Interaction of Freshwater Diatom with Gold Nanoparticles: Adsorption, Assimilation, and Stabilization by Cell Exometabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diatom Cultures
2.2. AuNPs Synthesis and Characterization
2.3. Short Term AuNPs Adsorption
2.4. Long Term AuNPS Assimilation
2.5. Analyses
2.6. Modeling
3. Results
3.1. Short-Term Kinetics of Adsorption
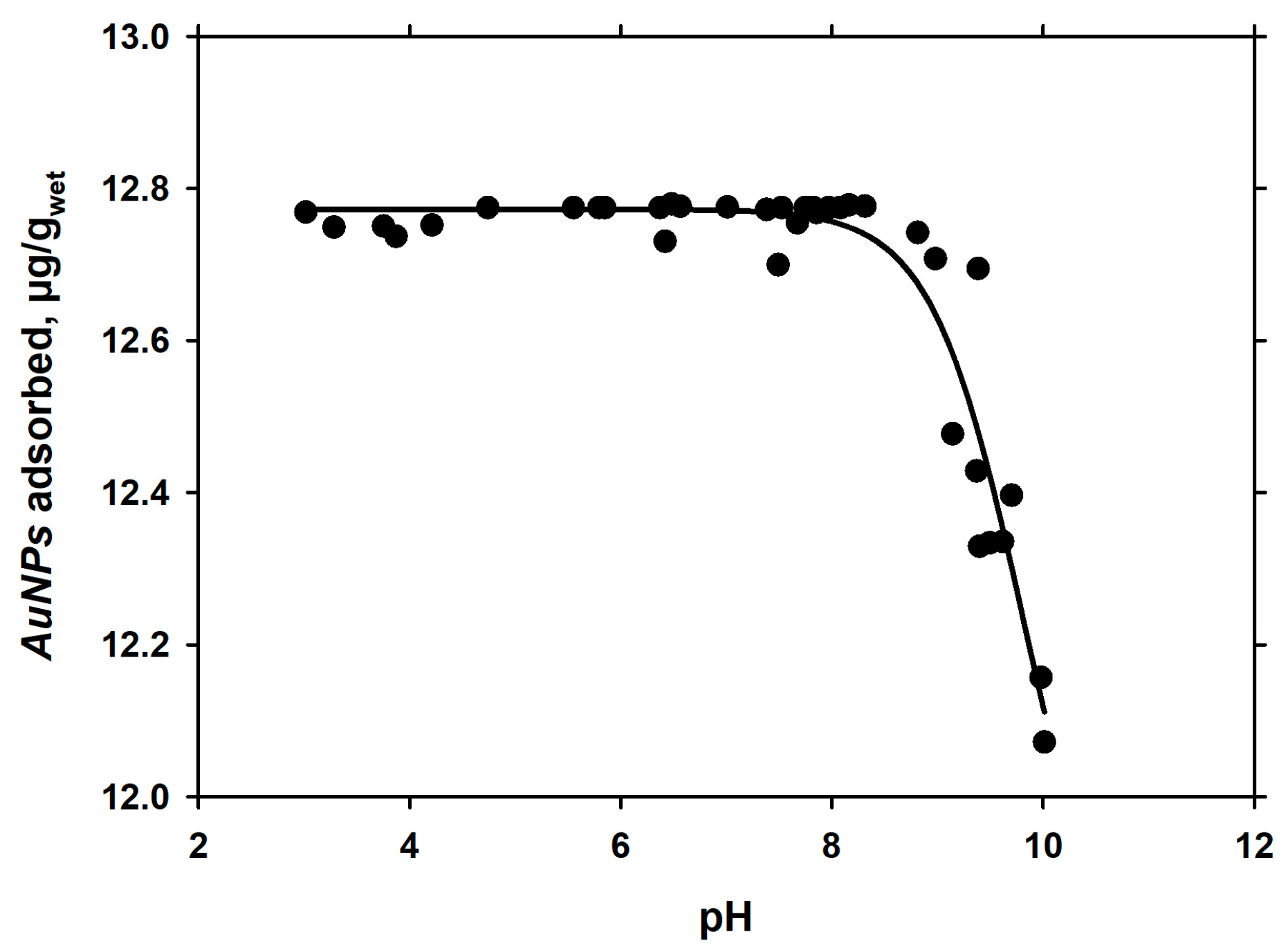
3.2. Dependence of Adsorption of pH
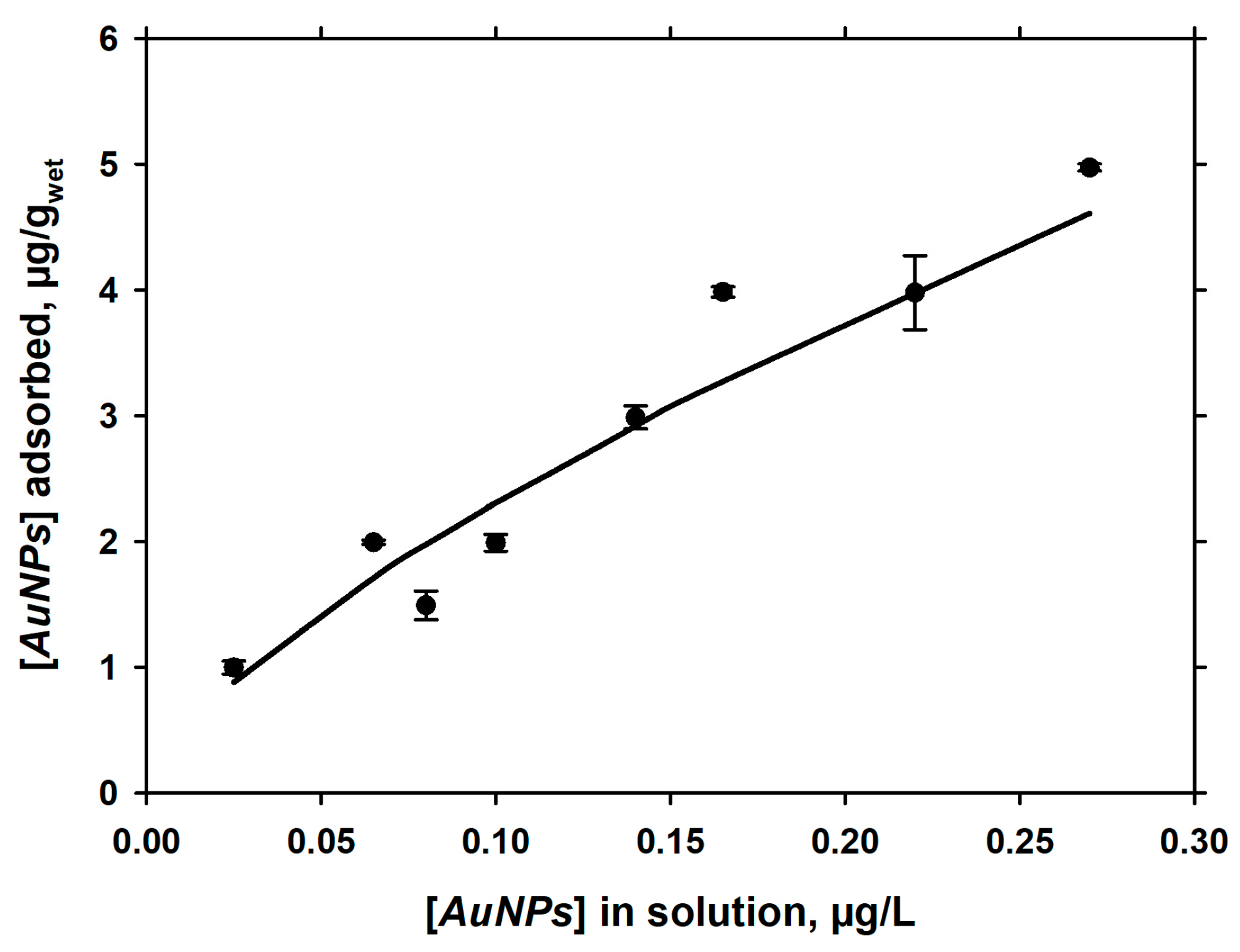
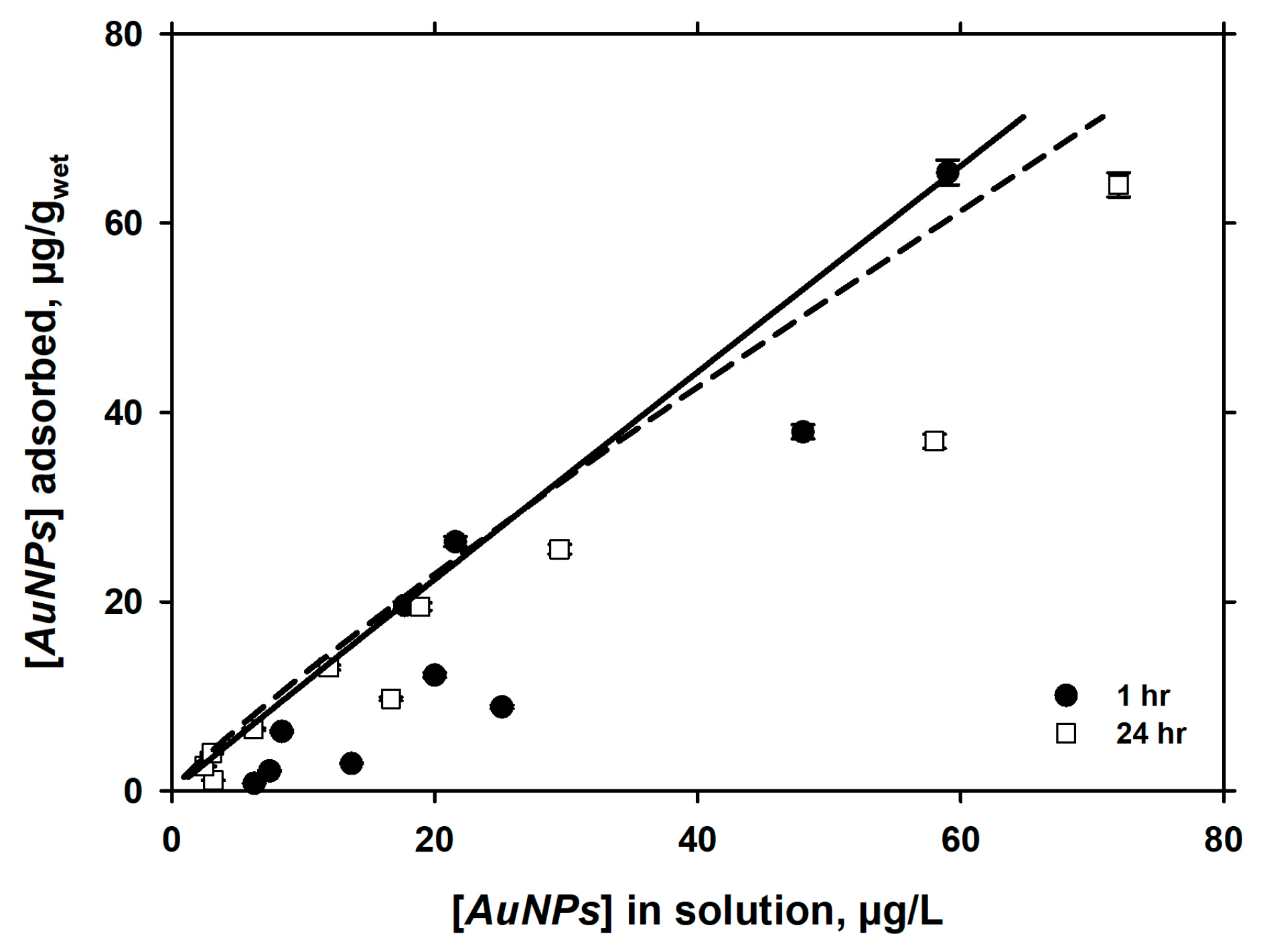
3.3. Langmuirian Adsorption at Constant pH, as a Function of AuNPs in Solution
