Sharyginite, Ca3TiFe2O8, A New Mineral from the Bellerberg Volcano, Germany
Abstract
:1. Introduction
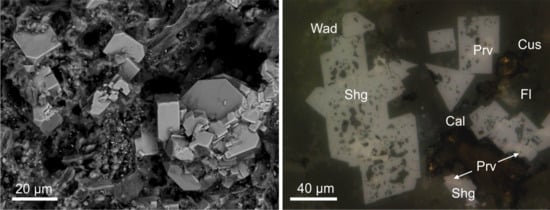
2. Occurrence, Geological Settings, Physical and Optical Properties of Sharyginite
3. Materials and Methods
4. Results
4.1. Chemical Composition
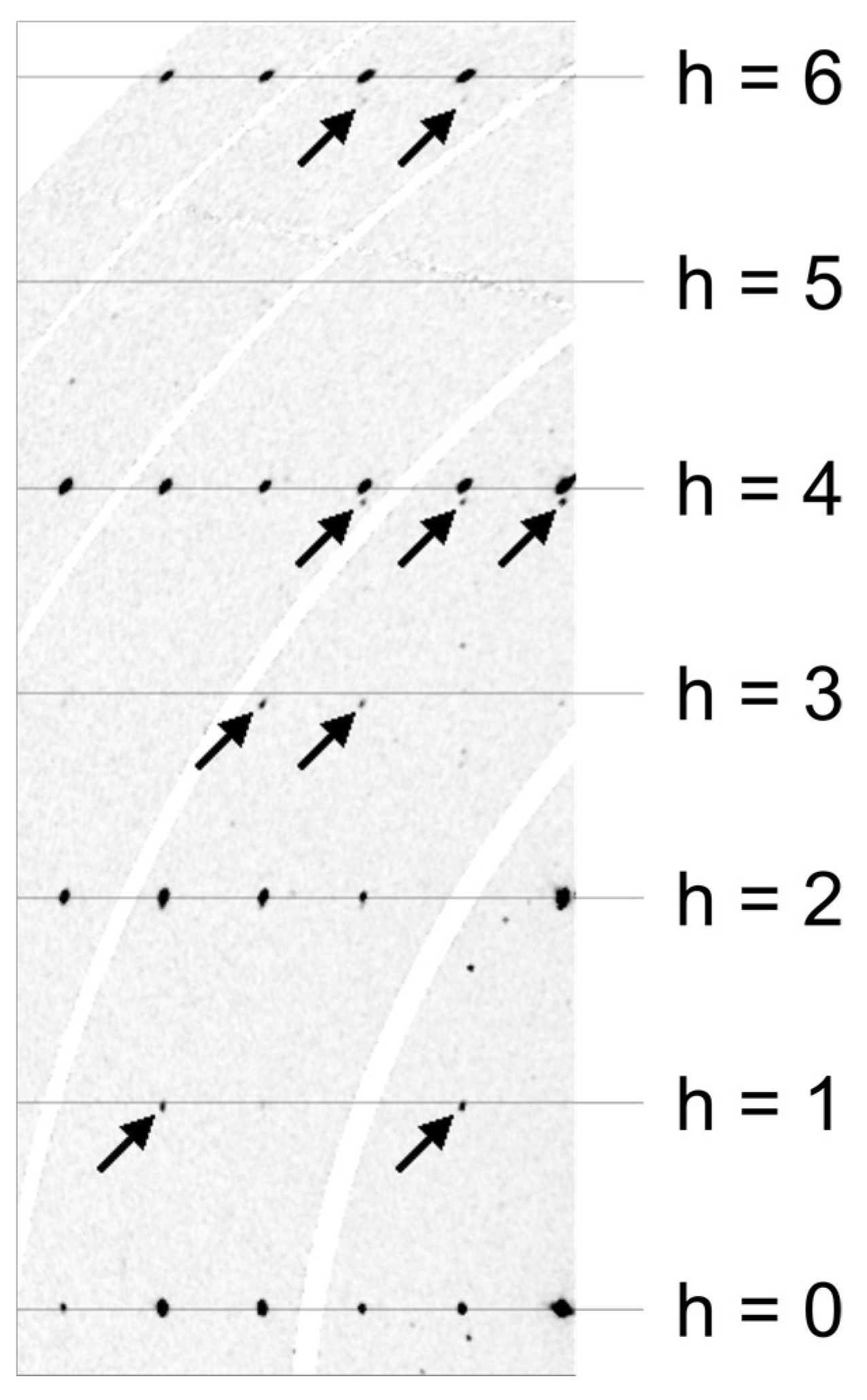
4.2. X-ray Crystallography
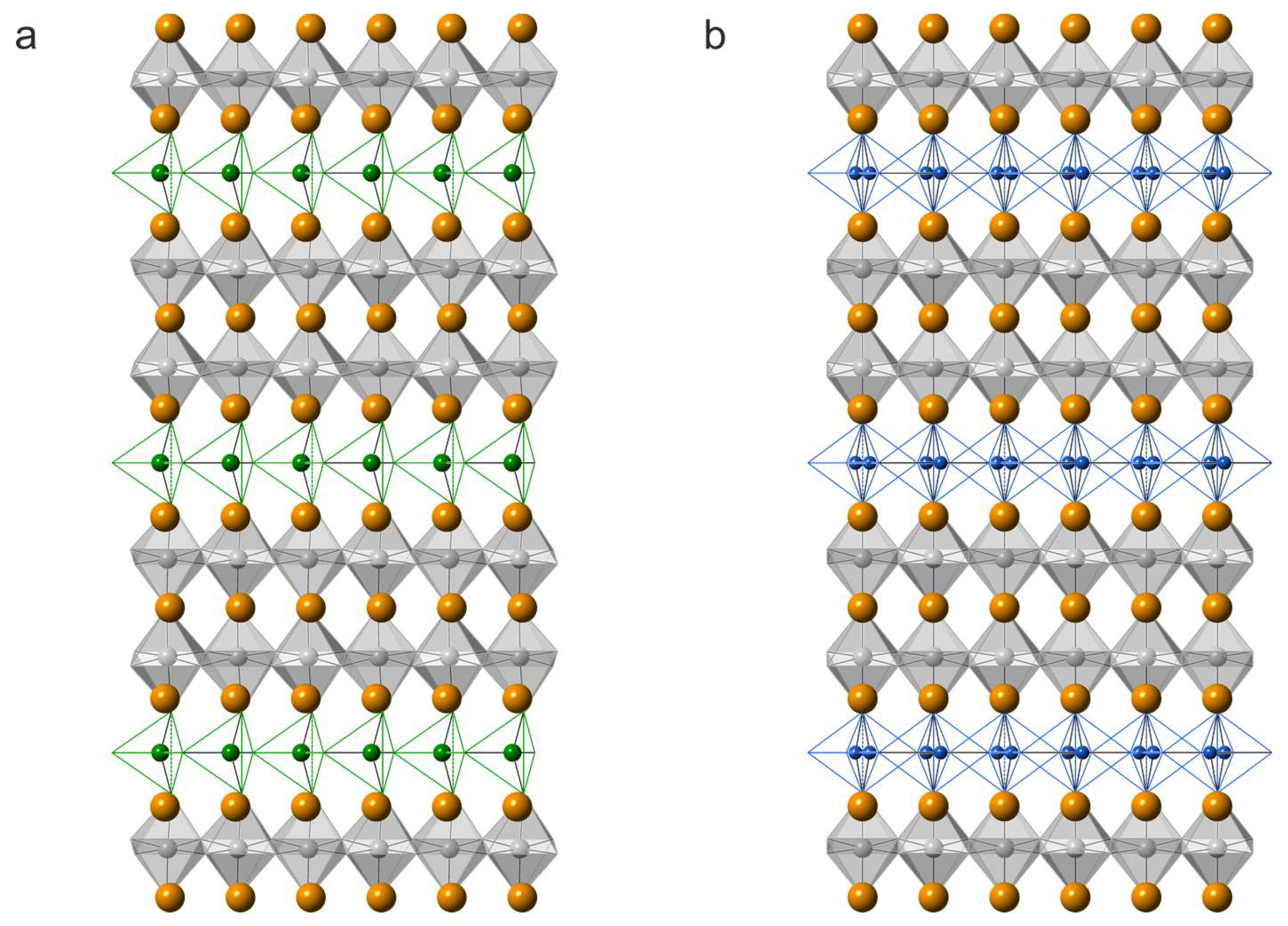
4.3. Crystal Structure
4.4. Raman Spectroscopy
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharygin, V.V.; Lazic, B.; Armbruster, T.M.; Murashko, M.N.; Wirth, R.; Galuskina, I.O.; Galuskin, E.V.; Vapnik, Y.; Britvin, S.N.; Logvinova, A.M. Shulamitite Ca3TiFe3+AlO8—A new perovskite-related mineral from Hatrurim Basin, Israel. Eur. J. Miner. 2013, 25, 97–111. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Welch, M.D.; Chakhmouradian, A.R.; Mills, S. Nomenclature of the perovskite supergroup: A hierarchical system of classification based on crystal structure and composition. Miner. Mag. 2017, 81, 411–461. [Google Scholar] [CrossRef] [Green Version]
- Sharygin, V.V.; Sokol, E.V.; Vapnik, Y. Minerals of the pseudobinary perovskite-brownmillerite series from combustion metamorphic larnite rocks of the Hatrurim Formation (Israel). Russ. Geol. Geophys. 2008, 49, 709–726. [Google Scholar] [CrossRef]
- Grenier, J.-C.; Darriet, J.; Pouchard, M.; Hagenmuller, P. Mise en evidence d’une nouvelle famille de phases de type perovskite lacunaire ordonnee de formule A3M3O8 (AMO2,67). Mater. Res. Bull. 1976, 11, 1219–1225. [Google Scholar] [CrossRef]
- Grenier, J.-C.; Pouchard, M.; Hagenmuller, P. Vacancy ordering in oxygen-deficient perovskite-related ferrites. In Ferrites Transitions Elements Luminescence. Structure and Bonding; Springer-Verlag: Berlin, Germany, 1981; Volume 47, pp. 1–25. [Google Scholar]
- Rodríguez-Carvajal, J.; Vallet-Regí, M.; Calbet, J.M.G. Perovskite threefold superlattices: A structure determination of the A3M3O8 phase. Mater. Res. Bull. 1989, 24, 423–430. [Google Scholar] [CrossRef]
- Causa, M.T.; Zysler, R.D.; Tovar, M.; Vallet-Regí, M.; González-Calbet, J.M. Magnetic properties of the CanFe2Tin−2O3n−1 perovskite related series: An EPR study. J. Solid State Chem. 1992, 98, 25–32. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gazeev, V.M.; Armbruster, T.; Zadov, A.E.; Galuskina, I.O.; Pertsev, N.N.; Dzierżanowski, P.; Kadiyski, M.; Gurbanov, A.G.; Wrzalik, R.; et al. Lakargiite CaZrO3: A new mineral of the perovskite group from the North Caucasus, Kabardino-Balkaria, Russia. Am. Miner. 2008, 93, 1903–1910. [Google Scholar] [CrossRef]
- Niedermayr, G.; Auer, C.; Bernhard, F.; Brandstätter, F.; Gröbner, J.; Hammer, V.M.F.; Knobloch, G.; Koch, G.; Kolitchs, U.; Konzett, J.; et al. Neue Mineralfunde aus Österreich LX. Carinth. II 2011, 201, 135–186. [Google Scholar]
- Sharygin, V.V. Lakargiite and Minerals of the Perovskite Brownmillerite Series in Metacarbonate Rocks from Donetsk Burned Dumps; Scientific works of Donetsk National Technical University, Mining and Geological Series; Donetsk National Technical University: Donetsk, Ukraine, 2011; Volume 15, pp. 114–125. [Google Scholar]
