Precision CNC Machining of Femoral Component of Knee Implant: A Case Study
Abstract
:1. Introduction
2. Design of the Implant Geometry and Machining Processes
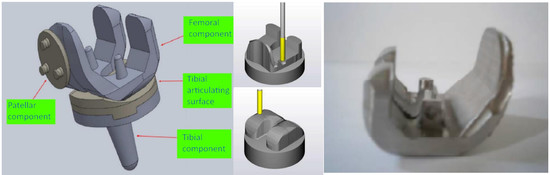
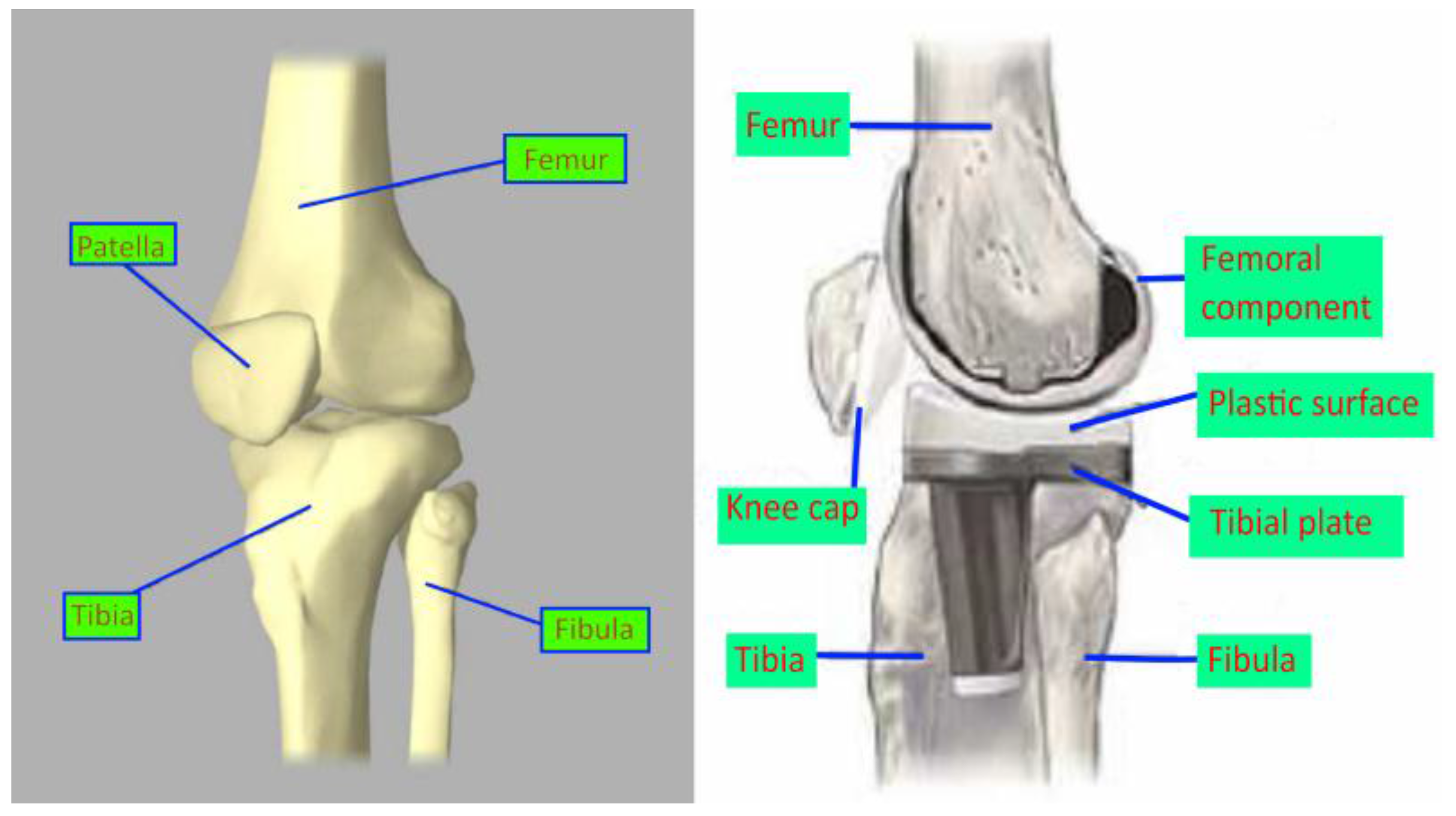
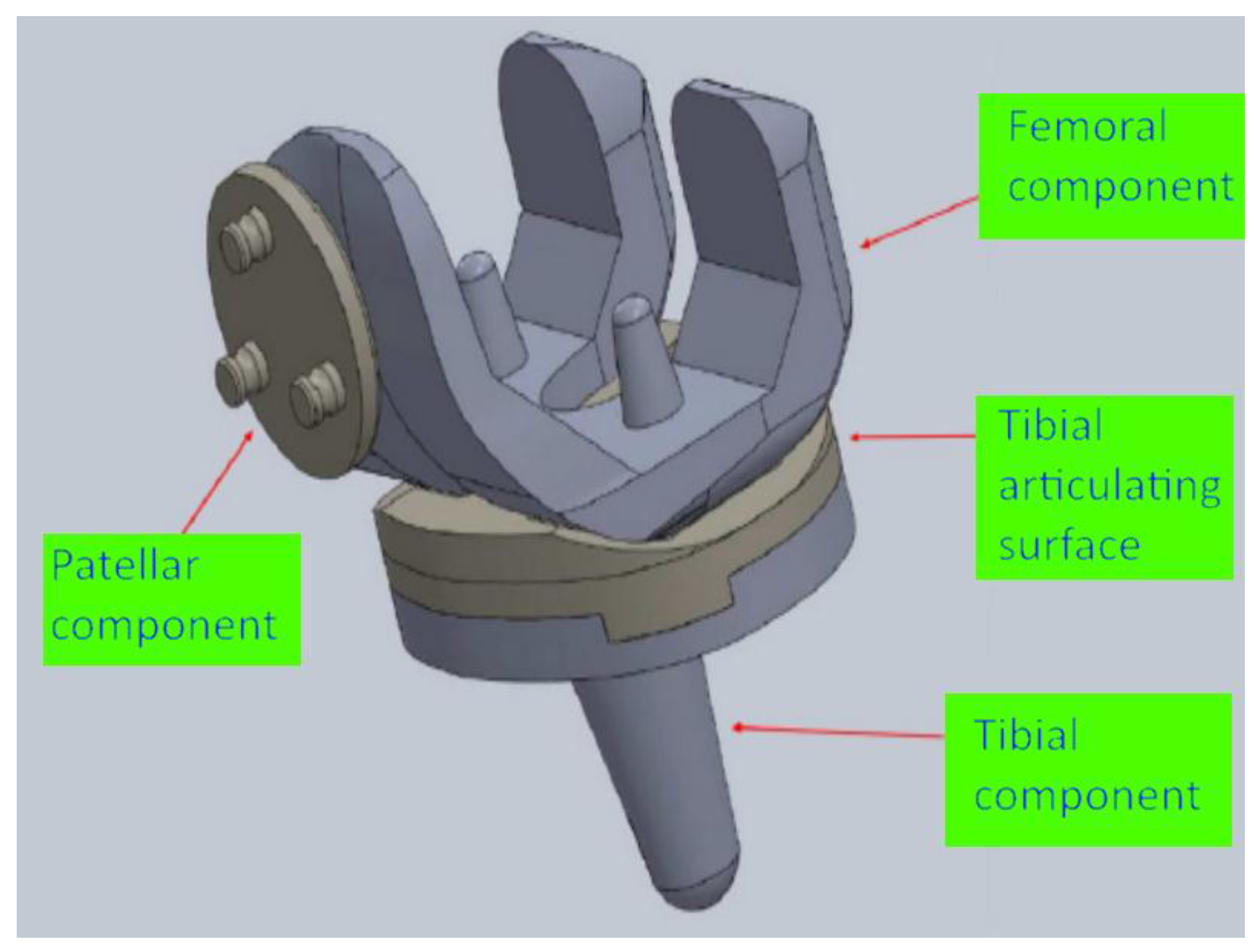
2.1. Design of the Knee Implant Components
2.2. Design of the Machining Process of the Femoral Component
2.2.1. First Phase of Machining
2.2.2. Second Phase of Machining
2.3. Initial Machining Test
3. Machining of the Implant and Measurement of Surface Roughness
3.1. Machining Process of the Implant
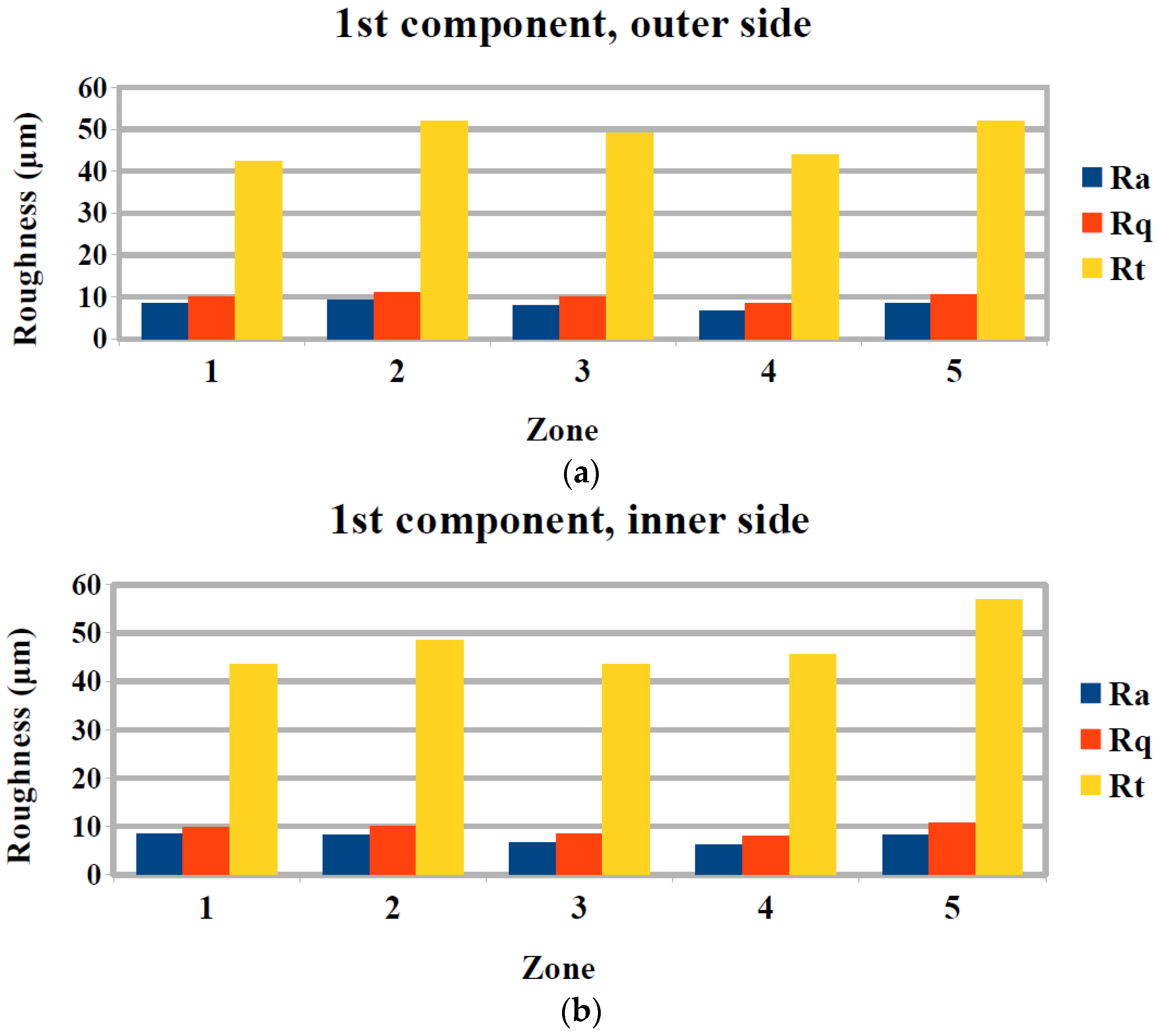
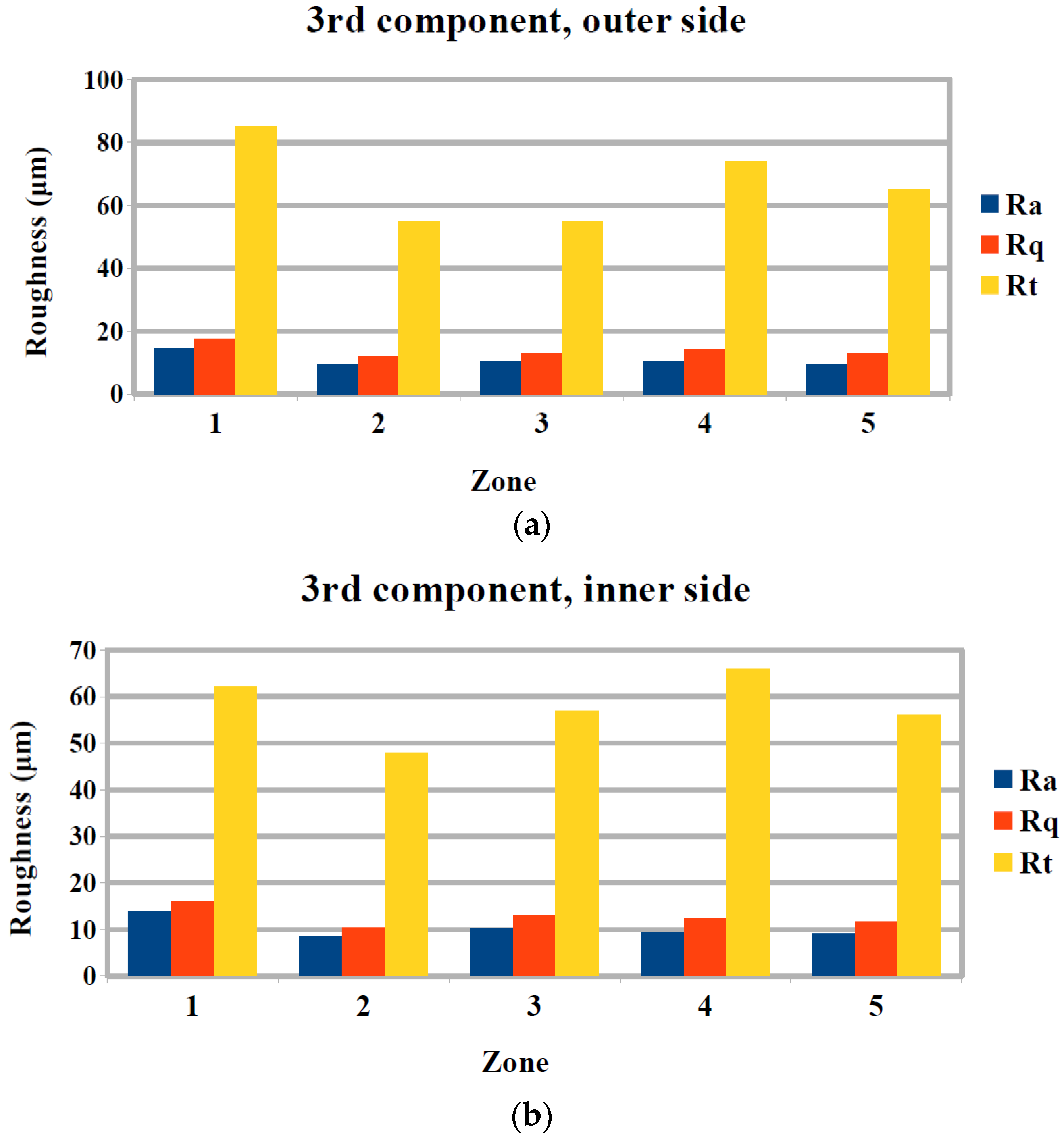
3.2. Surface Quality Evaluation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Carr, B.C.; Goswami, T. Knee implants—Review of models and biomechanics. Mater. Des. 2009, 30, 398–413. [Google Scholar] [CrossRef]
- Magdziak, M. The influence of a number of points on results of measurements of a turbine blade. Aircr. Eng. Aerosp. Technol. 2017, 89, 953–959. [Google Scholar] [CrossRef]
