Influence of Addition of Fluid Catalytic Cracking Residue (FCC) and the SiO2 Concentration in Alkali-Activated Ceramic Sanitary-Ware (CSW) Binders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Flow Chart, Mix Proportions and Curing Conditions
2.3. Sample Preparation and Characterization
3. Results and Discussion
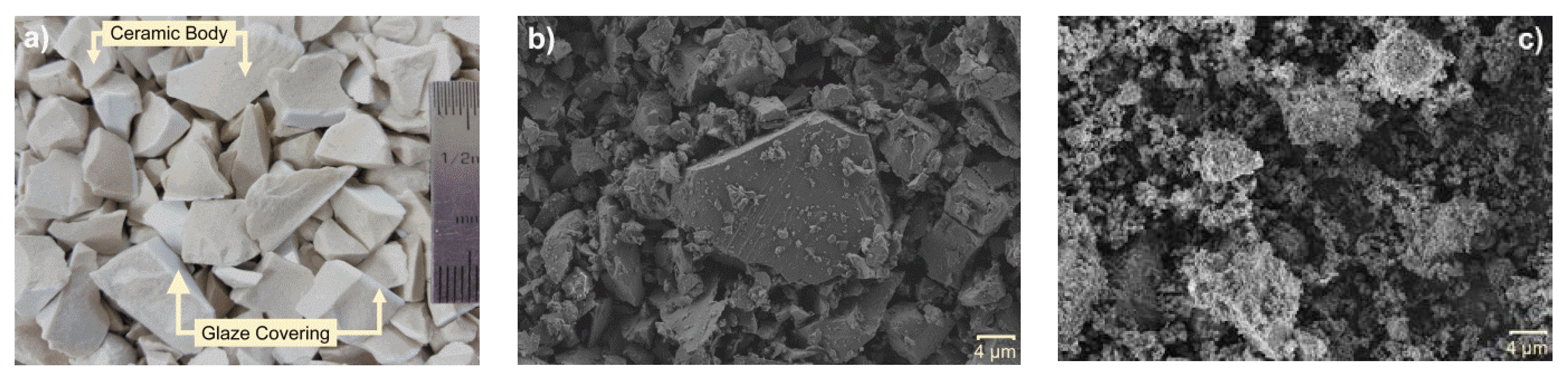
3.1. Properties of Raw Materials
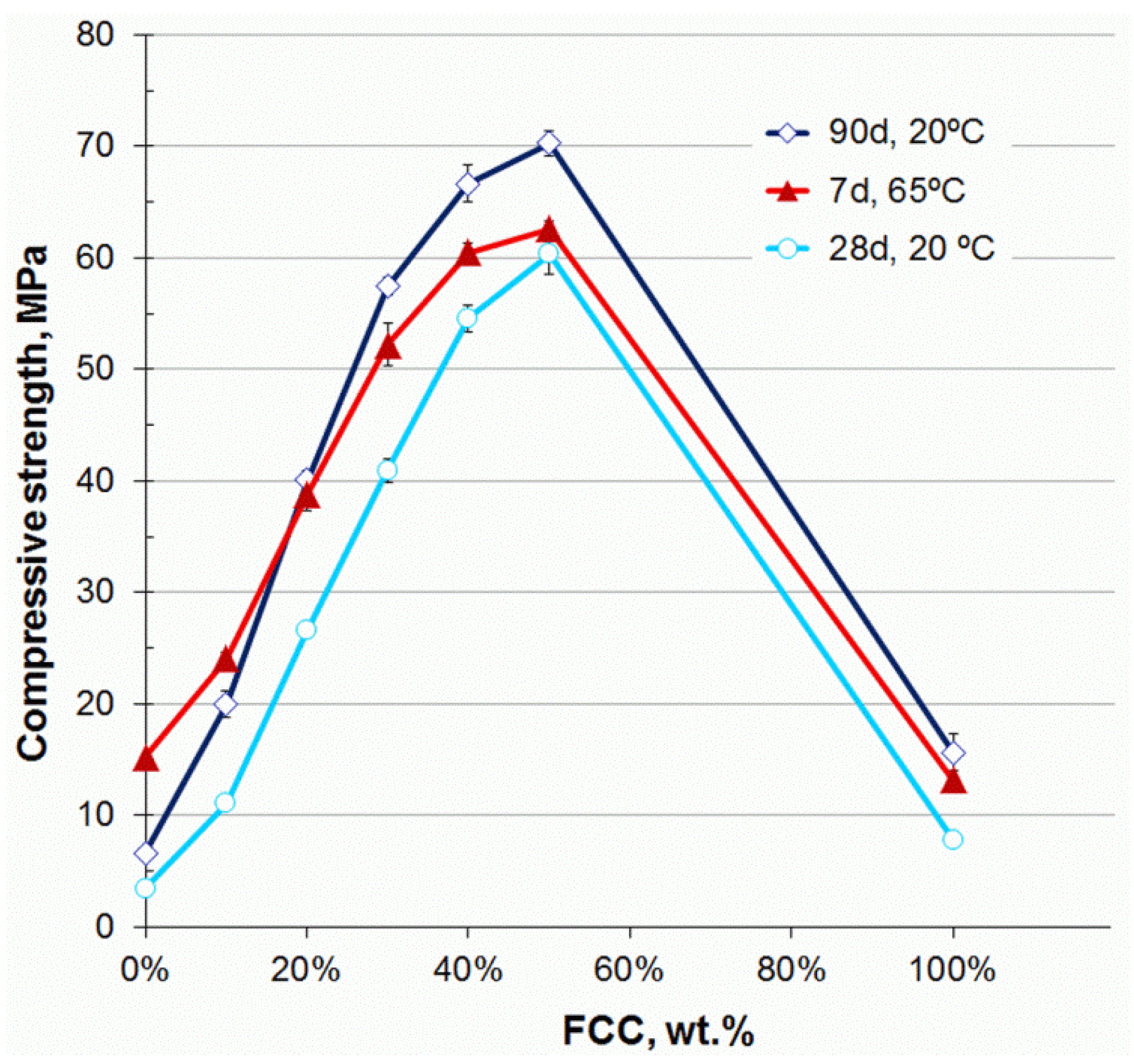
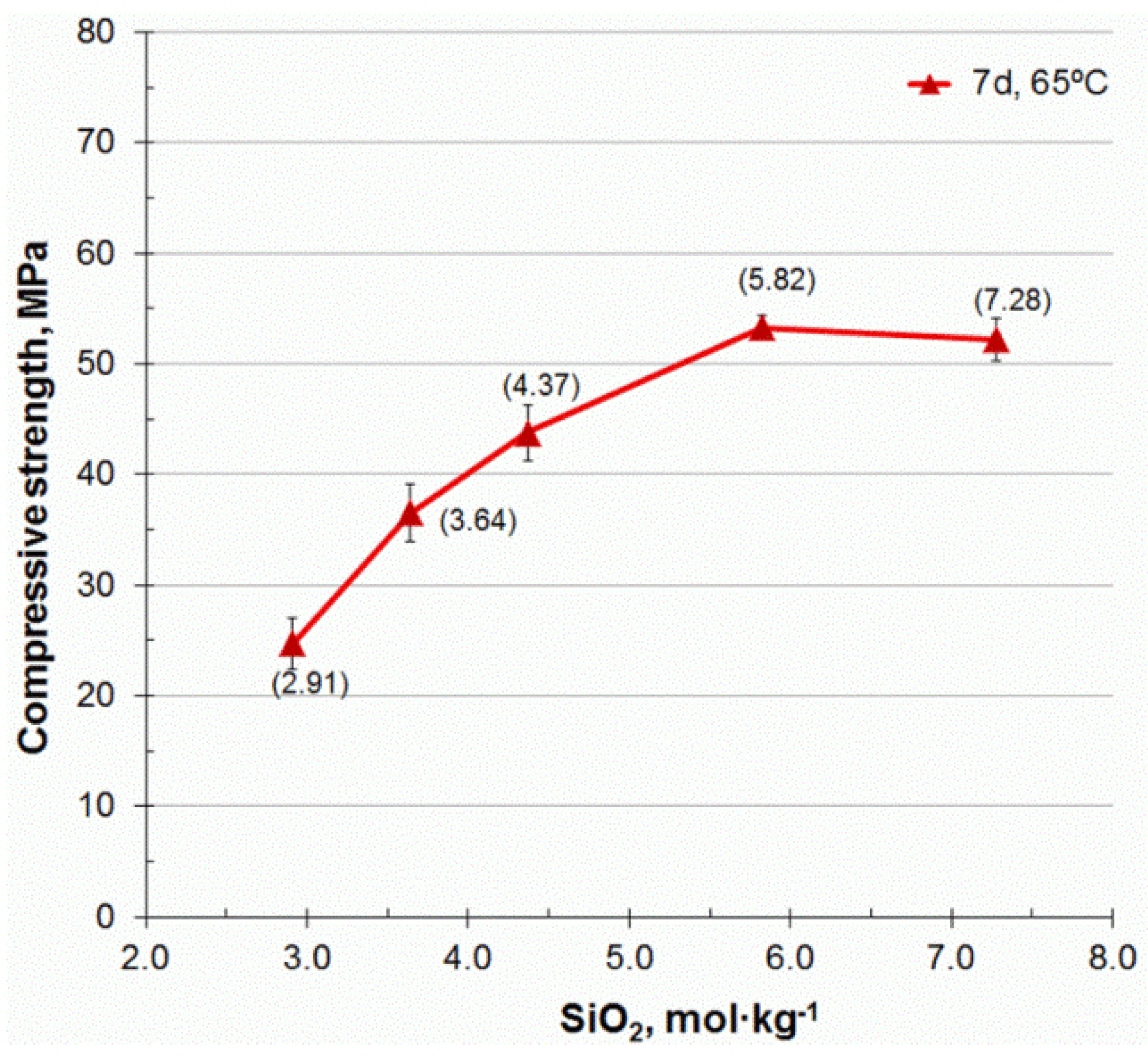
