Si-Disordering in MgAl2O4-Spinel under High P-T Conditions, with Implications for Si-Mg Disorder in Mg2SiO4-Ringwoodite
Abstract
:1. Introduction
2. Experimental and Analytical Methods
3. Results and Discussion
3.1. Synthetic MgAl2O4-Sp and Its SiO2
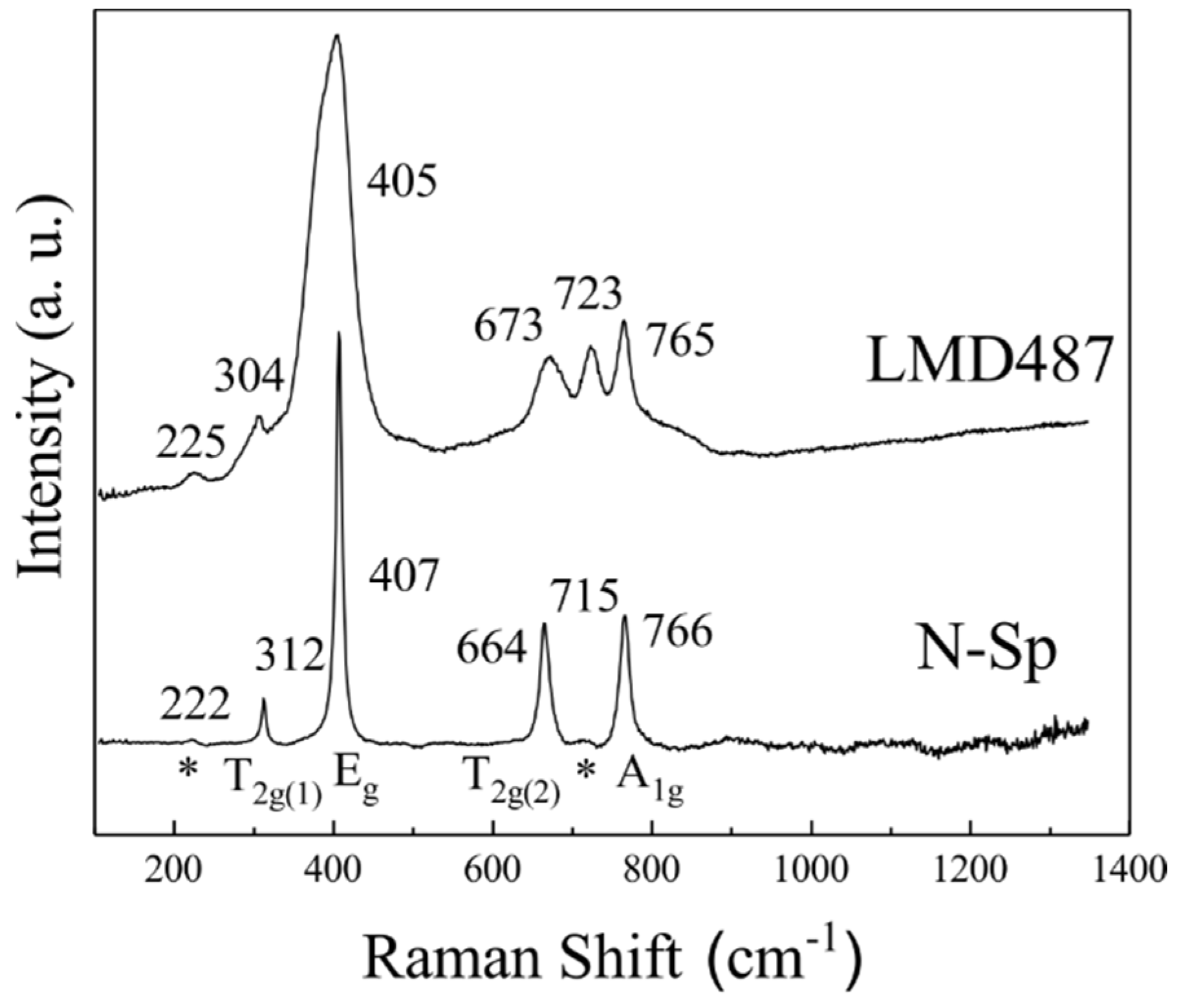
3.2. Raman Features of Nearly Normal MgAl2O4-Sp
3.3. Mg-Al Order-Disorder State of Synthetic MgAl2O4-Sp
3.4. Raman Features of Fully Disordered MgAl2O4-Sp
3.5. Raman Features of Si-Bearing Fully Disordered MgAl2O4-Sp
3.6. Si-Disordering in Fully-Disordered MgAl2O4-Sp
4. Implications
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Evans, B.W.; Frost, B.R. Chrome-spinel in progressive. Geochim. Cosmochim. Acta 1975, 39, 959–972. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Fabries, J. Spinel-olivine geothermometry in peridotites from ultramafic complexes. Contrib. Mineral. Petrol. 1979, 69, 329–336. [Google Scholar] [CrossRef]
- O’Neill, H.S.C. The transition between spinel lherzolite and garnet lherzolite, and its use as a geobarometer. Contrib. Mineral. Petrol. 1981, 77, 185–194. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen barometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib. Mineral. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Liang, Y.; Elthon, D. Evidence from chromium abundances in mantle rocks for extraction of picrite and komatiite melts. Nature 1990, 343, 551–553. [Google Scholar] [CrossRef]
- Liu, X.; O’Neill, H.S.C. The effect of Cr2O3 on the partial melting of spinel lherzolite in the system CaO-MgO-Al2O3-SiO2-Cr2O3 at 1.1 GPa. J. Petrol. 2004, 45, 2261–2286. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xiong, Z.; Zhang, Z. Compressional behavior of MgCr2O4 spinel from first-principles simulation. Sci. China Earth Sci. 2016, 59, 989–996. [Google Scholar] [CrossRef]
- Xie, X.; Chen, M.; Wang, D. Shock-related mineralogical features and P-T history of the Suizhou L6 chondrite. Eur. J. Mineral. 2001, 13, 1177–1190. [Google Scholar] [CrossRef]
- Wang, A.; Kuebler, K.E.; Jolliff, B.L.; Haskin, L.A. Raman spectroscopy of Fe-Ti-Cr-oxides, case study: Martian meteorite EETA 79001. Am. Mineral. 2004, 89, 665–680. [Google Scholar] [CrossRef]
- Fritz, J.; Greshake, A. High-pressure phases in an ultramafic rock from Mars. Earth Planet. Sci. Lett. 2009, 288, 619–623. [Google Scholar] [CrossRef]
- Lenaz, D.; Princivalle, F.; Schmitz, B. First crystal-structure determination of chromites from an acapulcoite and ordinary chondrites. Mineral. Mag. 2015, 79, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Sharp, T.G. High-pressure phases in shock-induced melt of the unique highly shocked LL6 chondrite Northwest Africa 757. Meteorit. Planet. Sci. 2016, 51, 1353–1369. [Google Scholar] [CrossRef]
- Pieters, C.M.; Besse, S.; Boardman, J.; Buratti, B.; Cheek, L.; Clark, R.N.; Combe, J.P.; Dhingra, D.; Goswami, J.N.; Green, R.O.; et al. Mg-spinel lithology: A new rock type on the lunar farside. J. Geophys. Res. Planets 2011, 116, 287–296. [Google Scholar] [CrossRef]