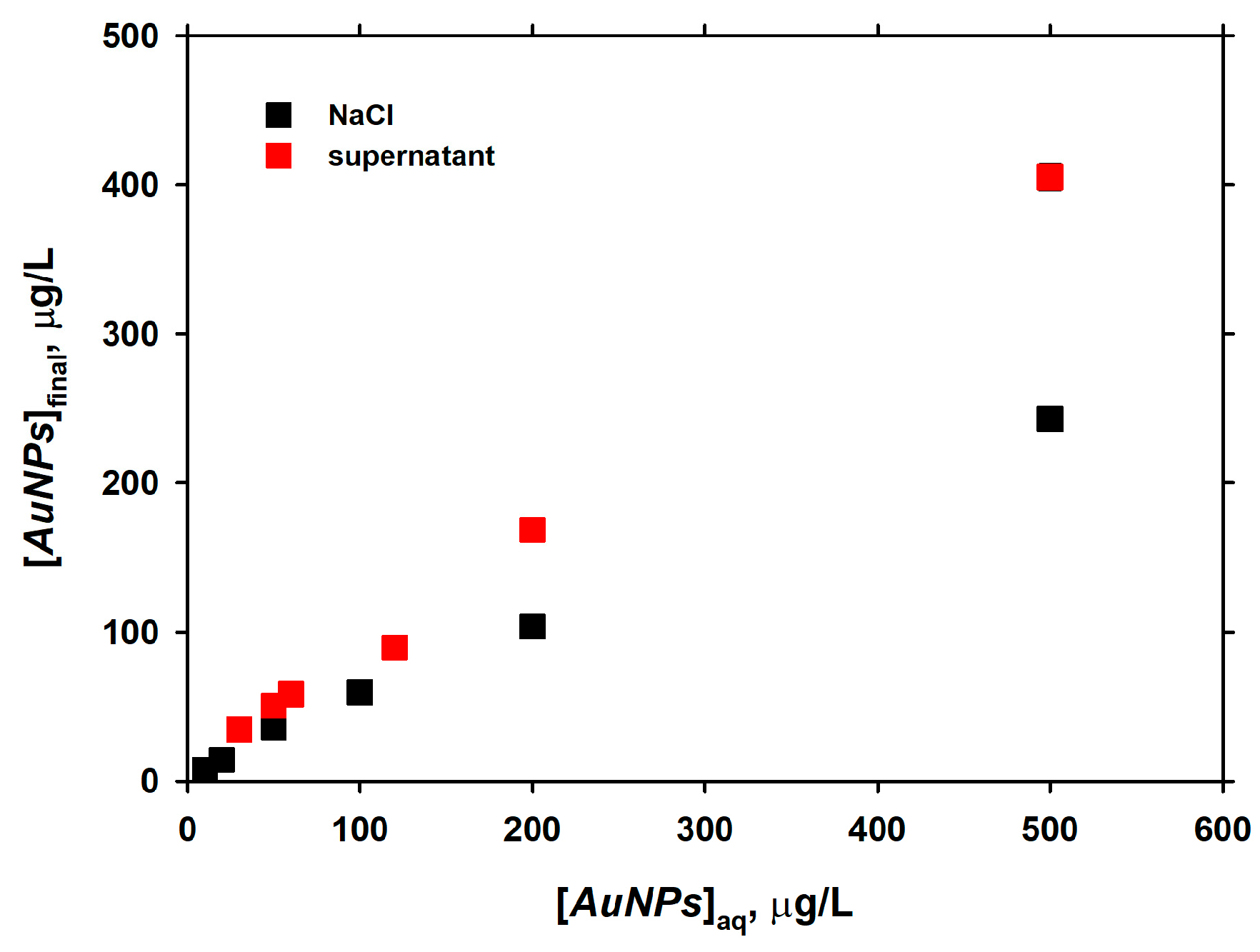
3.4. Long-Term Interaction of AuNPs with Live Diatom Cells
4. Conclusions and Natural Applications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatterjee, A.; Priyam, A.; Bhattacharya, S.C.; Saha, A. pH dependent interaction of biofunctionalized CdS nanoparticles with nucleobases and nucleotides: A fluorimetric study. J. Lumin. 2007, 126, 764–770. [Google Scholar] [CrossRef]
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry and Biology; World Scientific: Singapore, 2017; ISBN 1786341263. [Google Scholar]
- Chen, H.-C.; Liu, Y.-C. Creating functional water by treating excited gold nanoparticles for the applications of green chemistry, energy and medicine: A review. J. Ind. Eng. Chem. 2017, 60, 9–18. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.M.; Schürch, S.; Haenni, B.; Kapp, N.; Gehr, P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ. Sci. Technol. 2006, 40, 4353–4359. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.; Cresanti, R. Environmental Health and Safety Research Needs for Engineered Nanoscale Materials; Executive Office of the President Washington DC National Science and Technology Council: Washington, DC, USA, 2006.