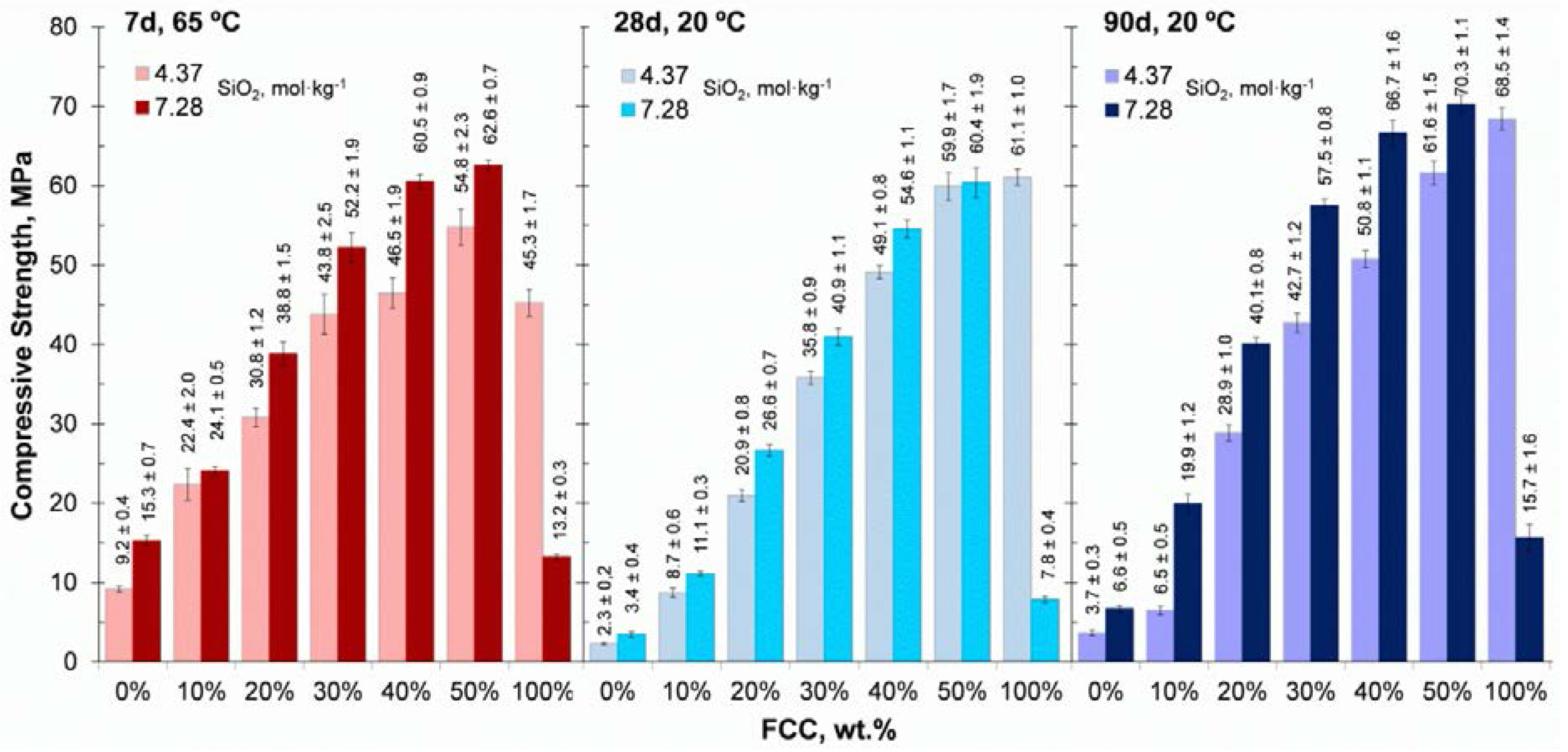
3.2. Compressive Strength
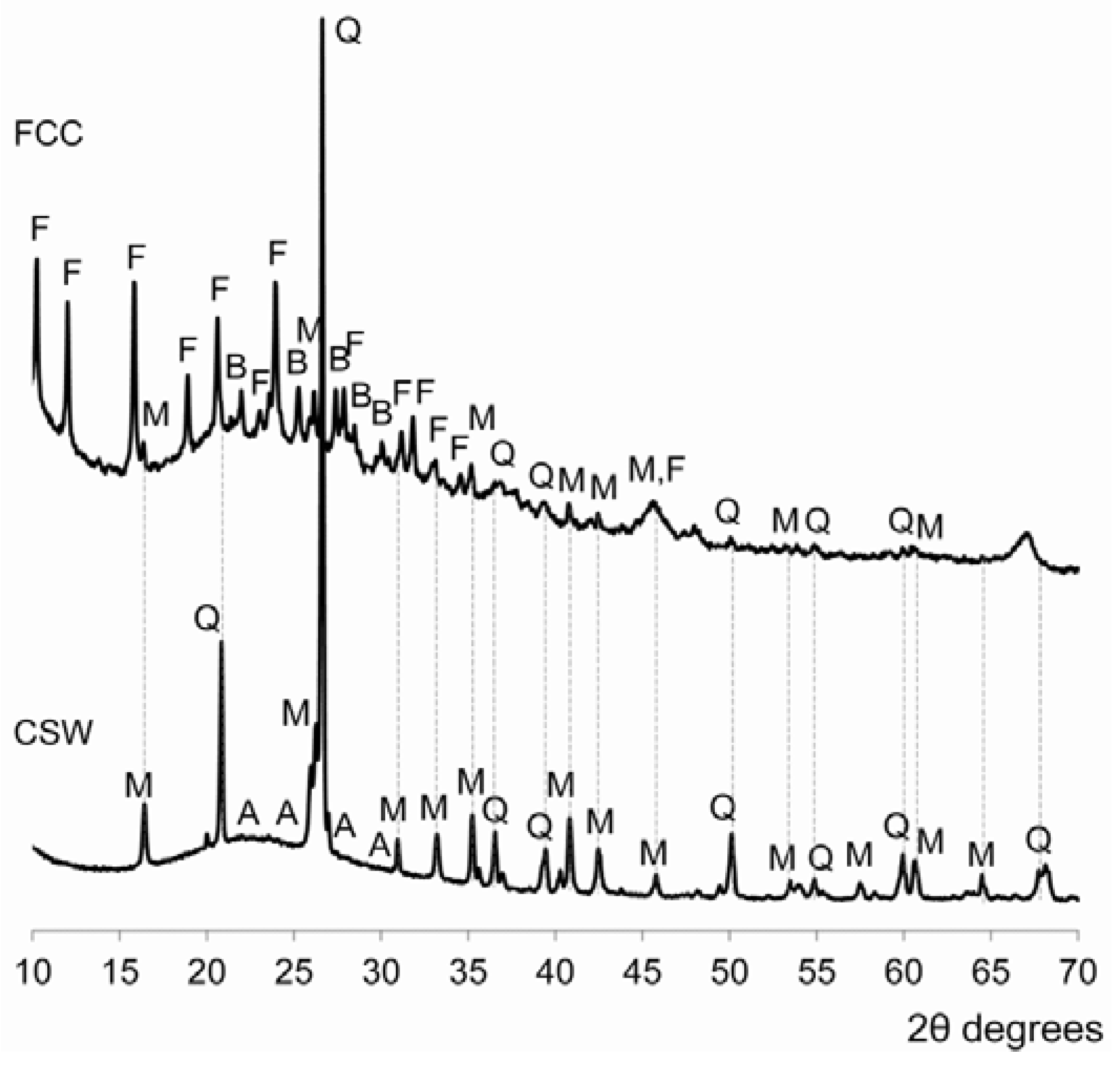
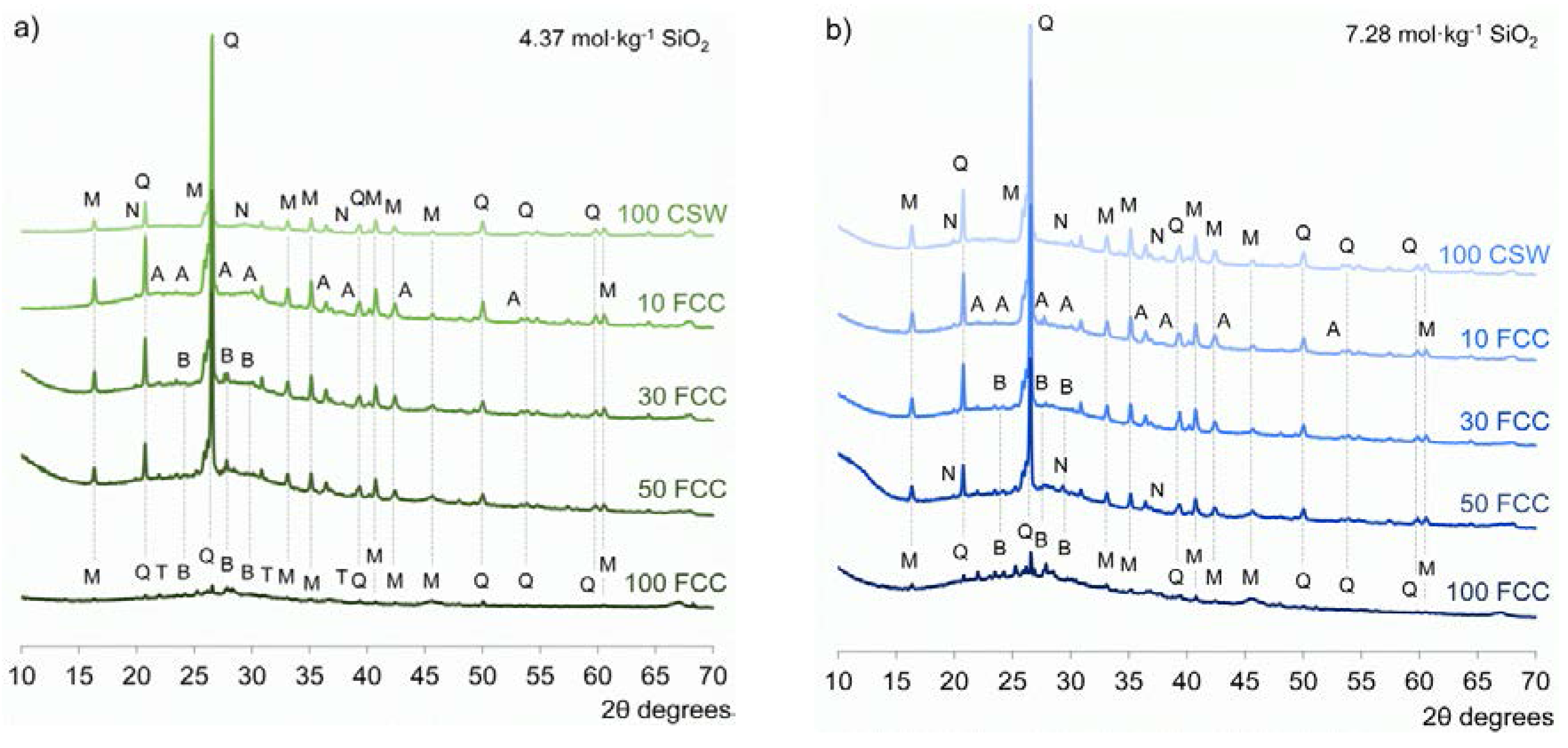
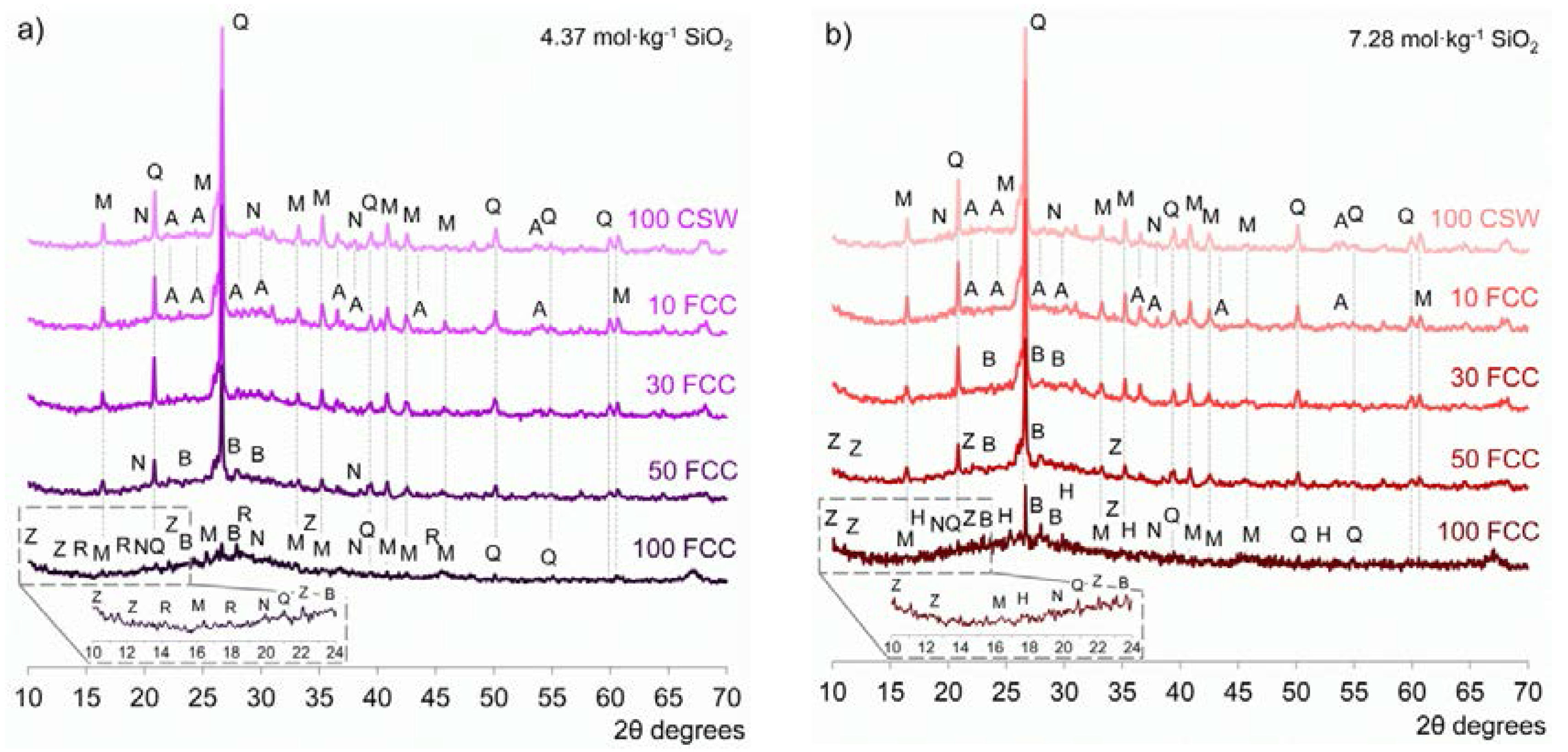
3.3. X-ray Diffraction (XRD)