- Felhő, C.; Kundrák, J. Comparison of theoretical and real surface roughness in face milling with octagonal and circular inserts. Key Eng. Mater. 2014, 581, 360–365. [Google Scholar] [CrossRef]
- Kundrák, J.; Varga, G. Possibility of Reducing Environmental Load in Hard Machining. Key Eng. Mater. 2012, 496, 205–210. [Google Scholar] [CrossRef]
- Kundrák, J.; Varga, G.; Deszpoth, I.; Molnar, V. Some Aspects of the Hard Machining of Bore Holes. Appl. Mech. Mater. 2013, 309, 126–132. [Google Scholar] [CrossRef]
- Illés, B.; Tamás, P.; Dobos, P.; Skapinyecz, R. New challenges for quality assurance of manufacturing processes in industry 4.0. Solid State Phenom. 2017, 261, 481–486. [Google Scholar] [CrossRef]
- Brand, R.A.; Mont, M.A.; Manring, M.M. Biographical sketch: Themistocles Gluck (1853-1942). Clin. Orthop. Relat. Res. 2011, 469, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.C.; Fajgenbaum, D.M.; Venters, G.C. Results of knee replacement with the Walldius and geometric prostheses. A comparative study. J. Bone Jt. Surg. Am. 1980, 62, 497–503. [Google Scholar] [CrossRef]