- Sharygin, V.V. Minerals of the Ca3TiFeAlO8–Ca3TiFeFeO8 series in natural and technogenic pyrometamorphic systems. In The Mineralogy of Technogenesis 2012; Institute of Mineralogy, Uralian Branch of Russian Academy of Sciences: Miass, Russia, 2012; pp. 29–49. [Google Scholar]
- Sharygin, V.V.; Wirth, R. Shulamitite and its Fe-analog in metacarbonate xenoliths from alkali basalts, E. Eifel, Germany. In Proceedings of the 29th International Conference Ore Potential of Alkaline, Kimberlite and Carbonatite Magmatism, “Alkaline magmatism of the Earth”, Sudak-Moscow, Ukraine-Russia, 14–22 September 2012. [Google Scholar]
- Mihajlović, T.; Lengauer, C.L.; Ntaflos, T.; Kolitsch, U.; Tillmanns, E. Two new minerals rondorfite, Ca8Mg[SiO4]4Cl2, and almarudite, K.(□,Na)2(Mn,Fe,Mg)2(Be,Al)3[Si12O30], and a study of iron-rich wadalite, Ca12[(Al8Si4Fe2)O32]Cl6, from the Bellerberg (Bellberg) volcano, Eifel, Germany. Neues Jahrb. Miner. 2004, 179, 265–294. [Google Scholar] [CrossRef]
- Hentschel, G. Die Mineralien der Eifelvulkane, 2nd ed.; Weise Verlag: München, Germany, 1987. [Google Scholar]
- Abraham, K.; Gebert, W.; Medenbach, O.; Schreyer, W.; Hentschel, G. Eifelite, KNa3Mg4Si12O30, a new mineral of the osumilite group with octahedral sodium. Contrib. Miner. Pet. 1983, 82, 252–258. [Google Scholar] [CrossRef]
- Irran, E.; Tillmanns, E.; Hentschel, G. Ternesite, Ca5(SiO4)2(SO4), a new mineral from the Ettringer Bellerberg/Eifel, Germany. Miner. Petrol. 1997, 60, 121–132. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Krüger, B.; Krüger, H.; Blass, G.; Widmer, R.; Galuskina, I.O. Wernerkrauseite, CaFe3+2Mn4+O6: The first nonstoichiometric post-spinel mineral, from Bellerberg volcano, Eifel, Germany. Eur. J. Miner. 2016, 28, 485–493. [Google Scholar] [CrossRef]
- Gazeev, V.M.; Zadov, A.E.; Gurbanov, A.G.; Pertsev, N.N.; Mokhov, A.V.; Dokuchaev, A.Y. Rare minerals from Verkhniechegemskaya caldera (in xenoliths of skarned limestone). Vestnik. Vladikavkazskogo Nauchnogo Centra. 2006, 6, 18–27. [Google Scholar]
- Grapes, R. Pyrometamorphism, 2nd ed.; Springer-Verlag: Berlin Heidelberg, Germany, 2010. [Google Scholar]
- Galuskin, E.V.; Gazeev, V.M.; Lazic, B.; Armbruster, T.; Galuskina, I.O.; Zadov, A.E.; Pertsev, N.N.; Wrzalik, R.; Dzierżanowski, P.; Gurbanov, A.G.; et al. Chegemite Ca7(SiO4)3(OH)2—A new humite-group calcium mineral from the Northern Caucasus, Kabardino-Balkaria, Russia. Eur. J. Miner. 2009, 21, 1045–1059. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Armbruster, T.; Lazic, B.; Kusz, J.; Dzierżanowski, P.; Gazeev, V.M.; Pertsev, N.N.; Prusik, K.; Zadov, A.E.; et al. Elbrusite-(Zr)—A new uranian garnet from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Am. Miner. 2010, 95, 1172–1181. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Lazic, B.; Armbruster, T.; Zadov, A.E.; Krzykawski, T.; Banasik, K.; Gazeev, V.M.; Pertsev, N.N. Rusinovite, Ca10(Si2O7)3Cl2: A new skarn mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Eur. J. Miner. 