| C1 | 28 | 4 | 5 | 0 | 0.266 |
| C2 (=A2) | 28 | 4 | 25 | 0 | 0.240 |
| C3 | 28 | 4 | 45 | 0 | 0.210 |
| Effect of CaCl2 concentration | |||||
| D1 (=A2) | 28 | 4 | 25 | 0 | 0.240 |
| D2 | 28 | 4 | 25 | 0.05 | 0.208 |
| D3 | 28 | 4 | 25 | 0.2 | 0.224 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Shang, J.Q. Micropore Structure of Cement-Stabilized Gold Mine Tailings. Minerals 2018, 8, 96. https://doi.org/10.3390/min8030096
Lee JK, Shang JQ. Micropore Structure of Cement-Stabilized Gold Mine Tailings. Minerals. 2018; 8(3):96. https://doi.org/10.3390/min8030096
Chicago/Turabian StyleLee, Joon Kyu, and Julie Q. Shang. 2018. "Micropore Structure of Cement-Stabilized Gold Mine Tailings" Minerals 8, no. 3: 96. https://doi.org/10.3390/min8030096