- Gross, J.; Treiman, A.H. Unique spinel-rich lithology in lunar meteorite ALHA81005: Origin and possible connection to M3 observations of the farside highlands. J. Geophys. Res. 2011, 116, E10009. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase transformations and their bearing on the constitution and dynamics of the mantle. Geochim. Cosmochim. Acta 1991, 55, 2083–2110. [Google Scholar] [CrossRef]
- Li, B.; Liebermann, R.C. Indoor seismology by probing the Earth’s interior by using sound velocity measurements at high pressures and temperatures. Proc. Natl. Acad. Sci. USA 2007, 104, 9145–9150. [Google Scholar] [CrossRef] [PubMed]
- Panero, W.R. Cation disorder in ringwoodite and its effects on wave speeds in the Earth’s transition zone. J. Geophys. Res. 2008, 113, B10204. [Google Scholar] [CrossRef]
- Stixrude, L.; Lithgow-Bertelloni, C. Thermodynamics of mantle minerals-II. Phase equilibria. Geophys. J. Int. 2011, 184, 1180–1213. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, Z.; Chang, L.; He, Q.; Wang, F.; Shieh, S.R.; Wu, C.; Li, B.; Zhang, L. Anhydrous ringwoodites in the mantle transition zone: Their bulk modulus, solid solution behavior, compositional variation, and sound velocity feature. Solid Earth Sci. 2016, 1, 28–47. [Google Scholar] [CrossRef]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, N.; Miyahara, M. High-pressure minerals in shocked meteorites. Meteorit. Planet. Sci. 2017, 52, 2017–2039. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X. The shock effects of olivine in the Yanzhuang chondrite. Acta Mineral. Sin. 1993, 13, 109–116. [Google Scholar]
- Kimura, M.; Chen, M.; Yoshida, T.; El Goresy, A.; Ohtani, E. Back-transformation of high-pressure phases in a shock melt vein of an H-chondrite during atmospheric passage: Implications for the survival of high-pressure phases after decompression. Earth Planet. Sci. Lett. 2003, 217, 141–150. [Google Scholar] [CrossRef]
- Bischoff, A. Discovery of purple-blue ringwoodite within shock veins of an LL6 ordinary chondrite from Northwest Africa. In Proceedings of the 33rd Lunar and Planetary Science Conference, Houston, TX, USA, 11–15 March 2002. [Google Scholar]
- Hollister, L.S.; Bindi, L.; Yao, N.; Poirier, G.R.; Andronicos, C.L.; MacPherson, G.J.; Lin, C.; Distler, V.V.; Eddy, M.P.; Kostin, A.; et al. Impact-induced shock and the formation of natural quasicrystals in the early solar system. Nat. Commun. 2014, 5, 4040. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Ohtani, E.; Yamaguchi, A. High-pressure polymorphs in Gujba CB type carbonaceous chondrite. In Proceedings of the Japan Geoscience Union Meeting, Chiba, Japan, 24–28 May 2015; pp. 20–22. [Google Scholar]
- Barrat, J.A.; Chaussidon, M.; Bohn, M.; Gillet, P.; Göpel, C.; Lesourd, M. Lithium behavior during cooling of a dry basalt: An ion-microprobe study of the lunar meteorite Northwest Africa 479 (NWA 479). Geochim. Cosmochim. Acta 2005, 69, 5597–5609. [Google Scholar] [CrossRef]
- Zhang, A.; Hsu, W.; Floss, C.; Li, X.; Li, Q.; Liu, Y.; Taylor, L.A. Petrogenesis of lunar meteorite Northwest Africa 2977: Constraints from in situ microprobe results. Meteorit. Planet. Sci. 2011, 45, 1929–1947. [Google Scholar] [CrossRef]