2011, 837–844. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Savelyeva, V.B.; Armbruster, T.; Lazic, B.; Galuskina, I.O.; Többens, D.M.; Zadov, A.E.; Dzierżanowski, P.; Pertsev, N.N.; et al. Pavlovskyite Ca8(SiO4)2(Si3O10): A new mineral of altered silicate-carbonate xenoliths from the two Russian type localities, Birkhin massif, Baikal Lake area and Upper Chegem caldera, North Caucasus. Am. Miner. 2012, 97, 503–512. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Lazic, B.; Armbruster, T.; Galuskina, I.O.; Pertsev, N.N.; Gazeev, V.M.; Włodyka, R.; Dulski, M.; Dzierżanowski, P.; Zadov, A.E.; et al. Edgrewite Ca9(SiO4)4F2-hydroxyledgrewite Ca9(SiO4)4(OH)2, a new series of calcium humite-group minerals from altered xenoliths in the ignimbrite of Upper Chegem caldera, Northern Caucasus, Kabardino-Balkaria, Russia. Am. Miner. 2012, 97, 1998–2006. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Kusz, J.; Dzierżanowski, P.; Prusik, K.; Gazeev, V.M.; Pertsev, N.N.; Dubrovinsky, L. Dzhuluite, Ca3SbSnFe3+3O12, a new bitikleite-group garnet from the Upper Chegem Caldera, Northern Caucasus, Kabardino-Balkaria, Russia. Eur. J. Miner. 2013, 231–239. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Krüger, B.; Galuskin, E.V.; Armbruster, T.; Gazeev, V.M.; Włodyka, R.; Dulski, M.; Dzierżanowski, P. Fluorchegemite, Ca7(SiO4)3F2, a new mineral from the edgrewitw-bearing endoskarn zone of an altered xenolith in ignimbrites from Upper Chegem Caldera, Northern Caucasus, Kabardino-Balkaria, Russia: Occurrence, crystal structure, and new data on the mineral assemblages. Can. Miner. 2015, 53, 325–344. [Google Scholar]
- Galuskin, E.V.; Galuskina, I.O.; Kusz, J.; Armbruster, T.; Marzec, K.M.; Dzierżanowski, P.; Murashko, M. Vapnikite Ca3UO6—A new double-perovskite mineral from pyrometamorphic larnite rocks of the Jabel Harmun, Palestinian Autonomy, Israel. Miner. Mag. 2014, 78, 571–581. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Vapnik, Y.; Lazic, B.; Armbruster, T.; Murashko, M.; Galuskin, E.V. Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel. Am. Miner. 2014, 99, 965–975. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Armbruster, T.; Galuskina, I.O.; Vapnik, Y.; Dulski, M.; Murashko, M.; Dzierżanowski, P.; Sharygin, V.V.; Krivovichev, S.V.; et al. Mayenite supergroup, part III: Fluormayenite, Ca12Al14O32[□4F2], and fluorkyuygenite, Ca12Al14O32[(H2O)4F2], two new minerals from pyrometamorphic rocks of the Hatrurim Complex, South Levant. Eur. J. Miner. 2015, 27, 123–136. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Armbruster, T.; Galuskina, I.O.; Vapnik, Y.; Murashko, M.; Włodyka, R.; Dzierżanowski, P. New minerals with a modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex. Part I. Nabimusaite, KCa12(SiO4)4(SO4)2O2F, from larnite rocks of Jabel Harmun, Palestinian Autonomy, Israel. Miner. Mag. 2015, 79, 1061–1072. