Micrometer-Scale Membrane Transition of Supported Lipid Bilayer Membrane Reconstituted with Cytosol of Dictyostelium discoideum
Abstract
:1. Introduction
2. Materials and Methods
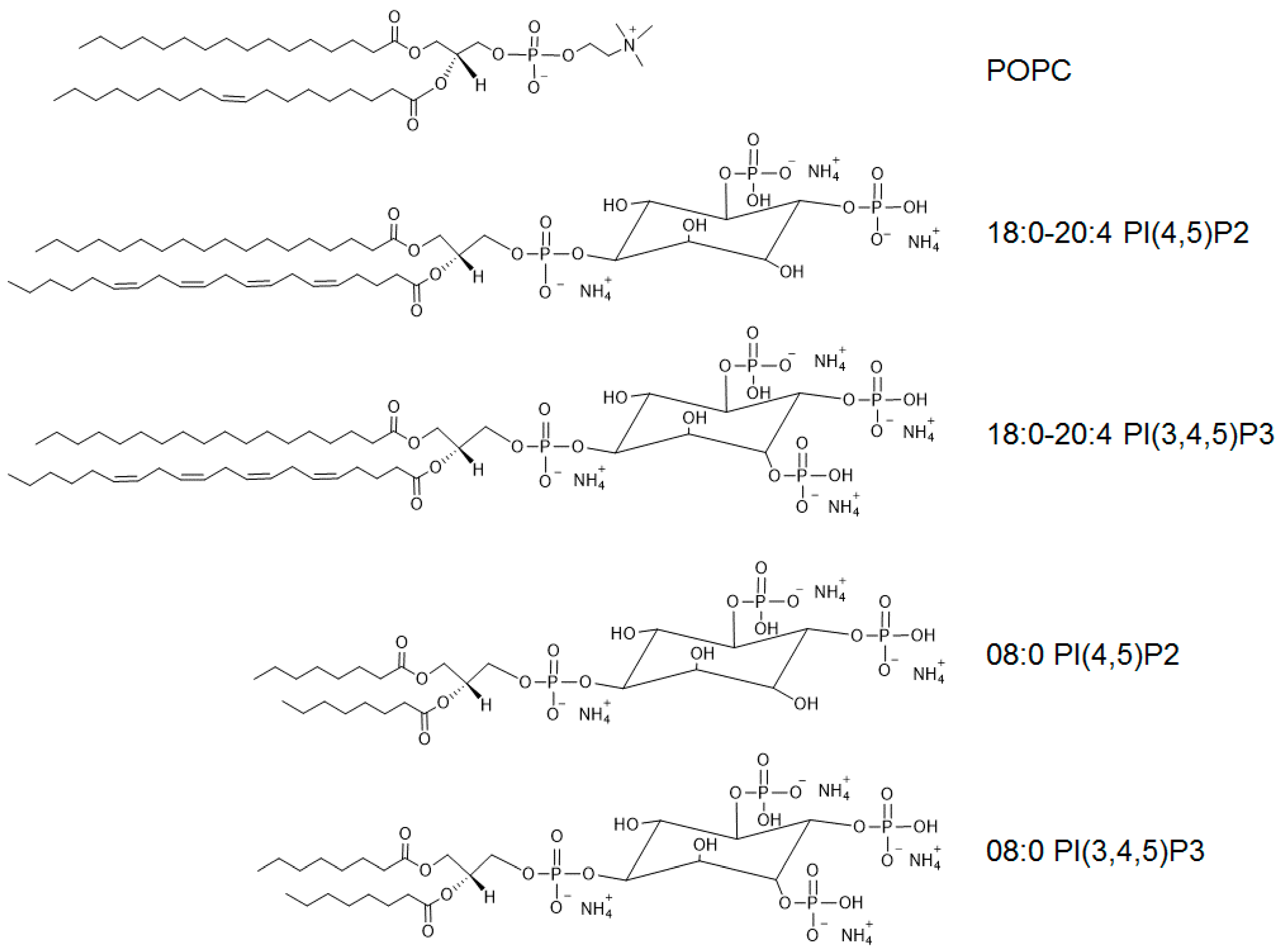