- Boonsue, S.; Spray, J. Shock-induced phase transformations in melt pockets within Martian meteorite NWA 4468. Spectrosc. Lett. 2012, 45, 127–134. [Google Scholar] [CrossRef]
- Baziotis, I.P.; Yang, L.; Paul, S.; DeCarli, H.; Melosh, J.; McSween, H.Y.; Bodnar, R.J.; Taylor, L.A. The Tissint Martian meteorite as evidence for the largest impact excavation. Nat. Commun. 2013, 4, 1404. [Google Scholar] [CrossRef] [PubMed]
- Greshake, A.; Fritz, J.; Böttger, U.; Goran, D. Shear-induced ringwoodite formation in the Martian shergottite Dar al Gani 670. Earth Planet. Sci. Lett. 2013, 375, 383–394. [Google Scholar] [CrossRef]
- Walton, E.L. Shock metamorphism of Elephant Moraine A79001: Implications for olivine-ringwoodite transformation and the complex thermal history of heavily shocked Martian meteorites. Geochim. Cosmochim. Acta 2013, 107, 299–315. [Google Scholar] [CrossRef]
- Walton, E.L.; Sharp, T.G.; Hu, J.; Filiberto, J. Heterogeneous mineral assemblages in Martian meteorite Tissint as a result of a recent small impact event on Mars. Geochim. Cosmochim. Acta 2014, 140, 334–348. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Liu, Y.; Rossman, G.R.; Zhuravlev, K.; Prakapenka, V.; Dera, P.; Taylor, L.A. Tissintite, (Ca, Na, □)AlSi2O6, a highly-defective, shock-induced, high-pressure clinopyroxene in the Tissint Martian meteorite. Earth Planet. Sci. Lett. 2015, 422, 194–205. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Liu, Y.; Rossman, G.R.; Sinogeikin, S.V.; Smith, J.S.; Taylor, L.A. Ahrensite, γ-Fe2SiO4, a new shock-metamorphic mineral from the Tissint meteorite: Implications for the Tissint shock event on Mars. Geochim. Cosmochim. Acta. 2016, 184, 240–256. [Google Scholar] [CrossRef]
- Miyahara, M.; Ohtani, E.; El Goresy, A.; Ozawa, S.; Gillet, P. Phase transition processes of olivine in the shocked Martian meteorite Tissint: Clues to origin of ringwoodite-, bridgmanite- and magnesiowüstite-bearing assemblages. Phys. Earth Planet. Inter. 2016, 259, 18–28. [Google Scholar] [CrossRef]
- Stöffler, D.; Keil, K.; Scott, E.R.D. Shock metamorphism of ordinary chondrites. Geochim. Cosmochim. Acta 1991, 55, 3845–3867. [Google Scholar] [CrossRef]
- Chen, M.; Sharp, T.G.; El Goresy, A.; Wopenka, B.; Xie, X. The majorite-pyrope+ magnesiowüstite assemblage: Constraints on the history of shock veins in chondrites. Science 1996, 271, 1570–1573. [Google Scholar] [CrossRef]
- Sharp, T.G.; DeCarli, P.S. Shock effects in meteorites. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Eds.; The University of Arizona Press: Tuscon, AZ, USA, 2006; pp. 653–677. [Google Scholar]
- Wood, B.J.; Kirkpatrick, R.J.; Montez, B. Order-disorder phenomena in MgAl2O4 spinel. Am. Mineral. 1986, 71, 999–1006. [Google Scholar]
- Hazen, R.M.; Navrotsky, A. Effects of pressure on order-disorder reactions. Am. Mineral. 1996, 81, 1021–1035. [Google Scholar] [CrossRef]
- Hazen, R.M.; Yang, H. Effects of cation substitution and order-disorder on P-V-T equations of state of cubic spinels. Am. Mineral. 1999, 84, 1956–1960. [Google Scholar] [CrossRef]