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Armbruster, T.; Krza¸tała, A.; Vapnik, Y.; Kusz, J.; Dulski, M.; Gardocki, M.; Gurbanov, A.G.; et al. New minerals with a modular structure derived from hatrurite from the pyrometamorphic rocks. Part III. Gazeevite, BaCa6(SiO4)2(SO4)2O, from Israel and the Palestine Autonomy, South Levant, and from South Ossetia, Greater Caucasus. Miner. Mag. 2017, 81, 499–513. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Prusik, K.; Vapnik, Y.; Juroszek, R.; Jeżak, L.; Murashko, M. Dzierżanowskite, CaCu2S2—A new natural thiocuprate from Jabel Harmun, Judean Desert, Palestine Autonomy, Israel. Miner. Mag. 2017, 81, 1073–1085. [Google Scholar] [CrossRef]
- Gross, S. The Mineralogy of the Hatrurim Formation Israel; Geological Survey of Israel: Jerusalem, Israel, 1977.
- Burg, A.; Starinsky, A.; Bartov, Y.; Kolodny, Y. Geology of the Hatrurim Formation (“Mottled Zone”) in the Hatrurim basin. Isr. J. Earth Sci. 1991, 40, 107–124. [Google Scholar]
- Sokol, E.V.; Novikov, I.S.; Vapnik, Y.; Sharygin, V.V. Gas fire from mud volcanoes as a trigger for the appearance of high-temperature pyrometamorphic rocks of the Hatrurim Formation (Dead Sea area). Dokl. Earth Sci. 2007, 413, 474–480. [Google Scholar] [CrossRef]
- Novikov, I.; Vapnik, Y.; Safonova, I. Mud volcano origin of the Mottled Zone, South Levant. Geosci. Front. 2013, 4, 597–619. [Google Scholar] [CrossRef]
- Waltersperger, S.; Olieric, V.; Pradervand, C.; Glettig, W.; Salathe, M.; Fuchs, M.R.; Curtin, A.; Wang, X.; Ebner, S.; Panepucci, E.; et al. PRIGo: A new multi-axis goniometer for macromolecular crystallography. J. Synchrotron Radiat. 2015, 22, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Wojdyla, J.A.; Kaminski, J.W.; Panepucci, E.; Ebner, S.; Wang, X.; Gabadinho, J.; Wang, M. DA+ data acquisition and analysis software at the Swiss Light Source macromolecular crystallography beamlines. J. Synchrotron Radiat. 2018, 25, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabsch, W. XDS. Acta Cryst. D Biol. Cryst. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vapnik, Y.; Galuskina, I.O.; Palchik, V.; Sokol, E.V.; Galuskin, E.V.; Lindsley-Griffin, N.; Stracher, G. Paralavas in combustion metamorphic complex at the Hatrurim Basin, Israel. In Coal and Peat Fires: A Global Perspective, 1st ed.; Elsevier: Amsterdam, Netherlands, 2014; Volume 3, pp. 281–316. [Google Scholar]
- Downs, R.T.; Bartelmehs, K.L.; Gibbs, G.V.; Boisen, M.B. Interactive software for calculating and displaying X-ray or neutron powder diffractometer patterns of crystalline materials. Am. Miner. 1993, 78, 1104–1107. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Abakumov, A.M.; Kalyuzhnaya, A.S.; Rozova, M.G.; Antipov, E.V.; Hadermann, J.; Van Tendeloo, G. Compositionally induced phase transition in the Ca2MnGa1−xAlxO5 solid solutions: Ordering of tetrahedral chains in brownmillerite structure. Solid State Sci. 2005, 7, 801–811. [Google Scholar] [CrossRef]
- Redhammer, G.J.; Tippelt, G.; Roth, G.; Amthauer, G. Structural variations in the brownmillerite series Ca2(Fe2−xAlx)O5: Single-crystal X-ray diffraction at 25 °C and high-temperature X-ray powder diffraction (25 °C ≤ T ≤ 1000 °C). Am. Miner. 2004, 89, 405–420. [Google Scholar] [CrossRef]