- Chang, M.R.; Lee, D.J.; Lai, J.Y. Nanoparticles in wastewater from a science-based industrial park-Coagulation using polyaluminum chloride. J. Environ. Manag. 2007, 85, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.; Bizi, M.; Guiraud, P.; Labille, J.; Janex-Habibi, M.-L. NANOSEP: Removal of nanoparticles using optimized conventional processes. In Proceedings of the Water Quality Technology Conference and Exposition, Phoenix, AZ, USA, 13–17 November 2011; pp. 995–1004. [Google Scholar]
- Bizi, M. Stability and flocculation of nanosilica by conventional organic polymer. Nat. Sci. 2012, 4, 372–385. [Google Scholar] [CrossRef]
- Patwa, A.; Labille, J.; Bottero, J.-Y.; Thiéry, A.; Barthélémy, P. Decontamination of nanoparticles from aqueous samples using supramolecular gels. Chem. Commun. 2015, 51, 2547–2550. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Exposure of the blue mussel, Mytilus edulis, to gold nanoparticles and the pro-oxidant menadione. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Baudrimont, M.; Andrei, J.; Mornet, S.; Gonzalez, P.; Mesmer-Dudons, N.; Gourves, P.Y.; Jaffal, A.; Dedourge-Geffard, O.; Geffard, A.; Geffard, O.; et al. Trophic transfer and effects of gold nanoparticles (AuNPs) in Gammarus fossarum from contaminated periphytic biofilm. Environ. Sci. Pollut. Res. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Christian, P.; Urrea, J.A.G.; Roos, N.; Hassellöv, M.; Tollefsen, K.E.; Thomas, K.V. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2010, 96, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Doyle, H.; Redmond, G.; Sheehan, D. Gold nanoparticles and oxidative stress in Mytilus edulis. Mar. Environ. Res. 2008, 66, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Renault, S.; Baudrimont, M.; Mesmer-Dudons, N.; Gonzalez, P.; Mornet, S.; Brisson, A. Impacts of gold nanoparticle exposure on two freshwater species: A phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea). Gold Bull. 2008, 41, 116–126. [Google Scholar] [CrossRef]
- Lapresta-Fernández, A.; Fernández, A.; Blasco, J. Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. TrAC Trends Anal. Chem. 2012, 32, 40–59. [Google Scholar] [CrossRef]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat. Res. Rev. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Fein, J.B.; Daughney, C.J.; Yee, N.; Davis, T.A. A chemical equilibrium model of metal adsorption onto bacterial surfaces. Geochim. Cosmochim. Acta 1997, 61, 3319–3328. [Google Scholar] [CrossRef]