- Méducin, F.; Redfern, S.A.T.; Godec, Y.L.; Stone, H.J.; Tucker, M.G.; Dove, M.T.; Marshall, W.G. Study of cation order-disorder in MgAl2O4 spinel by in situ neutron diffraction up to 1600 K and 3.2 GPa. Am. Mineral. 2004, 89, 981–986. [Google Scholar] [CrossRef]
- Li, L.; Carrez, P.; Weidner, D. Effect of cation ordering and pressure on spinel elasticity by ab initio simulation. Am. Mineral. 2007, 92, 174–178. [Google Scholar] [CrossRef]
- Schmocker, U.; Waldner, F. The inversion parameter with respect to the space group of MgAl2O4 spinels. J. Phys. C 1976, 9, L235–L237. [Google Scholar] [CrossRef]
- Peterson, R.C.; Lager, G.A.; Hitterman, R.L. A time-of-flight neutron powder diffraction study of MgAl2O4 at temperatures up to 1273 K. Am. Mineral. 1991, 76, 172–180. [Google Scholar]
- Cynn, H.; Sharma, S.K.; Cooney, T.F.; Nicol, M. High-temperature Raman investigation of order-disorder behavior in the MgAl2O4 spinel. Phys. Rev. B 1992, 45, 500–502. [Google Scholar] [CrossRef]
- Millard, R.L.; Peterson, R.C.; Hunter, B.K. Temperature dependence of cation disorder in MgAl2O4 spinel using 27Al and 17O magic-angle spinning NMR. Am. Mineral. 1992, 77, 44–52. [Google Scholar]
- Maekawa, H.; Kato, S.; Kawamura, K.; Yokokawa, T. Cation mixing in natural MgAl2O4 spinel: A high-temperature 27Al NMR study. Am. Mineral. 1997, 82, 1125–1132. [Google Scholar] [CrossRef]
- Pavese, A.; Artioli, G.; Hull, S. In situ powder neutron diffraction of cation partitioning vs. pressure in Mg0.94Al2.04O4 synthetic spinel. Am. Mineral. 1999, 84, 905–912. [Google Scholar] [CrossRef]
- Redfern, S.A.T.; Harrison, R.J.; O’Neill, H.St.C.; Wood, D.R.R. Thermodynamics and kinetics of cation ordering in MgAl2O4 spinel up to 1600 °C from in situ neutron diffraction. Am. Mineral. 1999, 84, 299–310. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Princivalle, F.; Skogby, H.; Della Giusta, A. Cation ordering and structural variations with temperature in MgAl2O4 spinel: An X-ray single-crystal study. Am. Mineral. 2000, 85, 1164–1171. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Princivalle, F. Kinetics of cation ordering in synthetic MgAl2O4 spinel. Am. Mineral. 2002, 87, 838–844. [Google Scholar] [CrossRef]
- Carbonin, S.; Martignago, F.; Menegazzo, G.; Nal Negro, A. X-ray single-crystal study of spinels: In situ heating. Phys. Chem. Miner. 2002, 29, 503–514. [Google Scholar] [CrossRef]
- Slotznick, S.P.; Shim, S.H. In situ Raman spectroscopy measurements of MgAl2O4 spinel up to 1400 °C. Am. Mineral. 2008, 93, 470–476. [Google Scholar] [CrossRef]
- Sreeja, V.; Smitha, T.S.; Nand, D.; Ajithkumar, T.G.; Joy, P.A. Size dependent coordination behavior and cation distribution in MgAl2O4 nanoparticles from 27Al solid state NMR studies. J. Phys. Chem. C 2008, 112, 14737–14744. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Hazen, R.M.; Downs, R.T.; Finger, L.W.; Ko, J. Crystal chemistry of ferromagnesian silicate spinels: Evidence for Mg-Si disorder. Am. Mineral. 1993, 78, 1320–1323. [Google Scholar]
- Stebbins, J.F.; Panero, W.R.; Smyth, J.R.; Frost, D.J. Forsterite, wadsleyite, and ringwoodite (Mg2SiO4): 29Si NMR constraints on structural disorder and effects of paramagnetic impurity ions. Am. Mineral. 2009, 94, 626–629. [Google Scholar] [CrossRef]
