Carbon Material with High Specific Surface Area Improves Complex Copper Ores’ Bioleaching Efficiency by Mixed Moderate Thermophiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Complex Copper Ores and Carbon Materials
2.2. Preparation of the Mixed Moderate Thermophiles
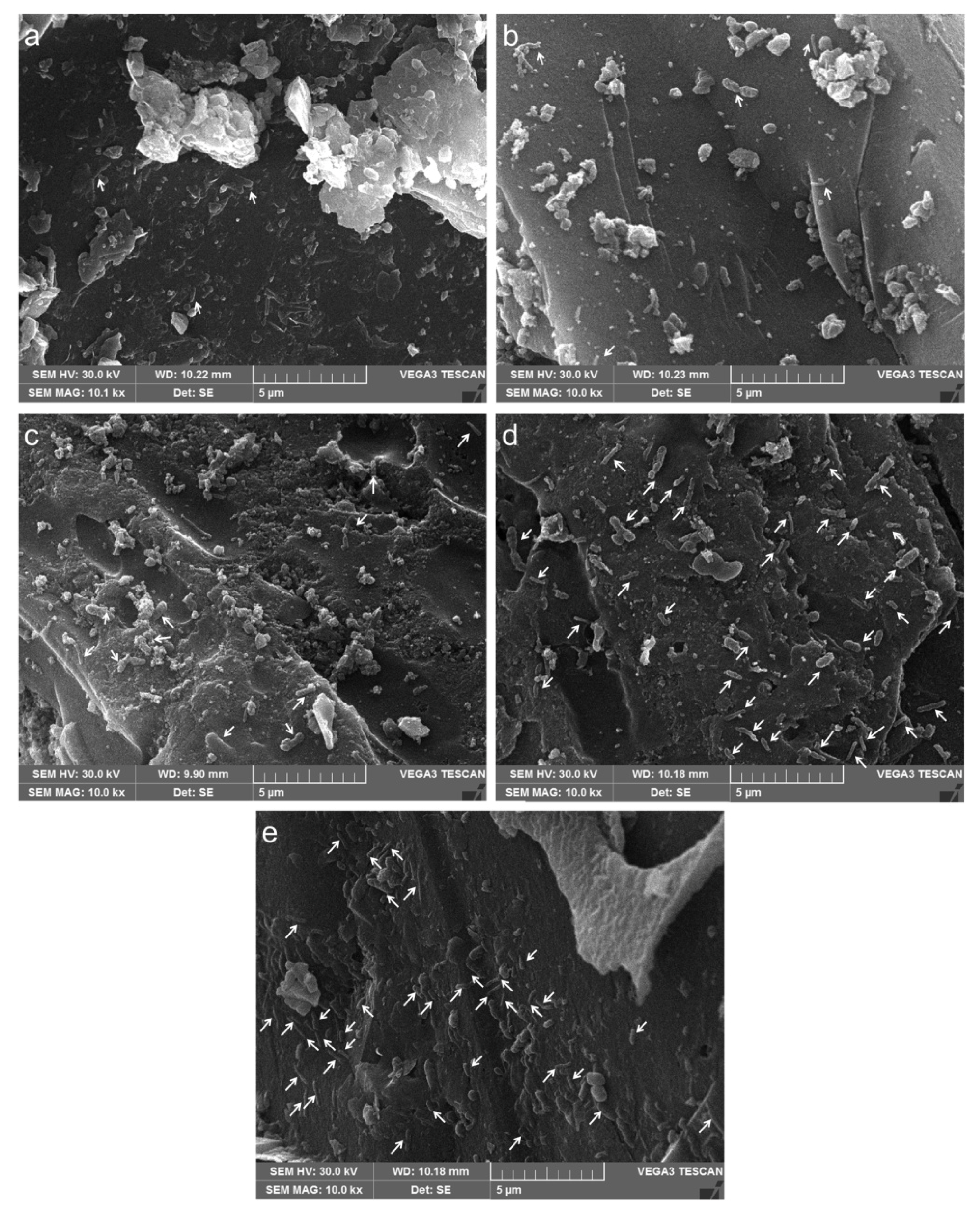
2.3. Bioleaching Experiments and Scanning Electron Microscope (SEM) Analysis
2.4. Physicochemical Analysis
2.5. Microbial Community Dynamic Analysis
2.5.1. Total Genomic DNA Extraction
2.5.2. Polymerase Chain Reaction (PCR) Amplification and Sequence Analysis
3. Results and Discussion
3.1. Effect of Carbon Materials with Different Specific Surface Area (SSA) on Copper Extractions
3.2. Effect of Carbon Materials with Different SSA on Leaching Parameters
3.3. Adsorption Behaviors of Bioleaching Microbes onto Complex Copper Ores and Carbon Materials
3.4. The Copper Phase Analysis of Bioleached Residues
3.5. Microbial Community Dynamics Analysis
3.6. A Model for the Effects of Activated Carbon with High SSA on Complex Copper Ores Bioleaching
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hirato, T.; Majima, H.; Awakura, Y. The leaching of chalcopyrite with ferric sulfate. Metall. Trans. B 1987, 18, 489–496. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Srinivasa Rao, K.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Yu, R.L.; Shi, L.J.; Gu, G.H.; Zhou, D.; You, L.; Chen, M.; Qiu, G.; Zeng, W. The shift of microbial community under the adjustment of initial and processing pH during bioleaching of chalcopyrite concentrate by moderate thermophiles. Bioresour. Technol. 2014, 162, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R.; Jiao, F.; Qin, W.Q. Co-bioleaching of chalcopyrite and silver-bearing bornite in a mixed moderately thermophilic culture. Minerals 2018, 8, 4. [Google Scholar] [CrossRef]
- Wang, Y.G.; Su, L.J.; Zhang, L.J.; Zeng, W.M.; Wu, J.Z.; Wan, L.L.; Qiu, G.Z.; Chen, X.H.; Zhou, H.B. Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium. Bioresour. Technol. 2012, 121, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Qiu, G.Z.; Wang, J.; Wang, D.Z. The effect of silver-bearing catalysts on bioleaching of chalcopyrite. Hydrometallurgy 2002, 64, 81–88. [Google Scholar]
- Zhang, R.Y.; Wei, D.Z.; Shen, Y.B.; Liu, W.G.; Lu, T.; Han, C. Catalytic effect of polyethylene glycol on sulfur oxidation in chalcopyrite bioleaching by Acidithiobacillus ferrooxidans. Miner. Eng. 2016, 95, 74–78. [Google Scholar] [CrossRef]
- Dreisinger, D.; Abed, N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Wei, J.C.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Karra, U.; Manickam, S.S.; McCutcheon, J.R.; Patel, N.; Li, B. Power generation and organics removal from wastewater using activated carbon nanofiber (ACNF) microbial fuel cells (MFCs). Int. J. Hydrog. Energy 2013, 38, 1588–1597. [Google Scholar] [CrossRef]