- Boyanov, M.I.; Kelly, S.D.; Kemner, K.M.; Bunker, B.A.; Fein, J.B.; Fowle, D.A. Adsorption of cadmium to Bacillus subtilis bacterial cell walls: A pH-dependent X-ray absorption fine structure spectroscopy study. Geochim. Cosmochim. Acta 2003, 67, 3299–3311. [Google Scholar] [CrossRef]
- Burnett, P.G.G.; Daughney, C.J.; Peak, D. Cd adsorption onto Anoxybacillus flavithermus: Surface complexation modeling and spectroscopic investigations. Geochim. Cosmochim. Acta 2006, 70, 5253–5269. [Google Scholar] [CrossRef]
- Guiné, V.; Spadini, L.; Sarret, G.; Muris, M.; Delolme, C.; Gaudet, J.-P.; Martins, J.M.F. Zinc sorption to three gram-negative bacteria: Combined titration, modeling, and EXAFS study. Environ. Sci. Technol. 2006, 40, 1806–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fein, J.B.; Martin, A.M.; Wightman, P.G. Metal adsorption onto bacterial surfaces: Development of a predictive approach. Geochim. Cosmochim. Acta 2001, 65, 4267–4273. [Google Scholar] [CrossRef]
- Martinez, R.E.; Ferris, F.G.G. Chemical equilibrium modeling techniques for the analysis of high-resolution bacterial metal sorption data. J. Colloid Interface Sci. 2001, 243, 73–80. [Google Scholar] [CrossRef]
- Martinez, R.E.; Pedersen, K.; Ferris, F.G. Cadmium complexation by bacteriogenic iron oxides from a subterranean environment. J. Colloid Interface Sci. 2004, 275, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Borrok, D.M.; Fein, J.B.; Kulpa, C.F.J. Cd and Proton adsorption onto bacterial grown from industrial wastes and contaminated geologic settings. Environ. Sci. Technol. 2004, 38, 5656–5664. [Google Scholar] [CrossRef] [PubMed]
- Borrok, D.; Turner, B.F.; Fein, J.B. A universal surface complexation framework for modeling proton binding onto bacterial surfaces in geologic settings. Am. J. Sci. 2005, 305, 826–853. [Google Scholar] [CrossRef]
- Gélabert, A.; Pokrovsky, O.S.; Viers, J.; Schott, J.; Boudou, A.; Feurtet-Mazel, A. Interaction between zinc and freshwater and marine diatom species: Surface complexation and Zn isotope fractionation. Geochim. Cosmochim. Acta 2006, 70, 839–857. [Google Scholar] [CrossRef]
- Gélabert, A.; Pokrovsky, O.S.; Schott, J.; Boudou, A.; Feurtet-Mazel, A. Cadmium and lead interaction with diatom surfaces: A combined thermodynamic and kinetic approach. Geochim. Cosmochim. Acta 2007, 71, 3698–3716. [Google Scholar] [CrossRef]