- Taran, M.N.; Koch-Müller, M.; Wirth, R.; Abs-Wurmbach, I.; Rhede, D.; Greshake, A. Spectroscopic studies of synthetic and natural ringwoodite, γ-(Mg, Fe)2SiO4. Phys. Chem. Miner. 2009, 36, 217–232. [Google Scholar] [CrossRef]
- Kiefer, B.; Stixrude, L.; Wentzcovitch, R. Normal and inverse ringwoodite at high pressures. Am. Mineral. 1999, 84, 288–293. [Google Scholar] [CrossRef]
- Sasaki, S.; Prewitt, C.T.; Sato, Y.; Ito, E. Single crystal x-ray study of γ-Mg2SiO4. J. Geophys. Res. 1982, 87, 7829–7832. [Google Scholar] [CrossRef]
- Sigurdsson, I.A.; Kamenetsky, V.S.; Crawford, A.J.; Eggins, S.M.; Zlobin, S.K. Primitive island arc and oceanic lavas from the hunter ridge-hunter fracture zone. Evidence from glass, olivine and spinel compositions. Miner. Petrol. 1993, 47, 149–169. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Nikogosian, I.K. Petrology of long-lived mantle plume magmatism: Hawaii, Pacific and Reunion Island, Indian Ocean. Petrology 1994, 2, 111–144. [Google Scholar]
- Kamenetsky, V.S.; Sobolev, A.V.; Joron, J.L.; Semet, M.P. Petrology and geochemistry of cretaceous ultramafic volcanics from eastern Kamchatka. J. Petrol. 1995, 36, 637–662. [Google Scholar] [CrossRef]
- Franz, L.; Wirth, R. Spinel inclusions in olivine of peridotite xenoliths from TUBAF seamount (Bismarck Archipelago/Papua New Guinea): Evidence for the thermal and tectonic evolution of the oceanic lithosphere. Contrib. Mineral. Petrol. 2000, 140, 283–295. [Google Scholar] [CrossRef]
- Chistyakova, S.; Latypov, R.; Zaccarini, F. Chromitite dykes in the Monchegorsk layered intrusion, Russia: In situ crystallization from chromite-saturated magma flowing in conduits. J. Petrol. 2016, 56, 2395–2424. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Tang, J.; He, Q.; Li, S.; Peng, F.; He, D.; Zhang, L.; Fei, Y. A large volume cubic press with a pressure-generating capability up to about 10 GPa. High Press. Res. 2012, 32, 239–254. [Google Scholar] [CrossRef]
- Bertka, C.M.; Fei, Y.W. Mineralogy of the Martian interior up to core-mantle boundary pressure. J. Geophys. Res. 1997, 102, 5251–5264. [Google Scholar] [CrossRef]
- He, Q.; Tang, J.; Wang, F.; Liu, X. High temperature stable assembly designed for cubic press. Chin. J. High Press. Phys. 2014, 28, 145–151. [Google Scholar]
- Carbonin, S.; Russo, U.; Della Giusta, A. Cation distribution in some natural spinels from X-ray diffraction and Mössbauer spectroscopy. Mineral. Mag. 1996, 60, 355–368. [Google Scholar] [CrossRef]
- Lavina, B.; Salviulo, G.; Della Giusta, A. Cation distribution and structural modeling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Princivalle, F.; Della Giusta, A.; Carbonin, S. Comparative crystal-chemistry of spinels from some suites of ultramafic rocks. Miner. Petrol. 1989, 40, 117–126. [Google Scholar] [CrossRef]
- Della Giusta, A.; Carbonin, S.; Ottonello, G. Temperature-dependent disorder in a natural Mg-Al-Fe2+-Fe3+-spinel. Mineral. Mag. 1996, 60, 603–616. [Google Scholar] [CrossRef]