- Aubriot, J.-H.; Deburge, A.; Genet, J.-P. GUEPAR hinge knee prosthesis. Orthop. Traumatol. Surg. Res. 2014, 100, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, C.J.; Hu, H.P.; Van Horn, J.R.; De Waal Malefijt, M.C. The Geomedic knee prosthesis. A long-term follow-up study. Acta Orthop. Belg. 1993, 59, 40–44. [Google Scholar] [PubMed]
- Coventry, M.B.; Finerman, G.A.; Riley, L.H.; Turner, R.H.; Upshaw, J.E. A new geometric knee for total knee arthroplasty. Clin. Orthop. Relat. Res. 1972, 83, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, K.; Wigren, A. Marmor Knee Arthroplasty: Clinical Results and Complications during an Observation Period of at least 3 Years. Acta Orthop. Scand. 1982, 53, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Lavernia, C.; C Alcerro, J.; Contreras Raygoza, J. Knee arthroplasty: Growing trends and future problems. Int. J. Clin. Rheumatol. 2010, 5, 565–579. [Google Scholar] [CrossRef]
- Goldberg, V.M.; Henderson, B.T. The Freeman-Swanson ICLH total knee arthroplasty. Complications and problems. JBJS 1980, 62, 1338–1344. [Google Scholar] [CrossRef]
- Mallory, T.H.; Smalley, D.; Danyi, J. Townley Anatomic Total Knee Arthroplasty Using Total Tibial Component with Cruciate Release. Clin. Orthop. Relat. Res. 1982, 169, 197–201. [Google Scholar] [CrossRef]
- Goodfellow, J.W.; Tibrewal, S.B.; Sherman, K.P.; O’Connor, J.J. Unicompartmental Oxford Meniscal knee arthroplasty. J. Arthroplast. 1987, 2, 1–9. [Google Scholar] [CrossRef]
- Buechel, F.F.S.; Buechel, F.F.J.; Pappas, M.J.; Dalessio, J. Twenty-year evaluation of the New Jersey LCS Rotating Platform Knee Replacement. J. Knee Surg. 2002, 15, 84–89. [Google Scholar] [PubMed]
- Buechel, F.F.; Pappas, M.J. Principles of Human Joint Replacement: Design and Clinical