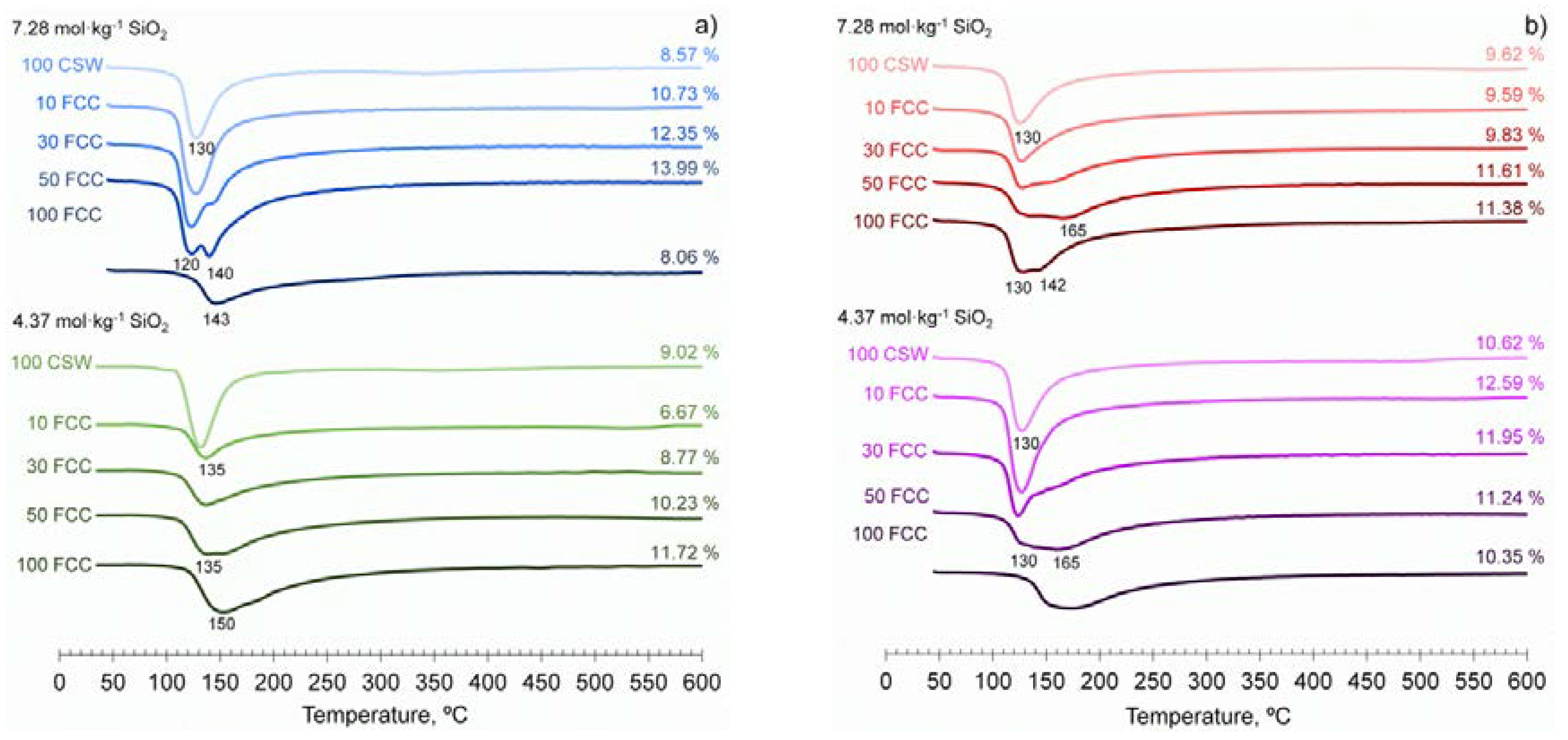
3.4. Thermal Analysis
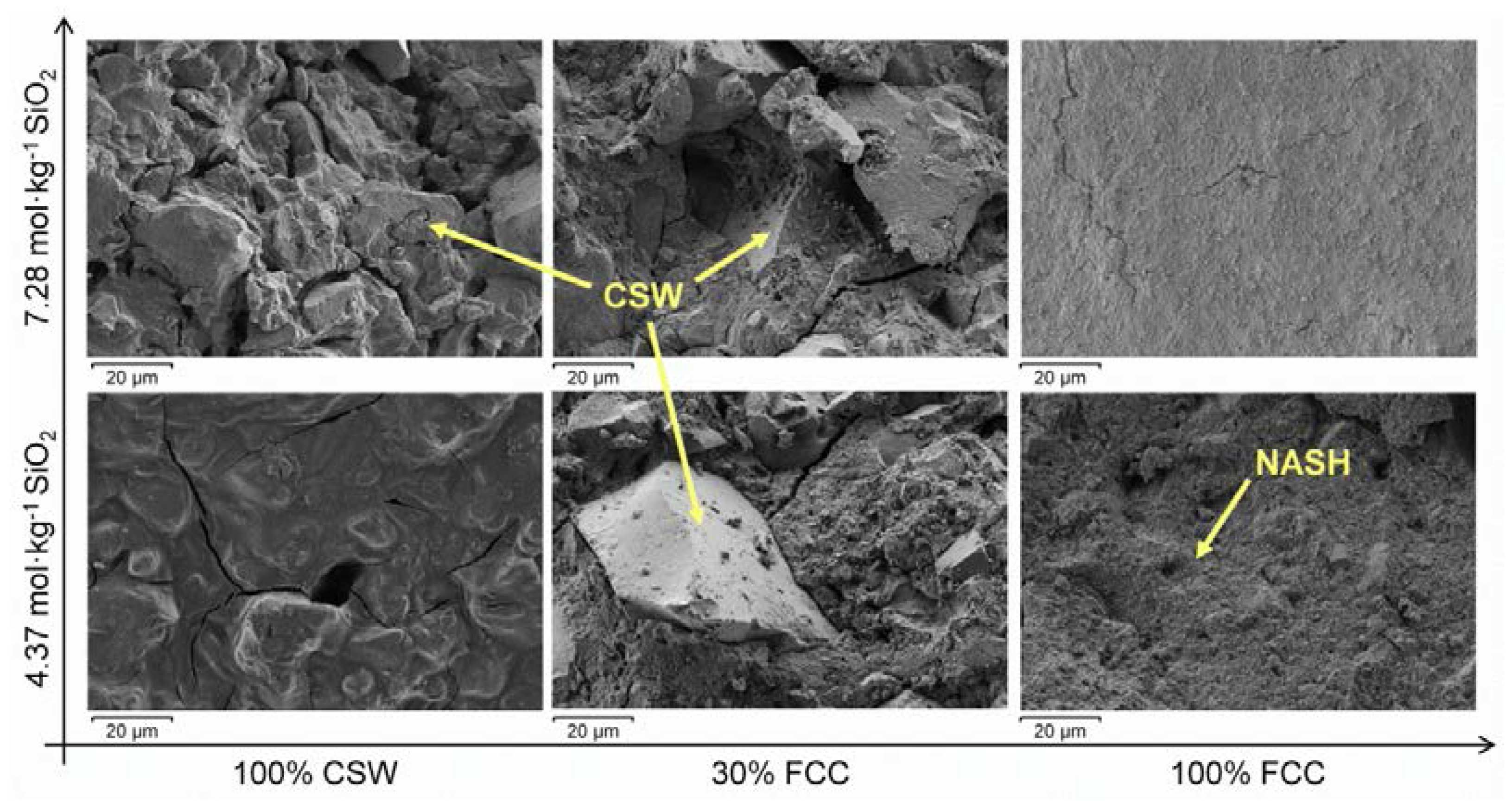
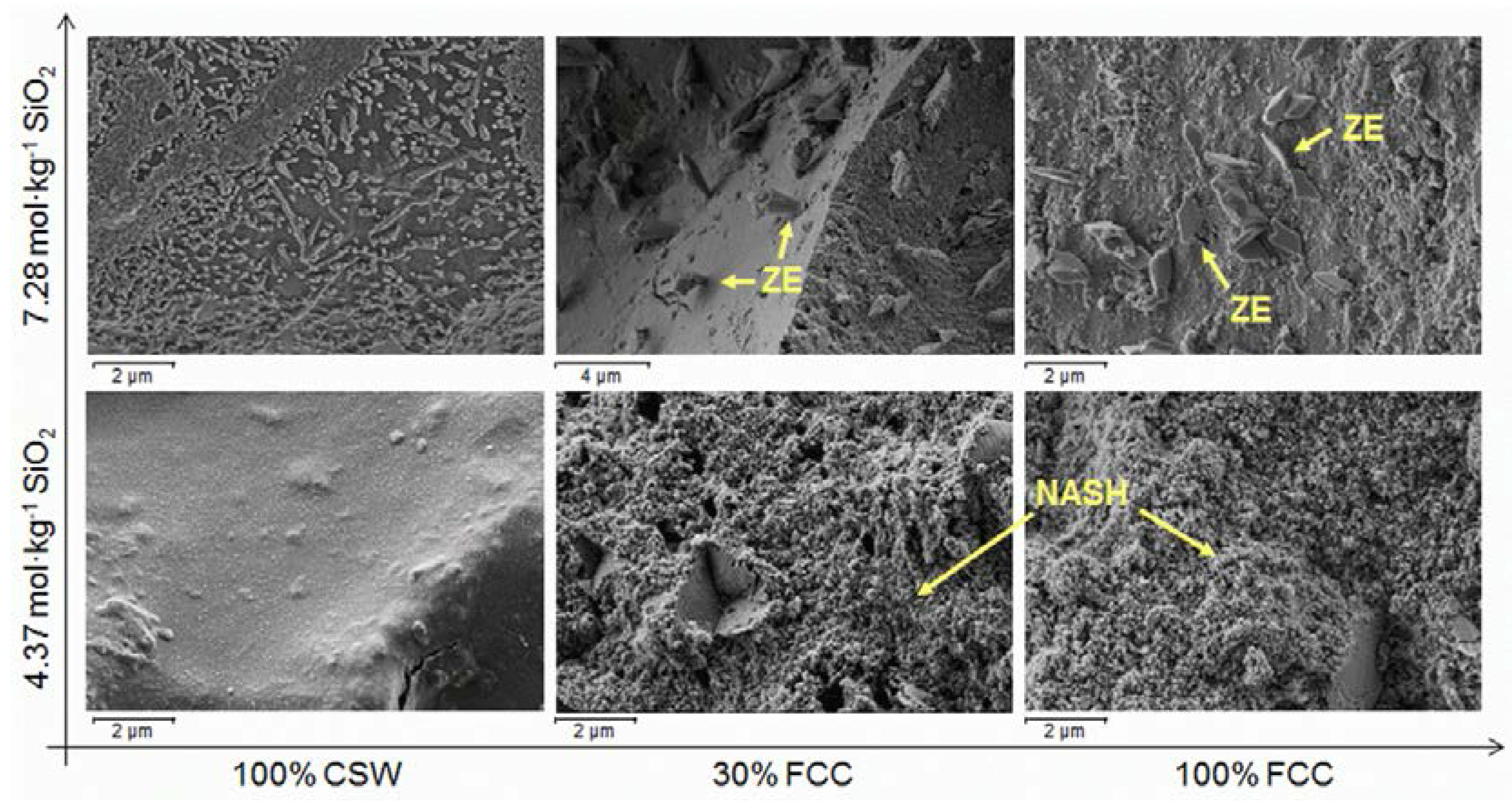
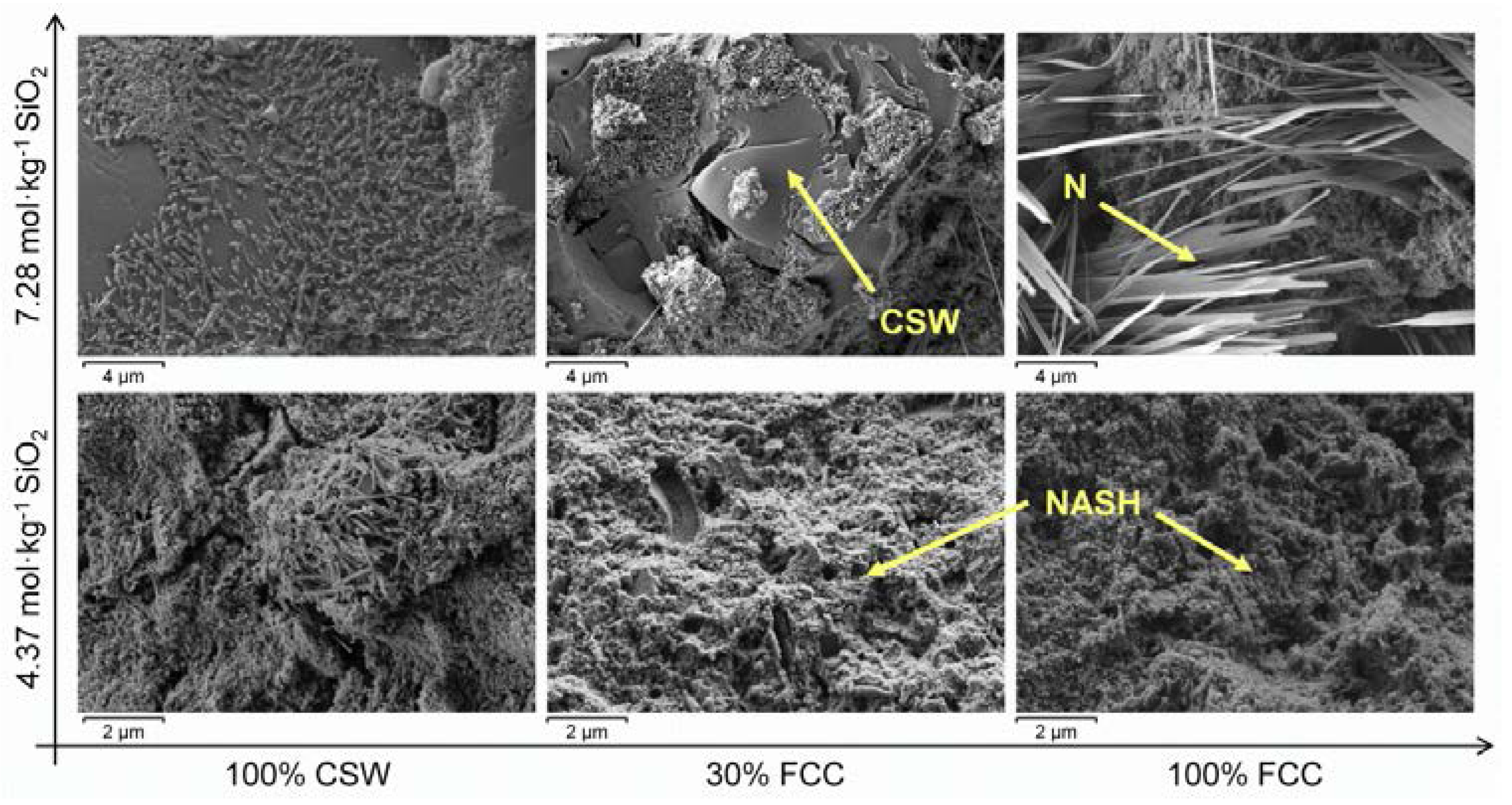
3.5. Field Mission Scanning Electron Microscopy (FESEM)
4. Conclusions
- -
- The mechanical properties of alkali-activated CSW binders significantly improved with FCC content. Strength values close to 40 MPa were obtained in the mortars blended with 20 wt % FCC cured at 65 °C for 7 days, and in those that contained 30 wt % FCC, cured at room temperature for 28 days. The compressive strength of these mortars was still higher than 30 MPa when the SiO2 concentration was lowered from 7.28 to 4.37 mol·kg−1.