- Uchida, H.; Lavina, B.; Downs, R.T.; Chesley, J. Single-crystal X-ray diffraction of spinels from San Carlos volcanic field, Arizona: Spinel as a geothermometer. Am. Mineral. 2005, 90, 1900–1908. [Google Scholar] [CrossRef]
- Chopelas, A.; Hofmeister, A.M. Vibrational spectroscopy of aluminate spinels at 1 atm and of MgAl2O4 to over 200 kbar. Phys. Chem. Miner. 1991, 18, 279–293. [Google Scholar] [CrossRef]
- Lauwers, H.A.; Herman, M.A. Force field of some AB2C4 spinel compounds. J. Phys. Chem. Solids 1980, 41, 223–230. [Google Scholar] [CrossRef]
- Sinha, M.M.; Singh, N.P.; Prasad, K.; Gupta, H.C. Effect of rattling on the interatomic interaction in oxide spinels MgAl2O4 and ZnGa2O4. Phys. Status Solidi B 1995, 190, K27–K29. [Google Scholar] [CrossRef]
- De Wijs, G.A.; Fang, C.M.; Kresse, G.; de With, G. First-principles calculation of the phonon spectrum of MgAl2O4 spinel. Phys. Rev. B 2002, 65, 094305. [Google Scholar] [CrossRef]
- Thibaudeau, P.; Gervais, F. Ab initio investigation of phonon modes in the MgAl2O4 spinel. J. Phys. Condens. Matter 2002, 14, 3543–3552. [Google Scholar] [CrossRef]
- Lazzeri, M.; Thibaudeau, P. Ab initio Raman spectrum of the normal and disordered MgAl2O4 spinel. Phys. Rev. B 2006, 74, 2952–2961. [Google Scholar] [CrossRef]
- Caracas, R.; Banigan, E.J. Elasticity and Raman and infrared spectra of MgAl2O4 spinel from density functional perturbation theory. Phys. Earth Planet. Inter. 2009, 174, 113–121. [Google Scholar] [CrossRef]
- Kushwaha, A.K. Vibrational and elastic properties of aluminate spinel MgAl2O4. Physica B 2010, 405, 2795–2798. [Google Scholar] [CrossRef]
- Cynn, H.; Anderson, O.L.; Nicol, M. Effects of cation disordering in a natural MgAl2O4 spinel observed by rectangular parallelepiped ultrasonic resonance and Raman measurements. Pure Appl. Geophys. 1993, 141, 415–444. [Google Scholar] [CrossRef]
- Van Minh, N.; Yang, I.-S. A Raman study of cation-disorder transition temperature of natural MgAl2O4 spinel. Vib. Spectrosc. 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Lenaz, D.; Musco, M.E.; Petrelli, M.; Caldeira, R.; Min, A.D.; Marzoli, A.; Mata, J.; Perugini, D.; Princivalle, F.; Boumehdi, M.A.; et al. Restitic or not? Insights from trace element content and crystal-structure of spinels in African mantle xenoliths. Lithos 2017, 278–281, 464–476. [Google Scholar] [CrossRef]
- Lucchesi, S.; Della Giusta, A. Crystal chemistry of a highly disordered Mg-Al natural spinel. Miner. Petrol. 1997, 59, 91–99. [Google Scholar] [CrossRef]
- Levy, D.; Pavese, A.; Hanfland, M. Synthetic MgAl2O4 (spinel) at high-pressure conditions (0.0001–30 GPa): A synchrotron X-ray powder diffraction study. Am. Mineral. 2003, 88, 93–98. [Google Scholar] [CrossRef]
- Nestola, F.; Ballaran, T.B.; Zunic, T.B.; Princivalle, F.; Secco, L.; Negro, A.D. Comparative compressibility and structural behavior of spinel MgAl2O4 at high pressures: The independency on the degree of cation order. Am. Mineral. 2007, 92, 1838–1843. [Google Scholar] [CrossRef]
- Bacon, G.E. A neutron-diffraction study of magnesium aluminium oxide. Acta Crystallogr. 1952, 5, 684–686. [Google Scholar] [CrossRef]