- Becerro, A.; McCammon, C.; Langenhorst, F.; Seifert, F.; Angel, R.J. Oxygen-vacancy ordering in CaTiO3-CaFeO2.5 perovskites: From isolated defects to infinite sheets. Phase Transit. 1999, 69, 133–146. [Google Scholar] [CrossRef]








| Rmax/Rmin (%) | λ (nm) | Rmax/Rmin (%) | λ (nm) |
|---|---|---|---|
| 18.7/17.6 | 400 | 14.8/14.2 | 560 |
| 18.3/17.4 | 420 | 14.7/14.1 | 580 |
| 17.0/16.0 | 440 | 14.6/14.1 | 589 (COM) |
| 16.4/15.6 | 460 | 14.6/14.1 | 600 |
| 16.1/15.5 | 470 (COM) | 14.6/14.0 | 620 |
| 15.9/15.4 | 480 | 14.6/14.0 | 640 |
| 15.5/14.9 | 500 | 14.5/13.9 | 650 (COM) |
| 15.2/14.5 | 520 | 14.4/13.7 | 660 |
| 15.0/14.3 | 540 | 14.3/13.5 | 680 |
| 14.9/14.2 | 546 (COM) | 14.1/13.4 | 700 |
| Crystal Data | |
| Chemical formula | Ca3TiFe1.68Al0.32O8 |
| Crystal system | orthorhombic |
| Space group | P21ma |
| Unit-cell dimensions | a = 5.423(2) Å |
| b = 11.150(8) Å | |
| c = 5.528(2) Å | |
| Unit cell volume | 334.3(3) Å3 |
| Formula weight | 398.6 |
| Density (calculated) | 3.943 g/cm3 |
| Z Crystal size | 2 60 × 40 × 40 µm |
| Data Collection | |
| Diffractometer | beamline X06DA, Swiss Light Source multi-axis goniometer PRIGo, PILATUS 2M-F detector |
| Radiation wavelength | 0.7085 Å |
| Detector to sample distance | 120 mm |
| Oscillation range | 0.1° |
| No. of frames measured | 1800 |
| Time of exposure | 0.1 s |
| Reflection ranges | −7 ≤ h ≤ 7; −16 ≤ k ≤ 10; −7 ≤ l ≤ 7 |
| Reflection measured | 1810 |
| Rint | 0.026 |
| Refinement of Structure | Full Matrix Least-squares on f |
| No. of unique reflections | 951 |
| No. of observed unique refl. [I > 3σ(I)] | 943 |
| Final R values [I > 3σ(I)] | R = 0.024; wR = 0.034 |
| Final R values (all data) | R = 0.024; wR = 0.034 |
| S (all data) | 1.62 |
| Refined parameters | 74 |
| Weighting scheme | w = 1/(σ2(F) + 0.0001F2) |
| Δρmin [e Å−3] | −0.62 |
| Δρmax [e Å−3] | 0.49 |
| Bellerberg (Holotype Locality) | Jabel Harmun (Hatrurim Complex) | Upper Chegem Caldera | |||||||
|---|---|---|---|---|---|---|---|---|---|
| wt % | Mean | S.D. | Range | Mean | S.D. | Range | Mean | S.D. | Range |
| n = 9 | n = 19 | n = 4 | |||||||
| Nb2O5 | n.d. | n.d. | n.d. | ||||||
| MnO2 | 2.27 | 0.71 | 1.04–3.22 | n.d. | n.d. | ||||
| SiO2 | 0.58 | 0.13 | 0.40–0.80 | 1.19 | 0.09 | 1.07–1.43 | 0.17 | 0.11 | 0.07–0.32 |
| SnO2 | n.d. | n.d. | 0.37 | 0.26 | 0.15–0.61 | ||||
| TiO2 | 17.04 | 0.89 | 16.29–19.34 | 17.97 | 0.61 | 16.65–18.76 | 17.38 | 0.38 | 16.97–17.76 |
| ZrO2 | 0.27 | 0.14 | 0.07–0.57 | 0.43 | 0.11 | 0.25–0.62 | 0.39 | 0.23 | 0.16–0.67 |
| Al2O3 | 2.49 | 0.53 | 2.22–3.89 | 3.83 | 0.14 | 3.62–4.13 | 1.86 | 0.45 | 1.36–2.29 |
| Cr2O3 | 0.20 | 0.11 | 0.07–0.42 | 0.25 | 0.14 | 0.00–0.47 | n.d. | ||
| Fe2O3 | 34.87 | 0.85 | 32.81–35.85 | 32.80 | 0.79 | 31.70–34.54 | 37.43 | 1.03 | 36.40–38.72 |
| CaO | 41.59 | 0.37 | 40.99–42.09 | 42.19 | 0.27 | 41.64–42.60 | 40.71 | 0.14 | 40.53–40.85 |
| FeO | n.d. | n.d. | n.d. | ||||||
| MgO | 0.13 | 0.05 | 0.08–0.24 | 0.08 | 0.02 | 0.06–0.11 | 0.05 | 0.01 | 0.04–0.06 |
| MnO | n.d. | n.d. | 0.09 | 0.06 | 0.01–0.13 | ||||