- González, A.G.; Pokrovsky, O.S.; Jiménez-Villacorta, F.; Shirokova, L.S.; Santana-Casiano, J.M.; González-Dávila, M.; Emnova, E.E. Iron adsorption onto soil and aquatic bacteria: XAS structural study. Chem. Geol. 2014, 372, 32–45. [Google Scholar] [CrossRef]
- González, A.G.; Jimenez-Villacorta, F.; Beike, A.K.; Reski, R.; Adamo, P.; Pokrovsky, O.S. Chemical and structural characterization of copper adsorbed on mosses (Bryophyta). J. Hazard. Mater. 2016, 308, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Gélabert, A.; Pokrovsky, O.S.; Schott, J.; Boudou, A.; Feurtet-Mazel, A.; Mielczarski, J.; Mielczarsky, E.; Mesmer-Dudons, N.; Spalla, O. Study of diatoms/aqueous solution interface. I. Acid-base equilibria, surface charge and spectroscopic observation of two freshwater peryphytic and two marine planctonic diatoms. Geochim. Cosmochim. Acta 2004, 68, 4039–4058. [Google Scholar] [CrossRef]
- Kim Tiam, S.; Feurtet-Mazel, A.; Delmas, F.; Mazzella, N.; Morin, S.; Daffe, G.; Gonzalez, P. Development of q-PCR approaches to assess water quality: Effects of cadmium on gene expression of the diatom Eolimna minima. Water Res. 2012, 46, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisset, S.; Tiam, S.K.; Feurtet-Mazel, A.; Morin, S.; Delmas, F.; Mazzella, N.; Gonzalez, P. Genetic and physiological responses of three freshwater diatoms to realistic diuron exposures. Environ. Sci. Pollut. Res. 2015, 22, 4046–4055. [Google Scholar] [CrossRef] [PubMed]
- Feurtet-Mazel, A.; Mornet, S.; Charron, L.; Mesmer-Dudons, N.; Maury-Brachet, R.; Baudrimont, M. Biosynthesis of gold nanoparticles by the living freshwater diatom Eolimna minima, a species developed in river biofilms. Environ. Sci. Pollut. Res. 2016, 23, 4334–4339. [Google Scholar] [CrossRef] [PubMed]
- Gold, C.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Impacts of Cd and Zn on the development of periphytic diatom communities in artificial streams located along a river pollution gradient. Arch. Environ. Contam. Toxicol. 2003, 44, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Martinez, R.E.; Golubev, S.V.; Kompantseva, E.I.; Shirokova, L.S. Adsorption of metals and protons on Gloeocapsa sp. cyanobacteria: A surface speciation approach. Appl. Geochem. 2008, 23, 2574–2588. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Martinez, R.E.; Kompantzeva, E.I.; Shirokova, L.S. Surface complexation of the phototrophic anoxygenic non-sulfur bacterium Rhodopseudomonas palustris. Chem. Geol. 2014, 383, 51–62. [Google Scholar] [CrossRef]