Application; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Walker, P.S.; Blunn, G.W.; Broome, D.R.; Perry, J.; Watkins, A.; Sathasivam, S.; Dewar, M.E.; Paul, J.P. A knee simulating machine for performance evaluation of total knee replacements. J. Biomech. 1997, 30, 83–89. [Google Scholar] [CrossRef]
- Harrysson, O.L.A.; Hosni, Y.A.; Nayfeh, J.F. Custom-designed orthopedic implants evaluated using finite element analysis of patient-specific computed tomography data: Femoral-component case study. BMC Musculoskelet. Disord. 2007, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chen, H.; Luo, C.-W.; Chang, K.-Y. Rapid Prototyping and Multi-axis NC Machining for The Femoral Component of Knee Prosthesis. Life Sci. J. 2010, 6, 73–77. [Google Scholar]
- Song, C.; Yang, Y.; Wang, Y.; Wang, D.; Yu, J. Research on rapid manufacturing of CoCrMo alloy femoral component based on selective laser melting. Int. J. Adv. Manuf. Technol. 2014, 75, 445–453. [Google Scholar] [CrossRef]
- Galanis, N.I.; Manolakos, D.E. Surface roughness prediction in turning of femoral head. Int. J. Adv. Manuf. Technol. 2010, 51, 79–86. [Google Scholar] [CrossRef]
- Galanis, N.; Manolakos, D. Surface roughness of manufactured femoral heads with high speed turning. Int J. Manuf. Mater. Mech. Eng. 2009, 5, 371–382. [Google Scholar] [CrossRef]
- Markopoulos, A.P.; Karkalos, N.E.; Galanis, N.I.; Manolakos, D.E. Design and Machining of the Femoral Component of Total Knee Implant. Solid State Phenom. 2017, 261, 313–320. [Google Scholar] [CrossRef]
- Denkena, B.; Köhler, J.; Turger, A.; Helmecke, P.; Correa, T.; Hurschler, C. Manufacturing conditioned wear of all-ceramic knee prostheses. Procedia CIRP 2013, 5, 179–184. [Google Scholar] [CrossRef]
- Otani, T.; Whiteside, L.A.; White, S.E.; McCarthy, D.S. Effects of femoral component material properties on cementless fixation in total hip arthroplasty. A comparison study between carbon composite, titanium alloy, and stainless steel. J. Arthroplast. 1993, 8, 67–74. [Google Scholar] [CrossRef]