- -
- The CSW/FCC binders exhibited a wide range of mechanical properties (up to 60.4 MPa after 28 curing days at 20 °C), which varied depending on the FCC replacing percentage and the silica content in the activating solution. Bigger FCC amounts or higher silica concentrations could be used for special applications, where high strength binders or the use of construction elements in shorter periods are required.
- -
- No significant amounts of new crystalline phases were identified in the alkali-activated CSW/FCC blended systems, where the main reaction product formed was a N-A-S-H/(C,N)-A-S-H binding gel. Zeolites were easily distinguished only in alkali-activated pastes developed with 50 wt % or 100 wt % FCC.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Geological Survey. Mineral Commodity Summaries 2017. 2017, pp. 44–45. Available online: https://doi.org/10.3133/70180197 (accessed on 14 March 2018).
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Fernández Jiménez, A.; Palomo, A. The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Puertas, F.; García-Díaz, I.; Barba, A.; Gazulla, M.F.; Palacios, M.; Gómez, M.P. Ceramic wastes as alternative raw materials for Portland cement Clinker production. Cem. Concr. Compos. 2008, 30, 798–805. [Google Scholar] [CrossRef]
- Puertas, F.; García-Díaz, I.; Palacios, M.; Gazulla, M.F.; Gómez, M.P.; Orduña, M. Clinkers and cements obtained from raw mix containing ceramic wastes as a raw materials. Characterization, hydration and leaching studies. Cem. Concr. Compos. 2010, 32, 175–186. [Google Scholar] [CrossRef]
- García de Lomas, M.; Sánchez de Rojas, M.I.; Frías, M. Pozzolanic reaction of a spent fluid catalytic craking catalyst in FCC-cement mortars. J. Therm. Anal. Calorim. 2007, 90, 443–447. [Google Scholar] [CrossRef]
- Frías, M.; Rodriguez, O.; Sánchez de Rojas, M.I.; Vilar-Cocina, E.; Rodrigues, M.; Savastano, H. Advances on the development of ternary cements elaborated with biomass ashes coming from different activation process. Constr. Build. Mater. 2017, 136, 73–80. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesing, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Srinivasula Reddy, M.; Dinakar, P.; Hanumantha Rao, B. A review of the influence of source material´s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 2016, 234, 12–23. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of alternative route. RSC Adv. 2014, 4, 23846–23852. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.A.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Marin, C.; Araiza, J.L.R.; Manzano, A.; Avalos, J.C.R.; Perez, J.J.; Muniz, M.S.; Ventura, E.; Vorobiev, Y. Synthesis and characterization of a concrete based on metakaolin geopolymer. Inorg. Mater. 2009, 45, 1429–1432. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.K.; Liang, S.; Hu, Y.; Hu, J.P.; Xiao, B.; Huang, Q.F. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Buil. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium additions on the compressive strength and microstructure of alkali-activated ceramic sanitary-ware. J. Am. Ceram. Soc. 2018. [Google Scholar] [CrossRef]
- Reig, L.; Borrachero, M.V.; Monzó, J.; Savastano, H.; Tashima, M.M.; Payá, J. Use of ceramic sanitaryware as an alternative for the development of nee sustainable binders. Key Eng. Mater. 2016, 668, 172–180. [Google Scholar] [CrossRef]
- Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Microstructure and properties of recycled concretes using ceramic sanitaryware industry waste as coarse aggregate. Constr. Build. Mater. 2012, 31, 112–118. [Google Scholar] [CrossRef]
- Baraldi, L. World sanitaryware production and exports. Ceram. World Rev. 2015, 114, 56–65. [Google Scholar]
- Trochez, J.J.; Mejía de Gutiérrez, R.; Rivera, J.; Bernal, S.A. Synthesis of geopolymer from spent FCC: Effect of SiO2/Al2O3 and Na2O/SiO2 molar ratios. Mater. Constr. 2015, 65, 65. [Google Scholar] [CrossRef]
- Schreiber, R.; Yonley, G. The use of spent catalyst as a raw material substitute in cement manufacturing. ACS Div. Petrol. Chem. 1993, 38, 97–99. [Google Scholar]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Velázquez, S. Evaluation of the pozzolanic activity of fluid catalytic cracking catalyst residue (FC3R). Thermogravimetric analysis studies on FC3R-Portland cement pastes. Cem. Concr. Res. 2003, 33, 603–609. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilinska, I.; Kubissa, J. Use of spent catalyst from catalytic cracking in fluidized bed as a new concrete additive. Therm. Acta 1998, 322, 175–181. [Google Scholar] [CrossRef]