2.1. Reagents
2.2. Vesicle Preparation
2.3. Cover Glass Washing
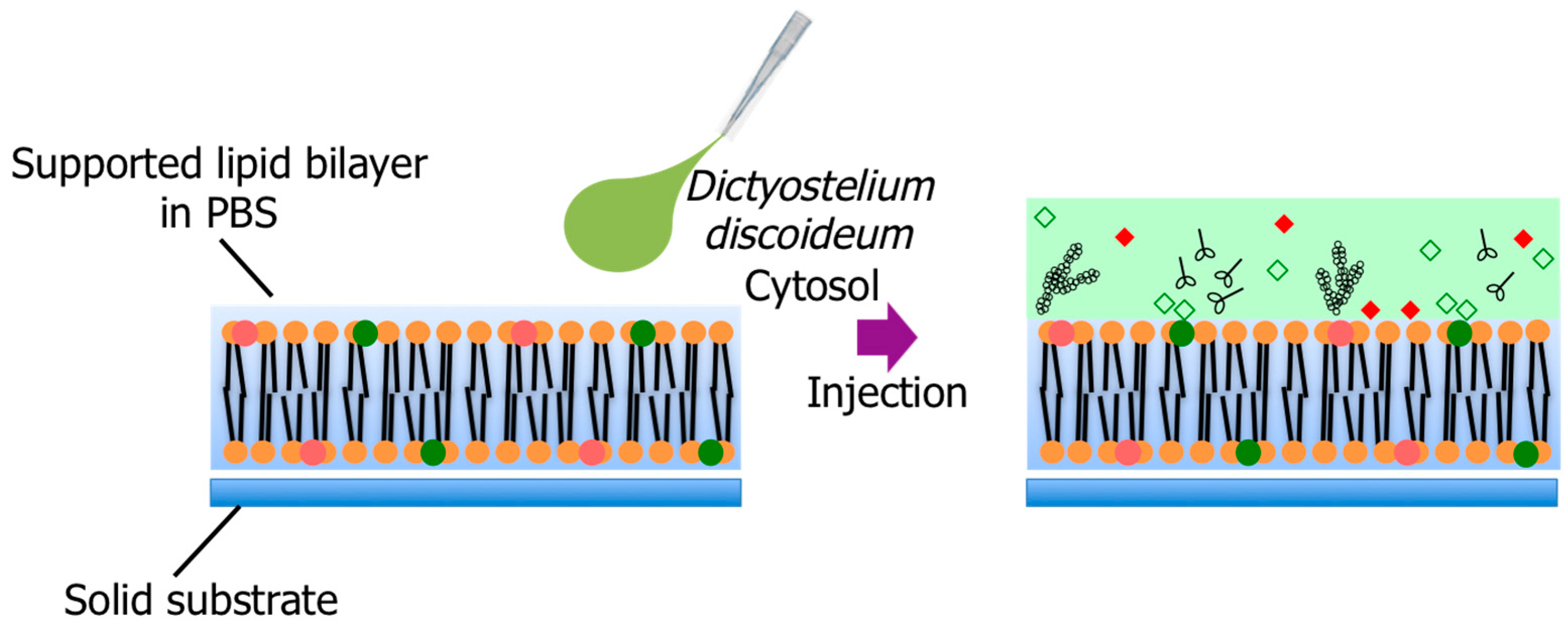
2.4. Supported Lipid Bilayer Membrane Preparation
2.5. Fluorescence Microscopy Observation