| SrO | n.d. | 0.18 | 0.05 | 0.08–0.32 | n.d. | ||||
| Total | 99.44 | 98.91 | 98.45 | ||||||
| Bellerberg (Holotype Locality) | Jabel Harmun (Hatrurim Complex) | Upper Chegem Caldera | |||||||
| Calculated on 8 O | |||||||||
| Ca2+ | 3.00 | 3.03 | 3.00 | ||||||
| Sr2+ | 0.01 | 0.00 | |||||||
| Sum A | 3.00 | 3.04 | 3.00 | ||||||
| Nb5+ | |||||||||
| Mn4+ | 0.11 | ||||||||
| Sn4+ | 0.01 | ||||||||
| Ti4+ | 0.86 | 0.91 | 0.90 | ||||||
| Zr4+ | 0.01 | 0.01 | 0.01 | ||||||
| Cr3+ | 0.01 | 0.01 | |||||||
| Fe3+ | 1.00 | 1.06 | 1.06 | ||||||
| Fe2+ | |||||||||
| Mg2+ | 0.01 | 0.01 | 0.01 | ||||||
| Mn2+ | 0.01 | ||||||||
| Sum B | 2.00 | 2.00 | 2.00 | ||||||
| Si4+ | 0.04 | 0.08 | 0.01 | ||||||
| Al3+ | 0.20 | 0.30 | 0.15 | ||||||
| Fe3+ | 0.76 | 0.59 | 0.87 | ||||||
| Sum T | 1.00 | 0.97 | 1.03 | ||||||
| Sample wt % | [A] | [B] | [C] | [D] | [E] | [F] | [G] | [H] | [I] |
|---|---|---|---|---|---|---|---|---|---|
| E-2011 | M7-184 | E-2-1 | H-201 | YV-411 | YV-568 | 42-17g | M-4 | M-4 | |
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | |
| n = 4 | n = 19 | n = 1 | n = 2 | n = 1 | n = 2 | n = 2 | n = 1 | n = 4 | |
| Nb2O5 | 0.17 | 0.18 | 0.17 | n.d. | n.d. | n.d. | 0.06 | 0.04 | 0.06 |
| SiO2 | 0.53 | 0.71 | 0.71 | 0.60 | 2.67 | 0.94 | 0.62 | 0.67 | 0.37 |
| TiO2 | 19.55 | 20.98 | 18.81 | 18.59 | 17.85 | 17.20 | 19.66 | 18.67 | 20.23 |
| ZrO2 | 0.19 | 0.03 | 0.12 | 0.35 | n.d. | n.d. | 0.45 | 0.31 | 0.41 |
| Al2O3 | 4.27 | 3.21 | 3.65 | 4.85 | 4.65 | 5.20 | 5.51 | 4.30 | 3.55 |
| Cr2O3 | 0.12 | 0.01 | 0.01 | 0.45 | n.d. | n.d. | 0.03 | 0.02 | 0.06 |
| Fe2O3 | 31.74 | 30.74 | 34.34 | 32.27 | 30.94 | 31.13 | 30.35 | 33.52 | 31.95 |
| V2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.18 | 0.10 |
| La2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 | n.d. | n.d. |
| Ce2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.17 | n.d. | n.d. |
| CaO | 41.12 | 41.64 | 41.68 | 42.48 | 42.76 | 42.79 | 42.73 | 41.21 | 41.10 |
| FeO | 0.06 | 0.51 | 0.03 | n.d. | n.d. | n.d. | 0.01 | n.d. | 0.48 |
| MgO | n.d. | 0.30 | n.d. | 0.12 | 0.45 | 0.06 | 0.07 | n.d. | n.d. |
| MnO | 0.09 | 0.38 | 0.22 | 0.05 | 0.54 | 0.95 | n.d. | n.d. | n.d. |
| SrO | 1.78 | 0.55 | 0.41 | 0.20 | n.d. | 0.15 | 0.16 | 1.41 | 1.29 |
| ZnO | n.d. | n.d. | n.d. | 0.07 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total | 99.62 | 99.24 | 100.15 | 100.03 | 99.86 | 98.42 | 99.95 | 100.33 | 99.60 |
| Sample | [A] | [B] | [C] | [D] | [E] | [F] | [G] | [H] | [I] |
| E-2011 | M7-184 | E-2-1 | H-201 | YV-411 | YV-568 | 42-17g | M-4 | M-4 | |
| Calculated on 8 O | |||||||||
| Ca2+ | 2.95 | 2.98 | 2.97 | 3.00 | 3.00 | 3.08 | 3.02 | 2.94 | 2.96 |
| Sr2+ | 0.07 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.05 | 0.05 | |
| La2++Ce2+ | 0.01 | ||||||||
| Sum A | 3.02 | 3.00 | 2.99 | 3.01 | 3.00 | 3.09 | 3.04 | 2.99 | 3.01 |
| Nb5+ | 0.01 | 0.01 | 0.01 | <0.01 | <0.01 | <0.01 | |||
| Ti4+ | 0.98 | 1.05 | 0.94 | 0.92 | 0.88 | 0.87 | 0.97 | 0.93 | 1.02 |
| Zr4+ | 0.01 | <0.01 | <0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Cr3+ | 0.01 | 0.02 | <0.01 | <0.01 | <0.01 | ||||