- Chen, H.L.; Tseng, Y.S.; Hsu, K.C. Spent FCC catalyst as a pozzolanic material for high-performance mortar. Cem. Concr. Comp. 2004, 26, 657–664. [Google Scholar] [CrossRef]
- Pacewska, B.; Nowacka, M.; Wilinska, I.; Kubissa, W.; Antonovich, V. Studies on the influence of spent FCC catalyst on hydration of calcium aluminate cements at ambient temperature. J. Therm. Anal. Calorim. 2011, 105, 129–140. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Castaldelli, V.N.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. New geopolymeric binder based on fluid catalytic cracking catalyst residue (FCC). Mater. Lett. 2012, 80, 50–52. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Melges, J.L.P.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. Alkali activated materials based on fluid catalytic cracking catalyst residue (FCC): Influence of SiO2/Na2O and H2O/FCC ratio on mechanical strength and microstructure. Fuel 2013, 108, 833–839. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Bernal, S.A.; Provis, J.L.; Gehman, J.D.; Monzó, J.; Payá, J. Geopolymers based on spent catalyst residue from a fluid catalytic cracking (FCC) process. Fuel 2013, 109, 493–502. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, K.L.; Cui, R.; Hwang, C.L.; Cheng, T.W.; Chang, T.M. Effect of solid-to-liquid ratios on the properties of waste catalyst-metakaolin based geopolymers. Constr. Build. Mater. 2015, 88, 74–83. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, K.L.; Cui, R.; Hwang, C.L.; Chang, Y.M.; Cheng, T.W. The effects of SiO2/Na2O molar ratio on the characteristics of alkali-activated waste catalyst-metakaolin based geopolymers. Constr. Build. Mater. 2015, 95, 710–720. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of the activator concentration and calcium hydroxide addition on the properties of alkali-activated porcelain stoneware. Constr. Build. Mater. 2014, 63, 214–222. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Reusing ceramic wastes in concrete. Constr. Build. Mater. 2010, 24, 832–838. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Bernal, S.A.; Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus andmetakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Hidalgo, A.; García, J.L.; Alonso, M.C.; Fernández, L.; Andrade, C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J. Therm. Anal. Calorim. 2009, 96, 335–345. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
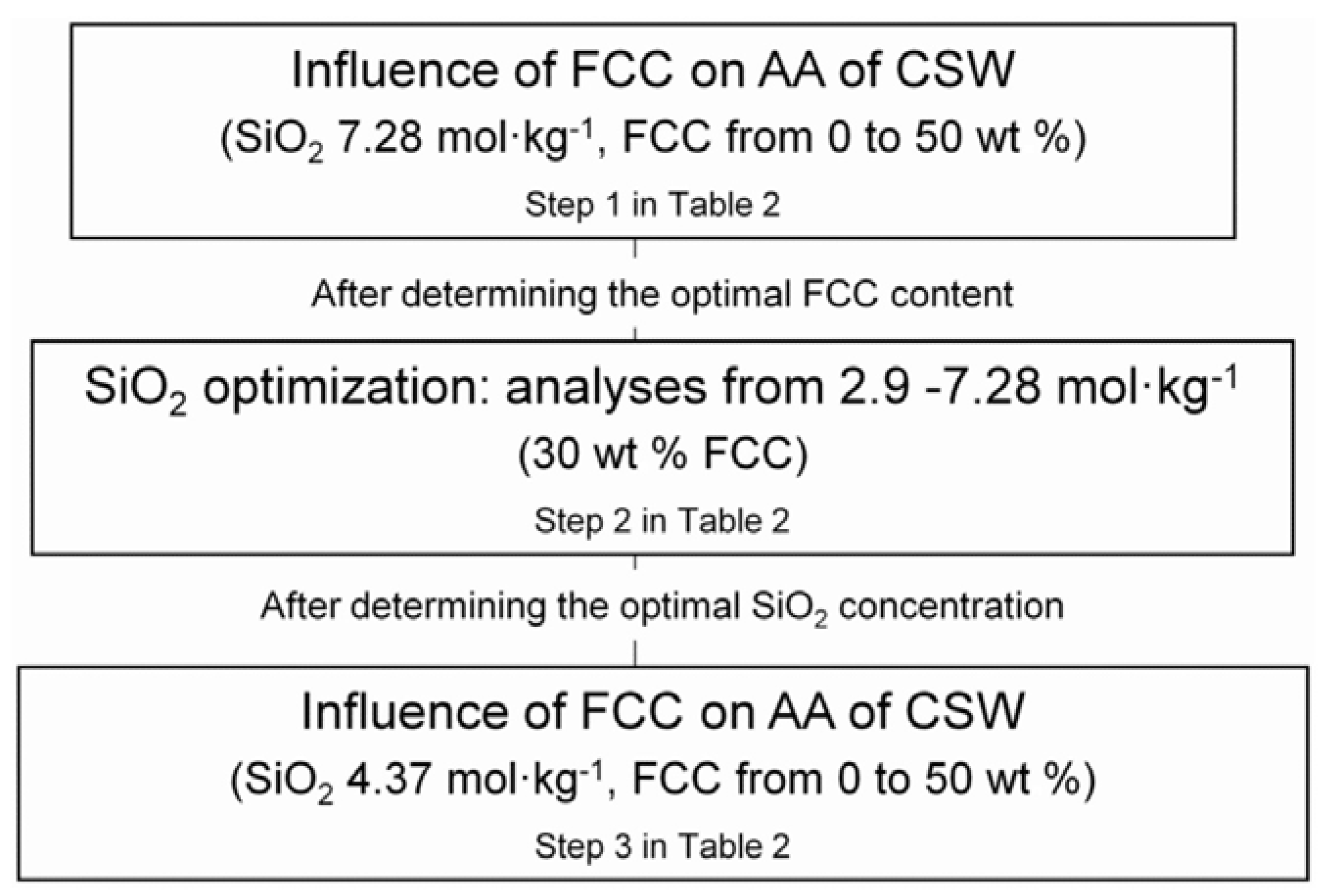

| Step | Designation | FCC wt % | w/b a | Activating Solution | Na2O mol·kgbinder−1 | SiO2 mol·kgbinder−1 | Ca(OH)2 b wt % | Curing Conditions | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Na2O mol·kg−1 | SiO2 mol·kg−1 | SiO2/Na2O Molar Ratio | ||||||||
| 1 | 0/7.28 10/7.28 20/7.28 30/7.28 40/7.28 50/7.28 100/7.28 | 0 10 20 30 40 50 100 | 7.28 | 1.94 | 1.69 | 3.28 | 4 0 | 7 days at 65 °C 28 and 90 days 20 °C | ||
| 2 | 30/2.91 30/3.64 30/4.37 30/5.82 30/7.28 | 30 | 0.45 | 3.75 | 2.91 3.64 4.37 5.82 7.28 | 0.78 0.97 1.16 1.55 1.94 | 1.69 | 1.31 1.64 1.97 2.62 3.28 | 4 | 7 days at 65 °C |
| 3 | 0/4.37 10/4.37 20/4.37 30/4.37 40/4.37 50/4.37 100/4.37 | 0 10 20 30 40 50 100 | 4.37 | 1.16 | 1.69 | 1.97 | 4 0 | 7 days at 65 °C 28 and 90 days 20 °C | ||
| Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | P2O5 | Other | LOI * | |
|---|---|---|---|---|---|---|---|---|---|
| FCC | 49.26 | 47.76 | 0.11 | 0.60 | 0.02 | 0.31 | 0.01 | 1.42 | 0.51 |
| CSW | 23.60 | 66.00 | 1.20 | 1.30 | 2.80 | 2.40 | 0.50 | 2.00 | 0.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. Influence of Addition of Fluid Catalytic Cracking Residue (FCC) and the SiO2 Concentration in Alkali-Activated Ceramic Sanitary-Ware (CSW) Binders. Minerals 2018, 8, 123. https://doi.org/10.3390/min8040123
Cosa J, Soriano L, Borrachero MV, Reig L, Payá J, Monzó JM. Influence of Addition of Fluid Catalytic Cracking Residue (FCC) and the SiO2 Concentration in Alkali-Activated Ceramic Sanitary-Ware (CSW) Binders. Minerals. 2018; 8(4):123. https://doi.org/10.3390/min8040123
Chicago/Turabian StyleCosa, Juan, Lourdes Soriano, María Victoria Borrachero, Lucía Reig, Jordi Payá, and José María Monzó. 2018. "Influence of Addition of Fluid Catalytic Cracking Residue (FCC) and the SiO2 Concentration in Alkali-Activated Ceramic Sanitary-Ware (CSW) Binders" Minerals 8, no. 4: 123. https://doi.org/10.3390/min8040123