- Sinclair, W.; Ringwood, A.E. Single crystal analysis of the structure of stishovite. Nature 1978, 272, 714–715. [Google Scholar] [CrossRef]
- Smyth, J.R.; Hazen, R.M. The crystal structures of forsterite and hortonolite at several temperatures up to 900 °C. Am. Mineral. 1973, 58, 588–593. [Google Scholar]
- Gibbs, R.E. Structure of α quartz. Proc. R. Soc. A 1926, 110, 443–455. [Google Scholar] [CrossRef]
- Zoltai, T.; Buerger, M.J. The crystal structure of coesite, the dense, high-pressure form of silica. Z. Kristallogr. 1959, 111, 129–141. [Google Scholar] [CrossRef]
- Chopelas, A. Single crystal Raman spectra of forsterite, fayalite, and monticellite. Am. Mineral. 1991, 76, 1101–1109. [Google Scholar]
- Katsura, T.; Yamada, H.; Nishikawa, O.; Song, M.; Kubo, A.; Shinmei, T.; Yokoshi, S.; Aizawa, Y.; Yoshino, T.; Walter, M.J.; et al. Olivine-wadsleyite transition in the system (Mg, Fe)2SiO4. J. Geophys. Res. 2004, 109, B02209. [Google Scholar] [CrossRef]
- Perinelli, C.; Bosi, F.; Andreozzi, G.B.; Conte, A.M.; Armienti, P. Geothermometric study of Cr-spinels of peridotite mantle xenoliths from northern Victoria Land (Antarctica). Am. Mineral. 2014, 99, 839–846. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Hutchinson, I.; Ingley, R. The ExoMars Raman spectrometer and identification of biogeological spectroscopic signatures using a flight-like prototype. Anal. Bioanal. Chem. 2012, 404, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.B.; Ingley, R.; Edwards, H.G.M.; Harris, L.; McHugh, M.; Malherbe, C.; Parnell, J. Raman spectroscopy on Mars: Identification of geological and bio-geological signatures in Martian analogues using miniaturized Raman spectrometers. Philos. Trans. R. Soc. 2014, 372, 20140204. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A.C.; Wood, B.J. The depth of the spinel to garnet transition at the peridotite solidus. Earth Planet. Sci. Lett. 1998, 164, 277–284. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. The origin of cratonic diamond-constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Kudoh, Y.; Kuribayashi, T.; Mizobata, H.; Ohtani, E.; Sasaki, S.; Tanaka, M. Pressure dependence of u parameter in ringwoodite up to 7.9 GPa. J. Miner. Petrol. Sci. 2007, 102, 8–11. [Google Scholar] [CrossRef]
- Ye, Y.; Brown, D.A.; Smyth, J.R.; Panero, W.R.; Jacobsen, S.D.; Chang, Y.Y.; Townsend, J.P.; Thomas, S.M.; Hauri, E.H.; Dera, P.; et al. Compressibility and thermal expansion of hydrous ringwoodite with 2.5(3) wt % H2O. Am. Mineral. 2012, 97, 573–582. [Google Scholar] [CrossRef]
- Kleppe, A.K.; Jephcoat, A.P.; Smyth, J.R. Raman spectroscopic study of hydrous γ-Mg2SiO4 to 56.5 GPa. Phys. Chem. Miner. 2002, 29, 473–476. [Google Scholar] [CrossRef]
- Chopelas, A.; Boehler, R.; Ko, T. Thermodynamics and behavior of γ-Mg2SiO4 at high pressure: Implications for Mg2SiO4 phase equilibrium. Phys. Chem. Miner. 1994, 21, 351–359. [Google Scholar] [CrossRef]
- Xie, Z.; Sharp, T.G. High-pressure phases in shock-induced melt veins of the Umbarger L6 chondrite: Constraints of shock pressure. Meteorit. Planet. Sci. 2004, 39, 2043–2054. [Google Scholar] [CrossRef]