- Zhang, S.J.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption kinetics of aromatic compounds on carbon nanotubes and activated carbons. Environ. Toxicol. Chem. 2012, 31, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.Y.; Miller, J.D.; Foley, J.; Pons, S. Electrochemical features of the ferric sulfate leaching of CuFeS2/C aggregates. In Proceedings of the International Symposium on Electrochemistry in Mineral and Metal Processing; Richardson, P.E., Srinivasan, S., Woods, R., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1984; pp. 391–416. [Google Scholar]
- Nakazawa, H.; Fujisawa, H.; Sato, H. Effect of activated carbon on the bioleaching of chalcopyrite concentrate. Int. J. Miner. Process. 1998, 55, 87–94. [Google Scholar] [CrossRef]
- Zhang, W.M.; Gu, S.F. Catalytic effect of activated carbon on bioleaching of low-grade primary copper sulfide ores. Trans. Nonferrous Met. Soc. China 2007, 17, 1123–1127. [Google Scholar] [CrossRef]
- Liang, C.L.; Xia, J.L.; Zhao, X.J.; Yang, Y.; Gong, S.Q.; Nie, Z.Y.; Ma, C.Y.; Zheng, L.; Zhao, Y.D.; Qiu, G.Z. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 2010, 105, 179–185. [Google Scholar] [CrossRef]
- Zeng, W.M.; Qiu, G.Z.; Zhou, H.B.; Peng, J.H.; Chen, M.; Tan, S.N.; Chao, W.L.; Liu, X.D.; Zhang, Y.S. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate. Bioresour. Technol. 2010, 101, 7068–7075. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, H.Q.; Liang, Y.L.; Hu, Q.; Zhou, X.S.; Xiao, Y.H.; Ma, L.Y.; Zhang, X.; Qiu, G.Z.; Liu, X.D. Comparative genome analysis reveals metabolic versatility and environmental adaptations of Sulfobacillus thermosulfidooxidans strain ST. PLoS ONE 2014, 9, e99417. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Wang, X.J.; Feng, X.; Liang, Y.L.; Xiao, Y.H.; Hao, X.D.; Yin, H.Q.; Liu, H.W.; Liu, X.D. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession. Bioresour. Technol. 2017, 223, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Zhang, R.; Hu, P.; Zeng, W.M.; Xie, Y.; Wu, C.; Qiu, G.Z. Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. J. Appl. Microbiol. 2008, 105, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.D.; Liang, Y.L.; Yin, H.Q.; Ma, L.Y.; Xiao, Y.H.; Liu, Y.Z.; Qiu, G.Z.; Liu, X.D. The effect of potential heap construction methods on column bioleaching of copper flotation tailings containing high levels of fines by mixed cultures. Miner. Eng. 2016, 98, 279–285. [Google Scholar] [CrossRef]