- González, A.G.; Shirokova, L.S.; Pokrovsky, O.S.; Emnova, E.E.; Martínez, R.E.; Santana-Casiano, J.M.; González-Dávila, M.; Pokrovski, G.S. Adsorption of copper on Pseudomonas aureofaciens: Protective role of surface exopolysaccharides. J. Colloid Interface Sci. 2010, 350, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Feurtet-Mazel, A.; Martinez, R.E.; Morin, S.; Baudrimont, M.; Duong, T.; Coste, M. Experimental study of cadmium interaction with periphytic biofilms. Appl. Geochem. 2010, 25, 418–427. [Google Scholar] [CrossRef]
- Stankus, D.P.; Lohse, S.E.; Hutchison, J.E.; Nason, J.A. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 2011, 45, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Louie, S.M.; Spielman-Sun, E.R.; Small, M.J.; Tilton, R.D.; Lowry, G.V. Correlation of the physicochemical properties of natural organic matter samples from different sources to their effects on gold nanoparticle aggregation in monovalent electrolyte. Environ. Sci. Technol. 2015, 49, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fein, J.B. Sulfhydryl binding sites within bacterial extracellular polymeric substances. Environ. Sci. Technol. 2016, 50, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Bundeleva, I.A.; Shirokova, L.S.; Pokrovsky, O.S.; Bénézeth, P.; Ménez, B.; Gérard, E.; Balor, S. Experimental modeling of calcium carbonate precipitation by cyanobacterium Gloeocapsa sp. Chem. Geol. 2014, 374–375, 44–60. [Google Scholar] [CrossRef]
- Rico, M.; López, A.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol. Oceanogr. 2013, 51, 144–152. [Google Scholar] [CrossRef]
- González, A.G.; Santana-Casiano, J.M.; González-Dávila, M.; Pérez-Almeida, N.; Suárez de Tangil, M. Effect of Dunaliella tertiolecta organic exudates on the Fe(II) oxidation kinetics in seawater. Environ. Sci. Technol. 2014, 48, 7933–7941. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Santana-Casiano, J.M.; González-Dávila, M.; Perez, N. Effect of organic exudates of Phaeodactylum tricornutum on the Fe(II) oxidation rate constant. Cienc. Mar. 2012, 38, 245–261. [Google Scholar] [CrossRef]
- Santana-Casiano, J.M.; González-Dávila, M.; González, A.G.; Rico, M.; Lopez, A.; Martel, A. Characterization of phenolic exudates from Phaeodactylum tricornutum and their effects on the chemistry of Fe(II)-Fe(III). Mar. Chem. 2014, 158, 10–16. [Google Scholar] [CrossRef]