- Xie, Z.; Sharp, T.G. Host rock solid-state transformation in a shock-induced melt vein of Tenham L6 chondrite. Earth Planet. Sci. Lett. 2007, 254, 433–445. [Google Scholar] [CrossRef]
- Nagy, S.; Józsa, S.; Gyollai, I.; Bérczi, S.; Bendõ, Z.; Stehlik, H. Ringwoodite microstructures in L-chondrite NWA 5011: Implications for transformation mechanism and source region in L parent body. Cent. Eur. Geol. 2011, 54, 233–248. [Google Scholar] [CrossRef]
- Sharp, T.G.; Xie, Z.; DeCarli, P.S.; Hu, J. A large shock vein in L chondrite Roosevelt County 106: Evidence for a long-duration shock pulse on the L chondrite parent body. Meteorit. Planet. Sci. 2015, 50, 1941–1953. [Google Scholar] [CrossRef]
- Tomioka, N.; Okuchi, T. A new high-pressure form of Mg2SiO4 highlighting diffusionless phase transition of olivine. Sci. Rep. 2017, 7, 17351. [Google Scholar] [CrossRef] [PubMed]







| Exp. # | P a | T a | t a | Phase Assemblage | MgO | Al2O3 | SiO2 | Total |
|---|---|---|---|---|---|---|---|---|
| LMD565 | 3 | 1500 | 36 | Sp(8) b + Melt | 28.66(25) c | 70.23(53) | 0.39(5) | 99.29(72) |
| LMD564 | 4 | 1500 | 36 | Sp(8) + Melt | 29.26(15) | 70.29(18) | 0.65(7) | 100.22(33) |
| LMD563 | 4 | 1550 | 24 | Sp(7) + Melt | 28.44(22) | 70.92(33) | 0.30(7) | 99.68(35) |
| LMD558 | 4 | 1550 | 36 | Sp(5) + Fo + Melt | 29.01(30) | 69.98(60) | 0.76(3) | 99.75(86) |
| LMD578 | 5 | 1630 | 12 | Sp(13) + Grt + Melt | 28.94(18) | 70.22(25) | 0.76(7) | 99.92(36) |
| LMD568 | 6 | 1650 | 12 | Sp(13) + Grt + Melt | 29.13(26) | 69.27(59) | 1.03(7) | 99.45(78) |
| LMD487 d | 5 | 1600 | 12 | Sp | - | - | - | - |
| LMD659 e | 5 | 1500 | 12 | Coe + Melt | - | - | - | - |
| PL1316 e | 14 | 1400 | 8 | St | - | - | - | - |
| Natural spinel | N-Sp(10) | 28.05(18) | 70.81(22) | 0.01(1) | 100.03(39) | |||
| Natural quartz | N-Qz(10) | 0.00(1) | 0.13(9) | 100.95(51) | 101.17(57) | |||
| Exp. # | P/T/SiO2 a | # b | (I823 + I856)/(I725 + I766) |
|---|---|---|---|
| LMD565 | 3/1500/0.39(5) c | 3 | 0.36(1) |
| LMD564 | 4/1500/0.65(7) | 3 | 0.77(29) |
| LMD563 | 4/1550/0.30(7) | 3 | 0.32(5) |
| LMD558 | 4/1550/0.76(3) | 8 | 0.40(10) |
| LMD578 d | 5/1630/0.76(7) | 4 | 0.22(13) |
| LMD568 | 6/1650/1.03(7) | 3 | 0.37(8) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, X.; Bao, X.; He, Q.; Yan, W.; Ma, Y.; He, M.; Tao, R.; Zou, R. Si-Disordering in MgAl2O4-Spinel under High P-T Conditions, with Implications for Si-Mg Disorder in Mg2SiO4-Ringwoodite. Minerals 2018, 8, 210. https://doi.org/10.3390/min8050210
Liu L, Liu X, Bao X, He Q, Yan W, Ma Y, He M, Tao R, Zou R. Si-Disordering in MgAl2O4-Spinel under High P-T Conditions, with Implications for Si-Mg Disorder in Mg2SiO4-Ringwoodite. Minerals. 2018; 8(5):210. https://doi.org/10.3390/min8050210
Chicago/Turabian StyleLiu, Liping, Xi Liu, Xinjian Bao, Qiang He, Wei Yan, Yunlu Ma, Mingyue He, Renbiao Tao, and Ruqiang Zou. 2018. "Si-Disordering in MgAl2O4-Spinel under High P-T Conditions, with Implications for Si-Mg Disorder in Mg2SiO4-Ringwoodite" Minerals 8, no. 5: 210. https://doi.org/10.3390/min8050210