- Gervais, B.; Vadean, A.; Raison, M.; Brochu, M. Failure analysis of a 316L stainless steel femoral orthopedic implant. Case Stud. Eng. Fail. Anal. 2016, 5, 30–38. [Google Scholar] [CrossRef]
- Affatato, S.; Ruggiero, A.; Grillini, L.; Falcioni, S. On the roughness measurement of the knee femoral components. In Proceedings of the 10th International Conference BIOMODLORE 2013, Vilnius, Lithuania, 20–22 September 2013; Vilnius Gediminas Technical University Press Technica: Vilnius, Lithuania, 2013; pp. 16–18. [Google Scholar] [CrossRef]
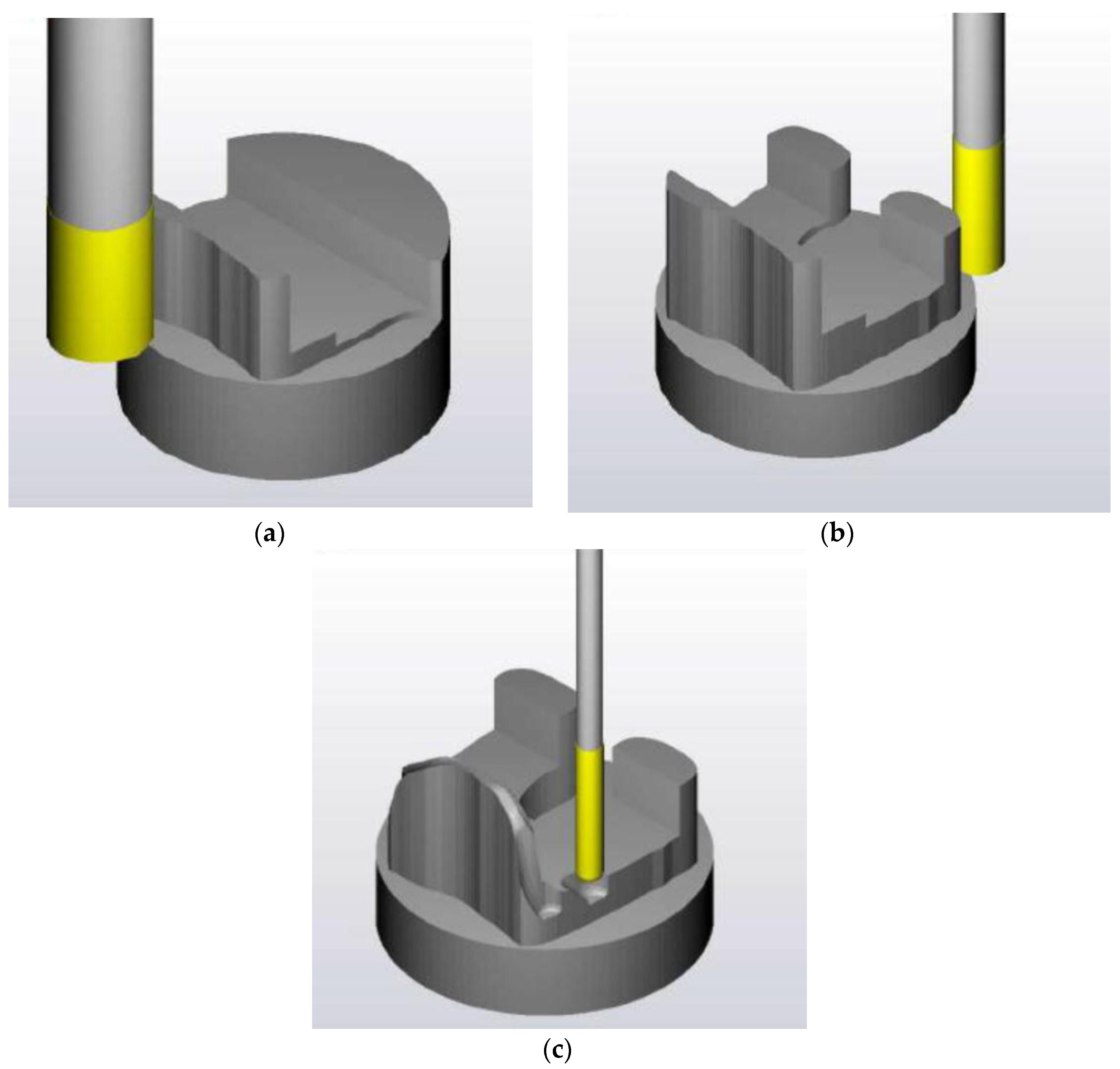
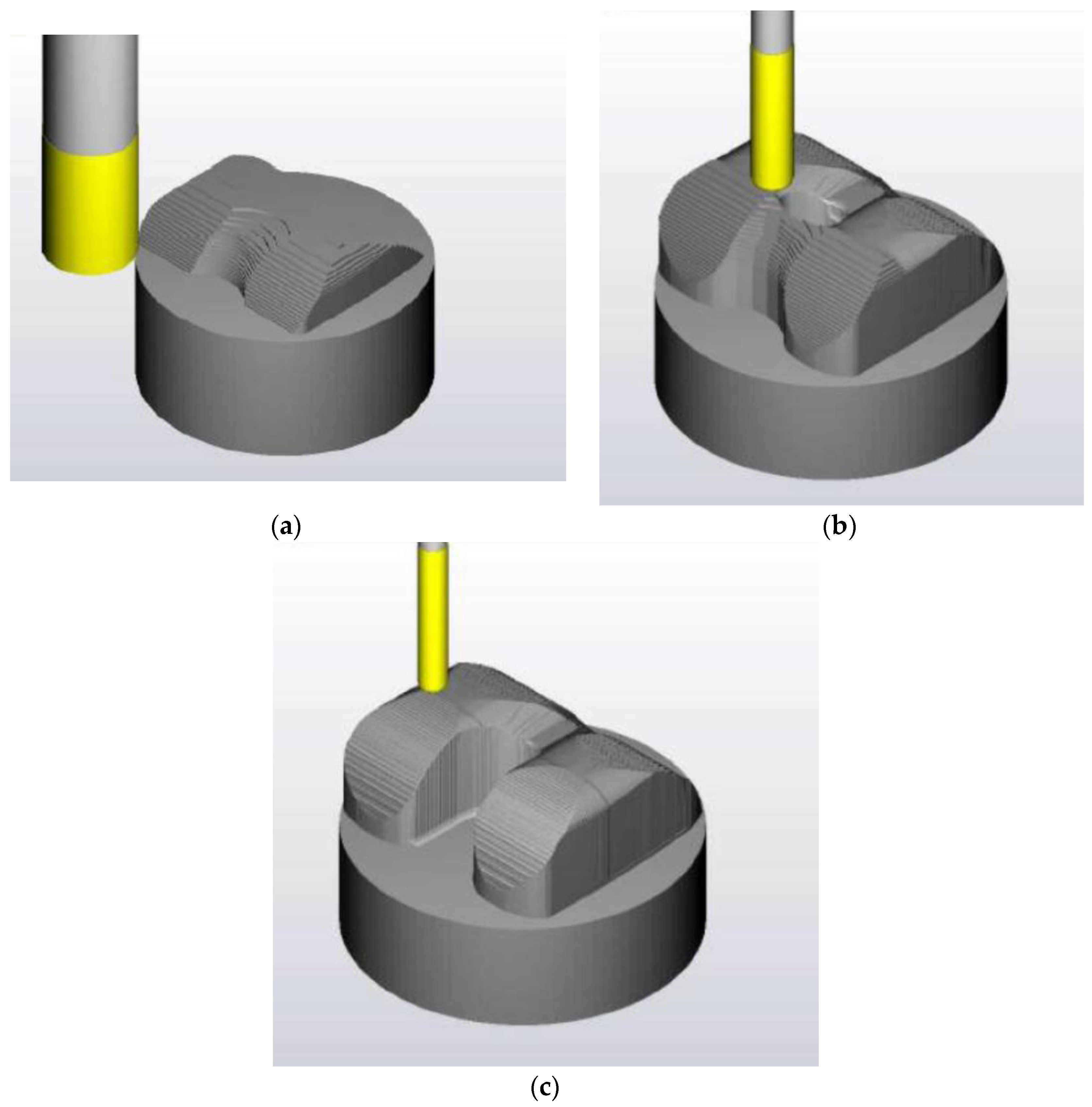




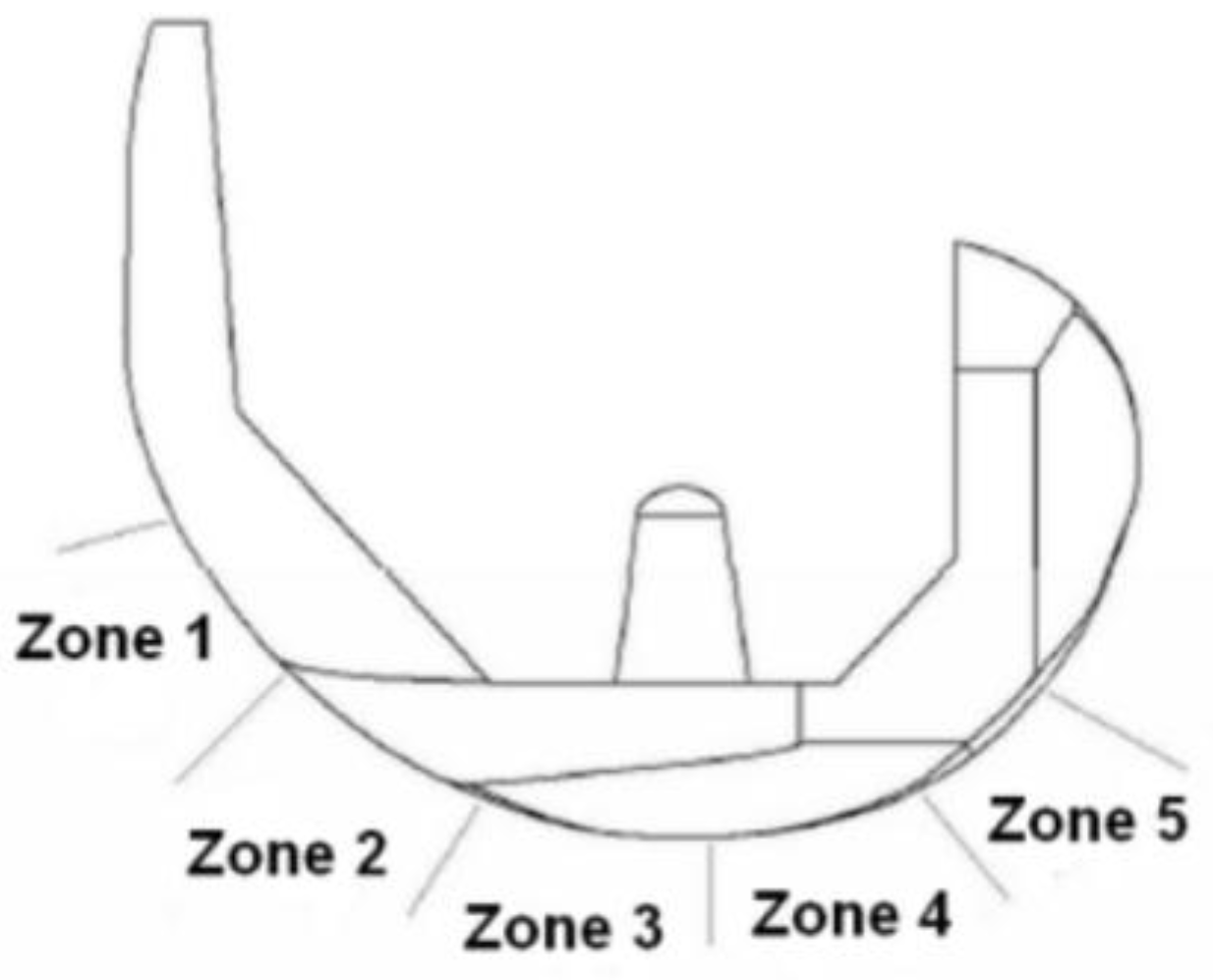

| No | Type | Diameter (mm) | Material |
|---|---|---|---|
| 1 | Flat end | 16 | carbide |
| 2 | Flat end | 6 | cobalt alloy |
| 3 | Ball end | 6 | cobalt alloy |
| 4 | Ball end | 4 | carbide |
| Zones of the Component | ||||||
|---|---|---|---|---|---|---|
| Component | 1 | 2 | 3 | 4 | 5 | |
| 1st | Feed rate (mm/min) | 100 | 100 | 100 | 100 | 100 |