| Fe3+ | 0.98 | 0.85 | 1.05 | 1.02 | 1.05 | 1.12 | 0.97 | 1.06 | 0.92 |
| V3+ | 0.01 | 0.01 | 0.01 | ||||||
| Fe2+ | 0.03 | <0.01 | <0.01 | 0.03 | |||||
| Mg2+ | 0.03 | 0.01 | 0.04 | 0.01 | 0.01 | ||||
| Mn2+ | 0.01 | 0.02 | 0.01 | <0.01 | 0.03 | ||||
| Zn2+ | <0.01 | ||||||||
| Sum B | 2.00 | 2.00 | 2.01 | 1.98 | 2.00 | 2.00 | 1.96 | 2.01 | 1.99 |
| Si4+ | 0.04 | 0.05 | 0.05 | 0.04 | 0.17 | 0.06 | 0.04 | 0.04 | 0.03 |
| Al3+ | 0.34 | 0.25 | 0.29 | 0.38 | 0.36 | 0.41 | 0.43 | 0.34 | 0.28 |
| Fe3+ | 0.62 | 0.69 | 0.67 | 0.58 | 0.47 | 0.46 | 0.53 | 0.62 | 0.69 |
| Sum T | 1.00 | 0.99 | 1.01 | 1.00 | 1.00 | 0.93 | 1.00 | 1.00 | 1.00 |
| Powder Data | Calculated from the Results of Single-crystal Structure Refinement | ||||||
|---|---|---|---|---|---|---|---|
| h | k | l | I | dmeas. | dcalc. | I | d |
| 0 | 1 | 0 | 9 | 11.0909 | 11.1641 | 7 | 11.1500 |
| 0 | 2 | 0 | 5 | 5.5991 | 5.5820 | 4 | 5.5750 |
| 0 | 1 | 1 | 3 | 4.9385 | 4.9544 | 3 | 4.9527 |
| 0 | 2 | 1 | 3 | 3.9160 | 3.9281 | 3 | 3.9254 |
| 1 | 0 | 1 | 8 | 3.8701 | 3.8716 | 11 | 3.8712 |
| 0 | 3 | 0 | 5 | 3.7127 | 3.7214 | 5 | 3.7167 |
| 1 | 2 | 1 | 4 | 3.1836 | 3.1813 | 4 | 3.1798 |
| 0 | 3 | 1 | 6 | 3.0783 | 3.0871 | 5 | 3.0844 |
| 0 | 0 | 2 | 32 | 2.7633 | 2.7643 | 28 | 2.7640 |
| 2 | 0 | 0 | 27 | 2.7119 | 2.7118 | 36 | 2.7115 |
| 1 | 3 | 1 | 100 | 2.6791 | 2.6830 | 100 | 2.6811 |
| 0 | 4 | 1 | 7 | 2.4874 | 2.4915 | 6 | 2.4890 |
| 1 | 0 | 2 | 4 | 2.4578 | 2.4628 | 4 | 2.4626 |
| 1 | 4 | 1 | 7 | 2.2612 | 2.2641 | 6 | 2.2621 |
| 0 | 3 | 2 | 3 | - | 2.2191 | 2 | 2.2179 |
| 2 | 3 | 0 | 3 | 2.1868 | 2.1916 | 3 | 2.1905 |
| 2 | 3 | 1 | 2 | 2.0386 | 2.0374 | 2 | 2.0365 |
| 2 | 0 | 2 | 36 | 1.9359 | 1.9358 | 41 | 1.9356 |
| 1 | 5 | 1 | 4 | - | 1.9342 | 4 | 1.9323 |
| 2 | 1 | 2 | 3 | 1.9080 | 1.9074 | 3 | 1.9071 |
| 0 | 6 | 0 | 19 | 1.8566 | 1.8607 | 18 | 1.8583 |
| 1 | 4 | 2 | 2 | 1.8511 | 1.8467 | 2 | 1.8455 |
| 2 | 2 | 2 | 3 | 1.8286 | 1.8290 | 3 | 1.8285 |
| 0 | 2 | 3 | 3 | 1.7554 | 1.7500 | 2 | 1.7496 |
| 1 | 6 | 1 | 2 | 1.6859 | 1.6771 | 2 | 1.6753 |
| 0 | 3 | 3 | 4 | 1.6507 | 1.6515 | 4 | 1.6509 |
| 3 | 2 | 1 | 2 | 1.6393 | 1.6423 | 3 | 1.6419 |
| 1 | 3 | 3 | 18 | 1.5800 | 1.5798 | 17 | 1.5793 |
| 3 | 3 | 1 | 12 | 1.5592 | 1.5600 | 16 | 1.5596 |
| 0 | 6 | 2 | 8 | 1.5450 | 1.5436 | 8 | 1.5422 |
| 2 | 6 | 0 | 8 | - | 1.5342 | 9 | 1.5329 |
| 3 | 4 | 1 | 2 | - | 1.4632 | 2 | 1.4626 |
| 2 | 3 | 3 | 3 | 1.4064 | 1.4105 | 3 | 1.4101 |
| 0 | 0 | 4 | 3 | - | 1.3821 | 3 | 1.3820 |
| 4 | 0 | 0 | 3 | 1.3559 | 1.3559 | 4 | 1.3557 |
| 2 | 6 | 2 | 11 | 1.3410 | 1.3415 | 13 | 1.3405 |
| 3 | 3 | 3 | 4 | 1.2193 | 1.2193 | 5 | 1.2190 |
| 4 | 0 | 2 | 2 | 1.2176 | 1.2173 | 2 | 1.2172 |
| 1 | 9 | 1 | 3 | 1.1806 | 1.1813 | 4 | 1.1799 |
| Site | Atom | x | y | z | Uiso | Occupancy |
|---|---|---|---|---|---|---|
| Ti1 | Ti | 0.25010(10) | 0.16862(4) | 0.23966(9) | 0.00672(15) | 0.5 |
| Fe1 | Fe | 0.25010(10) | 0.16862(4) | 0.23966(9) | 0.00672(15) | 0.465(5) |
| Al1 | Al | 0.25010(10) | 0.16862(4) | 0.23966(9) | 0.00672(15) | 0.035(5) |