- Pond, S.K.; Wadhawan, S.; Chiaromonte, F.; Ananda, G.; Chung, W.Y.; Taylor, J.; Nekrutenko, A. Windshield splatter analysis with the Galaxy metagenomic pipeline. Genome Res. 2009, 19, 2144–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.D.; Liang, Y.L.; Yin, H.Q.; Liu, H.W.; Zeng, W.M.; Liu, X.D. Thin-layer heap bioleaching of copper flotation tailings containing high levels of fine grains and microbial community succession analysis. Int. J. Miner. Metall. Mater. 2017, 24, 360–368. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J. Catalytic effect of activated charcoal on microbial extraction of arsenic and heavy metals from mine tailings. Geosci. J. 2014, 18, 355–363. [Google Scholar] [CrossRef]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. Control of the redox potential by oxygen limitation improves bacterial leaching of chalcopyrite. Biotechnol. Bioeng. 2002, 78, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Wang, J.; Gan, X.W.; Zheng, X.H.; Tao, L.; Hu, M.H.; Li, Y.N.; Qin, W.Q.; Qiu, G.Z. Effects of pyrite and bornite on bioleaching of two different types of chalcopyrite in the presence of Leptospirillum ferriphilum. Bioresour. Technol. 2015, 194, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate—chloride and sulfate—nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Dong, Y.B.; Lin, H.; Xu, X.F.; Zhang, Y.; Gao, Y.J.; Zhou, S.S. Comparative study on the bioleaching, biosorption and passivation of copper sulfide minerals. Int. Biodeter. Biodegr. 2013, 84, 29–34. [Google Scholar] [CrossRef]
- Xia, L.X.; Liu, X.X.; Zeng, J.; Yin, C.; Gao, J.; Liu, J.S.; Qiu, G.Z. Mechanism of enhanced bioleaching efficiency of Acidithiobacillus ferrooxidans after adaptation with chalcopyrite. Hydrometallurgy 2008, 92, 95–101. [Google Scholar] [CrossRef]
- Devasia, P.; Natarajan, K.A. Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces. Int. J. Miner. Process. 2010, 94, 135–139. [Google Scholar] [CrossRef]
- Florian, B.; Noël, N.; Thyssen, C.; Felschau, I.; Sand, W. Some quantitative data on bacterial attachment to pyrite. Miner. Eng. 2011, 24, 1132–1138. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.Y.; Song, Y.; Tong, L.L. Catalytic effects of activated carbon and surfactants on bioleaching of cobalt ore. Hydrometallurgy 2015, 152, 69–75. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.G.; Schippers, A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Tang, X.H.; Guo, K.; Li, H.R.; Du, Z.W.; Tian, J.L. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes. Bioresour. Technol. 2011, 102, 3558–3560. [Google Scholar] [CrossRef] [PubMed]
- Sandström, Å.; Shchukarev, A.; Paul, J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential. Miner. Eng. 2005, 18, 505–515. [Google Scholar] [CrossRef]
- Vilcáez, J.; Suto, K.; Inoue, C. Response of thermophiles to the simultaneous addition of sulfur and ferric ion to enhance the bioleaching of chalcopyrite. Miner. Eng. 2008, 21, 1063–1074. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria. Hydrometallurgy 2008, 93, 106–115. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Majuste, D.; Ciminelli, V.S.T.; Eng, P.J.; Osseo-Asare, K. Applications of in situ synchrotron XRD in hydrometallurgy: Literature review and investigation of chalcopyrite dissolution. Hydrometallurgy 2013, 131–132, 54–66. [Google Scholar] [CrossRef]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. The role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching. Hydrometallurgy 2000, 57, 225–233. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 2006, 83, 40–49. [Google Scholar] [CrossRef]