| Spindle speed (rpm) | 2500 | 2500 | 2500 | 2500 | 2500 | |
| 2nd | Feed rate (mm/min) | 90 | 70 | 50 | 100 | 90 |
| Spindle speed (rpm) | 2500 | 2500 | 2500 | 2250 | 2250 | |
| 3rd | Feed rate (mm/min) | 70 | 50 | 100 | 90 | 70 |
| Spindle speed (rpm) | 2250 | 2250 | 2000 | 2000 | 2000 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markopoulos, A.P.; Galanis, N.I.; Karkalos, N.E.; Manolakos, D.E. Precision CNC Machining of Femoral Component of Knee Implant: A Case Study. Machines 2018, 6, 10. https://doi.org/10.3390/machines6010010
Markopoulos AP, Galanis NI, Karkalos NE, Manolakos DE. Precision CNC Machining of Femoral Component of Knee Implant: A Case Study. Machines. 2018; 6(1):10. https://doi.org/10.3390/machines6010010
Chicago/Turabian StyleMarkopoulos, Angelos P., Nikolaos I. Galanis, Nikolaos E. Karkalos, and Dimitrios E. Manolakos. 2018. "Precision CNC Machining of Femoral Component of Knee Implant: A Case Study" Machines 6, no. 1: 10. https://doi.org/10.3390/machines6010010