- González, A.G.; Fernández-Rojo, L.; Leflaive, J.; Pokrovsky, O.S.; Rols, J.L. Response of three biofilm-forming benthic microorganisms to Ag nanoparticles and Ag+: The diatom Nitzschia palea, the green alga Uronema confervicolum and the cyanobacteria Leptolyngbya sp. Environ. Sci. Pollut. Res. 2016, 23, 22136–22150. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Biomineralization in diatoms mediated through peptide-and polyamine-assisted condensation of silica. Angew. Chem. Int. Ed. 2002, 41, 3167–3169. [Google Scholar] [CrossRef]
- Wallace, A.F.; DeYoreo, J.J.; Dove, P.M. Kinetics of silica nucleation on carboxyl- and amine-terminated surfaces: Insights for biomineralization. J. Am. Chem. Soc. 2009, 131, 5244–5250. [Google Scholar] [CrossRef] [PubMed]
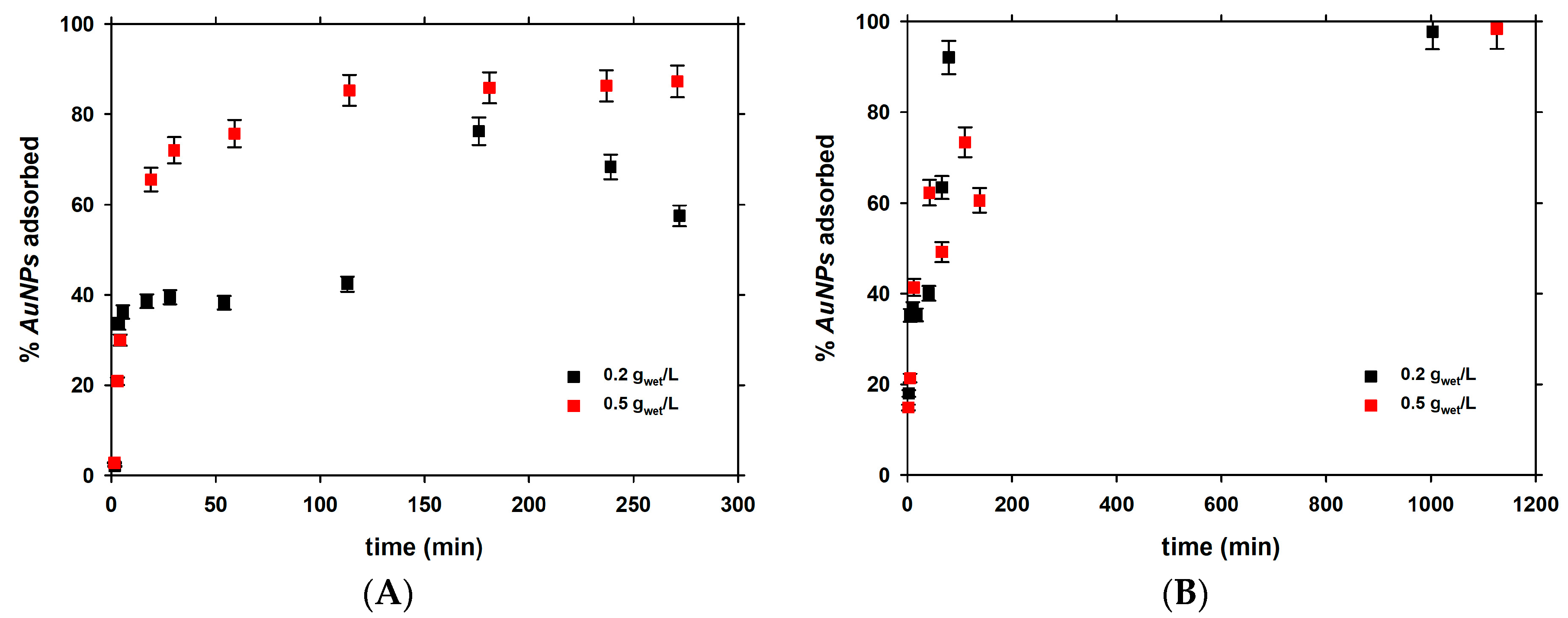
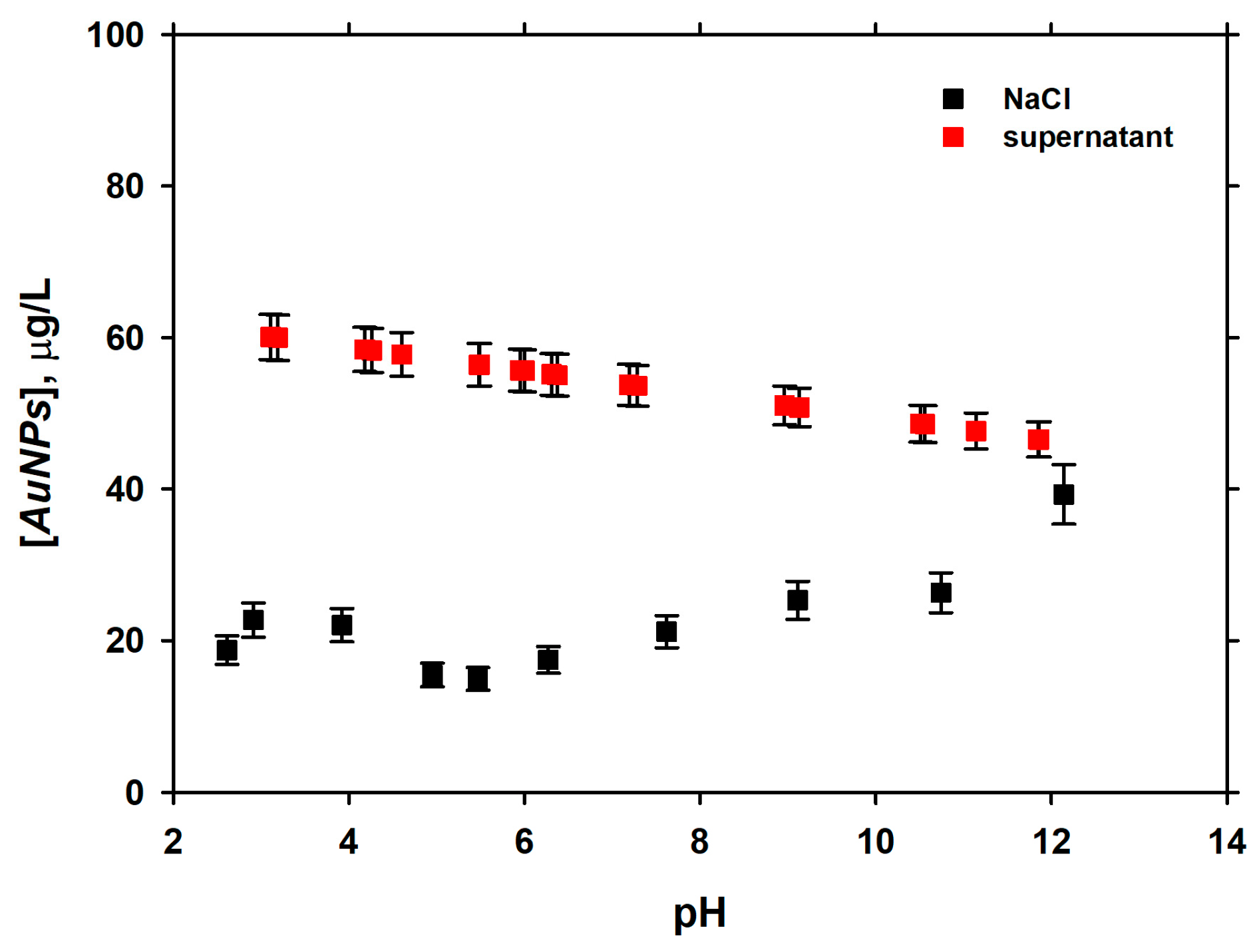
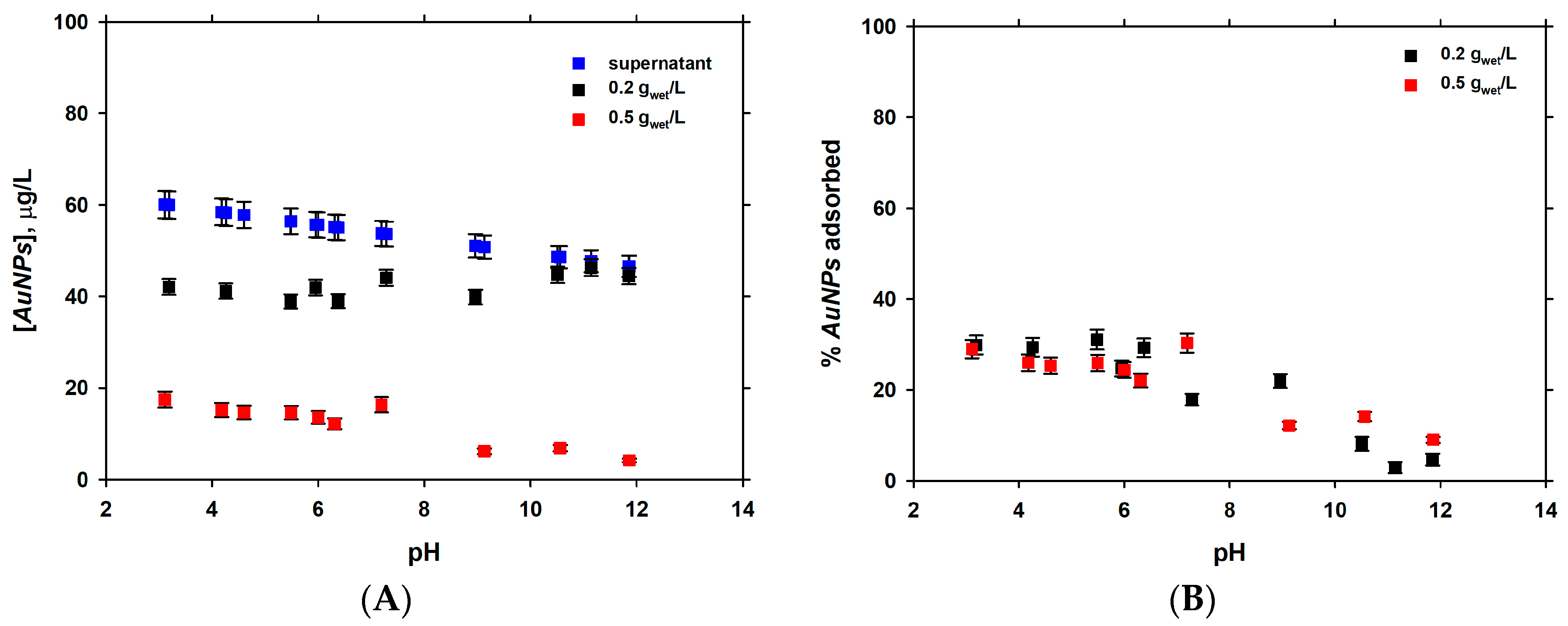
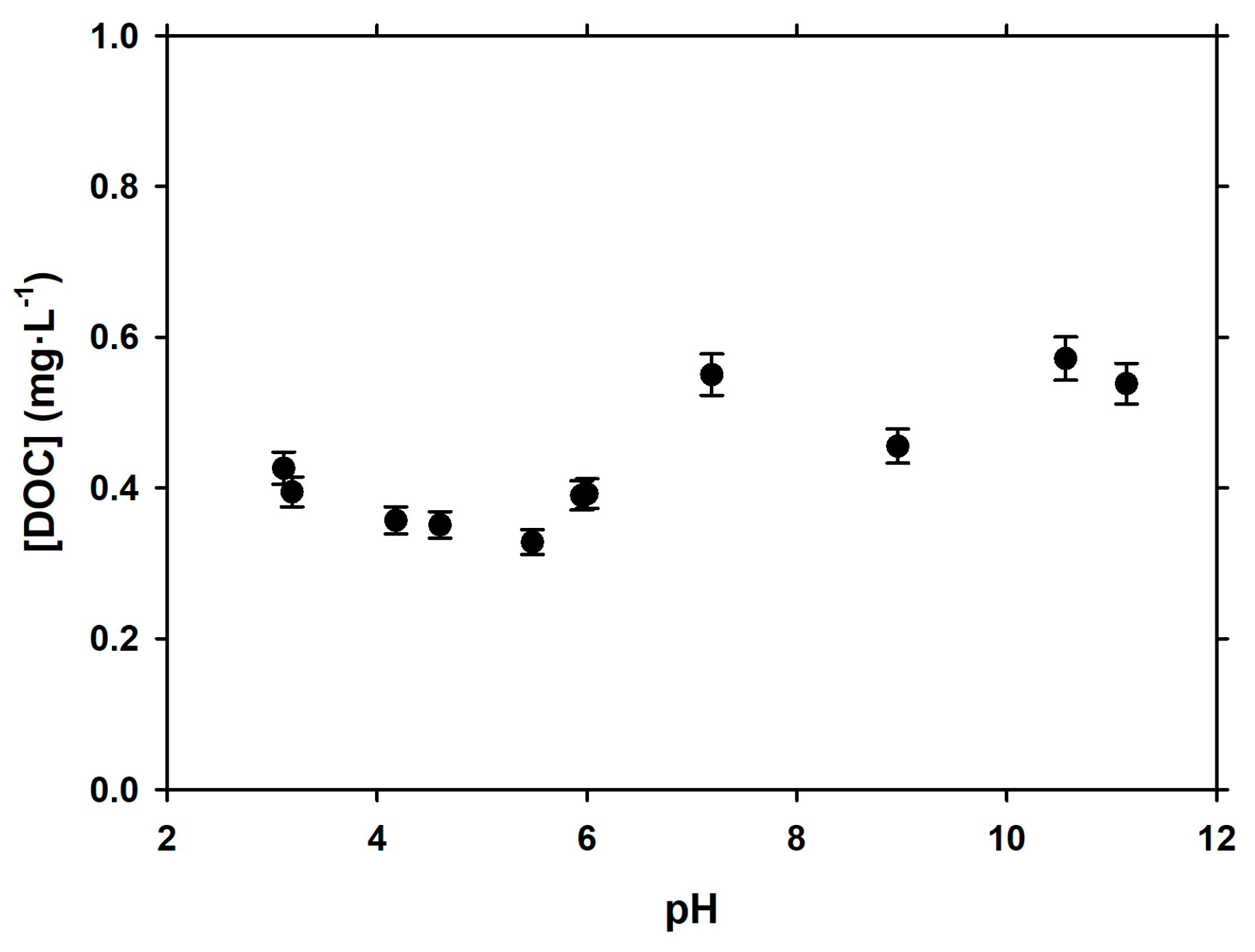



| Experiment | Initial AuNPs (µg·L−1) | k (L·µg−1·min−1) | t1/2 (min) |
|---|---|---|---|
| low AuNPs and 0.2 gwet/L EOMI | 3.60 | 7.50 × 10−3 | 37.0 |
| low AuNPs and 0.5 gwet/L EOMI | 8.50 | 1.05 × 10−2 | 11.2 |
| high AuNPs and 0.2 gwet/L EOMI | 115 | 4.00 × 10−4 | 21.8 |
| high AuNPs and 0.5 gwet/L EOMI | 111 | 4.00 × 10−4 | 22.5 |
| Parameter | Coefficient | Std. Error |
|---|---|---|
| min | 11.69 | 0.189 |
| max | 12.77 | 0.0118 |
| log EC50 | 9.81 | 0.142 |
| Medium | pH | Contact Time (h) | log kF | kF (mol/L) | 1/n | n (mol/L) |
|---|---|---|---|---|---|---|
| 5 mM NaCl + 5 mM NaHCO3 | 8.56 ± 0.05 | 2 | −0.459 | 0.347 | 0.696 | 1.436 |
| 10 mM NaCl | 7.40 ± 0.06 | 1 | 0.621 | 4.177 | 1.115 | 0.897 |
| 7.10 ± 0.06 | 24 | 0.035 | 1.085 | 1.014 | 0.986 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, A.G.; Pokrovsky, O.S.; Ivanova, I.S.; Oleinikova, O.; Feurtet-Mazel, A.; Mornet, S.; Baudrimont, M. Interaction of Freshwater Diatom with Gold Nanoparticles: Adsorption, Assimilation, and Stabilization by Cell Exometabolites. Minerals 2018, 8, 99. https://doi.org/10.3390/min8030099
González AG, Pokrovsky OS, Ivanova IS, Oleinikova O, Feurtet-Mazel A, Mornet S, Baudrimont M. Interaction of Freshwater Diatom with Gold Nanoparticles: Adsorption, Assimilation, and Stabilization by Cell Exometabolites. Minerals. 2018; 8(3):99. https://doi.org/10.3390/min8030099
Chicago/Turabian StyleGonzález, Aridane G., Oleg S. Pokrovsky, Irina S. Ivanova, Olga Oleinikova, Agnes Feurtet-Mazel, Stephane Mornet, and Magalie Baudrimont. 2018. "Interaction of Freshwater Diatom with Gold Nanoparticles: Adsorption, Assimilation, and Stabilization by Cell Exometabolites" Minerals 8, no. 3: 99. https://doi.org/10.3390/min8030099