- Ahmadi, A.; Schaffie, M.; Manafi, Z.; Ranjbar, M. Electrochemical bioleaching of high grade chalcopyrite flotation concentrates in a stirred bioreactor. Hydrometallurgy 2010, 104, 99–105. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Johnson, D.B. Biodiversity and interactions of acidophiles: Key to understanding and optimizing microbial processing of ores and concentrates. Trans. Nonferrous Met. Soc. China 2008, 18, 1367–1373. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R.; Watkin, E.L.J.; Ralph, D.E. The resilience and versatility of acidophiles that contribute to the bio-assisted extraction of metals from mineral sulphides. Environ. Technol. 2010, 31, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.S.; Yang, H.L.; Wang, W. Insights into the enhancement mechanism coupled with adapted adsorption behavior from mineralogical aspects in bioleaching of copper-bearing sulfide ore by Acidithiobacillus sp. RSC Adv. 2015, 5, 98057–98066. [Google Scholar] [CrossRef]
- Ma, Y.L.; Liu, H.C.; Xia, J.L.; Nie, Z.Y.; Zhu, H.R.; Zhao, Y.D.; Ma, C.Y.; Zheng, L.; Hong, C.H.; Wen, W. Relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture at 65 °C. Trans. Nonferrous Met. Soc. China 2017, 27, 1374–1384. [Google Scholar] [CrossRef]





| Carbon Materials | Specific Surface Area (m2/g) | Eelectrical Conductivity (S/m) | pH | S (%) | Fe (%) |
|---|---|---|---|---|---|
| Graphite (C2) | 2 | 0.6 | 5.2 | 0.9 | 0.9 |
| Activated carbon I (C400) | 400 | 0.1 | 6.2 | 0.4 | 0.8 |
| Activated carbon II (C800) | 800 | 0.1 | 6.9 | 0.5 | 0.3 |
| Activated carbon III (C1200) | 1200 | 0.1 | 5.9 | 0.2 | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Liu, X.; Zhu, P.; Chen, A.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Carbon Material with High Specific Surface Area Improves Complex Copper Ores’ Bioleaching Efficiency by Mixed Moderate Thermophiles. Minerals 2018, 8, 301. https://doi.org/10.3390/min8070301
Hao X, Liu X, Zhu P, Chen A, Liu H, Yin H, Qiu G, Liang Y. Carbon Material with High Specific Surface Area Improves Complex Copper Ores’ Bioleaching Efficiency by Mixed Moderate Thermophiles. Minerals. 2018; 8(7):301. https://doi.org/10.3390/min8070301
Chicago/Turabian StyleHao, Xiaodong, Xueduan Liu, Ping Zhu, Aijia Chen, Hongwei Liu, Huaqun Yin, Guanzhou Qiu, and Yili Liang. 2018. "Carbon Material with High Specific Surface Area Improves Complex Copper Ores’ Bioleaching Efficiency by Mixed Moderate Thermophiles" Minerals 8, no. 7: 301. https://doi.org/10.3390/min8070301