| Fe2 | Fe | 0.19825(16) | 0.5 | 0.18318(13) | 0.00750(19) | 0.751(7) |
| Al2 | Al | 0.19825(16) | 0.5 | 0.18318(13) | 0.00750(19) | 0.249(7) |
| Ca1 | Ca | −0.26824(16) | 0.31319(6) | 0.27602(11) | 0.01048(18) | 1 |
| Ca2 | Ca | 0.2676(3) | 0 | −0.26624(16) | 0.0141(3) | 1 |
| O1 | O | 0.2758(5) | 0.35744(18) | 0.3210(3) | 0.0136(6) | 1 |
| O2 | O | 0.3553(6) | 0.5 | −0.1222(5) | 0.0127(8) | 1 |
| O3 | O | 0.0161(4) | 0.15042(14) | 0.5135(5) | 0.0105(5) | 1 |
| O4 | O | 0.2444(6) | 0 | 0.1700(5) | 0.0132(8) | 1 |
| O5 | O | −0.0146(5) | 0.20160(16) | 0.0157(5) | 0.0127(6) | 1 |
| Site | Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|---|
| Ti1 | Ti | 0.0055(3) | 0.0109(2) | 0.0038(3) | −0.0001(3) | −0.00028(18) | 0.00037(14) |
| Fe1 | Fe | 0.0055(3) | 0.0109(2) | 0.0038(3) | −0.0001(3) | −0.00028(18) | 0.00037(14) |
| Al1 | Al | 0.0055(3) | 0.0109(2) | 0.0038(3) | −0.0001(3) | −0.00028(18) | 0.00037(14) |
| Fe2 | Fe | 0.0071(4) | 0.0094(3) | 0.0060(4) | 0 | 0.0003(3) | 0 |
| Al2 | Al | 0.0071(4) | 0.0094(3) | 0.0060(4) | 0 | 0.0003(3) | 0 |
| Ca1 | Ca | 0.0131(4) | 0.0115(3) | 0.0069(3) | −0.0012(4) | 0.0015(3) | −0.00057(18) |
| Ca2 | Ca | 0.0166(6) | 0.0142(4) | 0.0116(4) | 0 | −0.0004(4) | 0 |
| O1 | O | 0.0176(12) | 0.0131(9) | 0.0099(9) | 0.0016(8) | −0.0004(9) | 0.0011(7) |
| O2 | O | 0.0128(15) | 0.0130(13) | 0.0124(14) | 0 | 0.0032(13) | 0 |
| O3 | O | 0.0105(9) | 0.0129(7) | 0.0080(9) | 0.0001(8) | 0.0014(8) | −0.0008(8) |
| O4 | O | 0.0159(17) | 0.0122(12) | 0.0115(13) | 0 | −0.0009(13) | 0 |
| O5 | O | 0.0134(11) | 0.0177(8) | 0.0071(9) | 0.0007(8) | −0.0022(8) | −0.0005(9) |
| Atom | Atom | distance (Å) |
|---|---|---|
| Ti1 | O1 | 2.157(4) |
| O3 | 1.985(4) | |
| O3 | 1.996(3) | |
| O4 | 1.919(3) | |
| O5 | 1.931(3) | |
| O5 | 1.938(3) | |
| Mean | 1.988 | |
| Fe2 | O1 × 2 | 1.812(3) |
| O2 | 1.891(3) | |
| O2 | 1.890(3) | |
| Mean | 1.851 | |
| Ca2 | O3 × 2 | 2.481(3) |
| O3 × 2 | 2.549(3) | |
| O4 | 2.887(4) | |
| O4 | 2.640(4) | |
| O4 | 2.415(3) | |
| O5 × 2 | 2.892(3) | |
| Mean | 2.643 | |
| Ca1 | O1 | 2.534(3) |
| O1 | 2.294(3) | |
| O2 | 2.347(3) | |
| O3 | 2.719(3) | |
| O3 | 2.453(3) | |
| O5 | 2.348(3) | |
| O5 | 2.436(3) | |
| Mean | 2.448 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juroszek, R.; Krüger, H.; Galuskina, I.; Krüger, B.; Jeżak, L.; Ternes, B.; Wojdyla, J.; Krzykawski, T.; Pautov, L.; Galuskin, E. Sharyginite, Ca3TiFe2O8, A New Mineral from the Bellerberg Volcano, Germany. Minerals 2018, 8, 308. https://doi.org/10.3390/min8070308
Juroszek R, Krüger H, Galuskina I, Krüger B, Jeżak L, Ternes B, Wojdyla J, Krzykawski T, Pautov L, Galuskin E. Sharyginite, Ca3TiFe2O8, A New Mineral from the Bellerberg Volcano, Germany. Minerals. 2018; 8(7):308. https://doi.org/10.3390/min8070308
Chicago/Turabian StyleJuroszek, Rafał, Hannes Krüger, Irina Galuskina, Biljana Krüger, Lidia Jeżak, Bernd Ternes, Justyna Wojdyla, Tomasz Krzykawski, Leonid Pautov, and Evgeny Galuskin. 2018. "Sharyginite, Ca3TiFe2O8, A New Mineral from the Bellerberg Volcano, Germany" Minerals 8, no. 7: 308. https://doi.org/10.3390/min8070308