2.6. Cell Culture
2.7. Cytosol Extraction
3. Results and Discussion
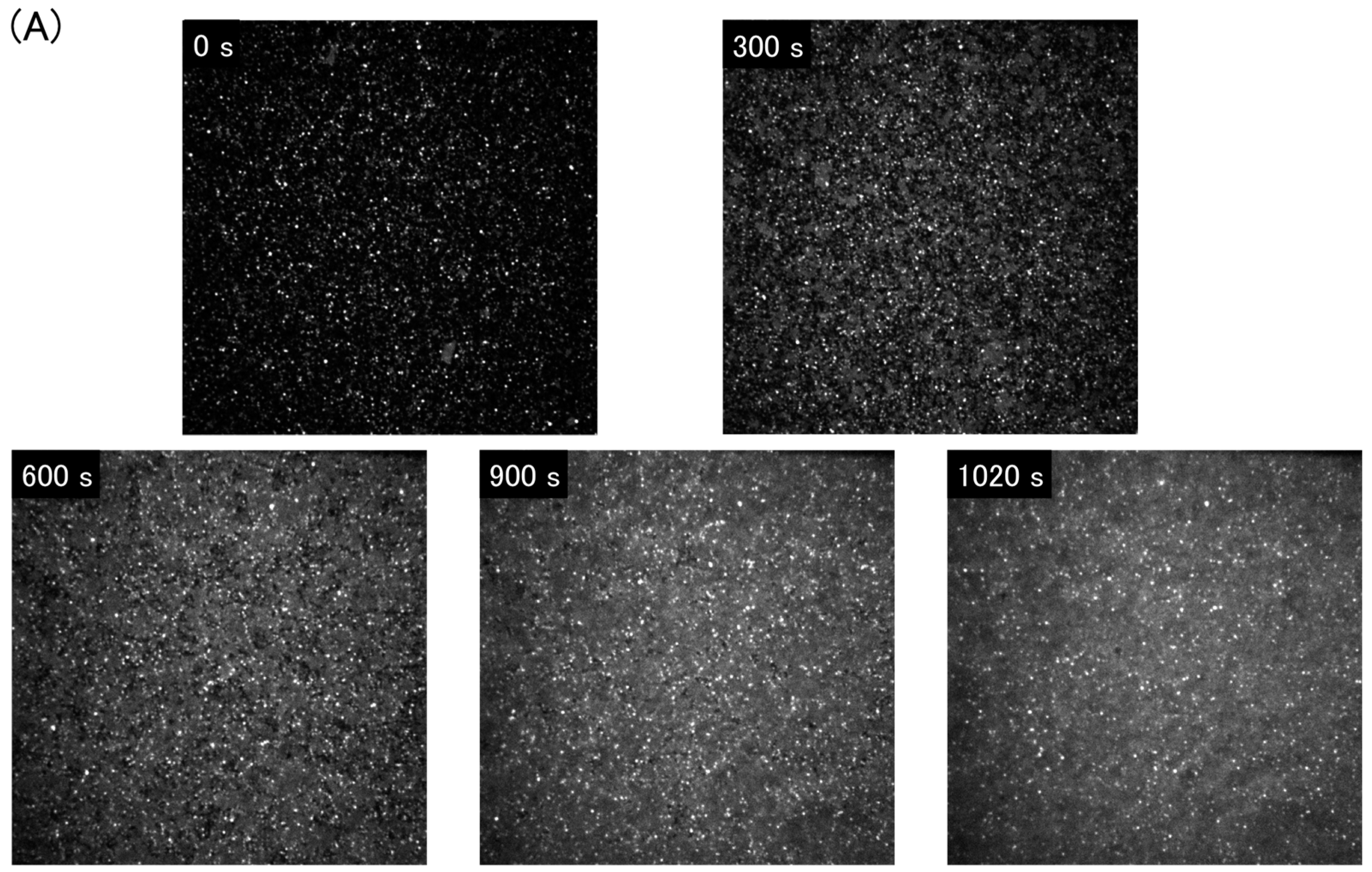
3.1. Supported Lipid Bilayer Membrane Formation by Rupturing Vesicles on Glass
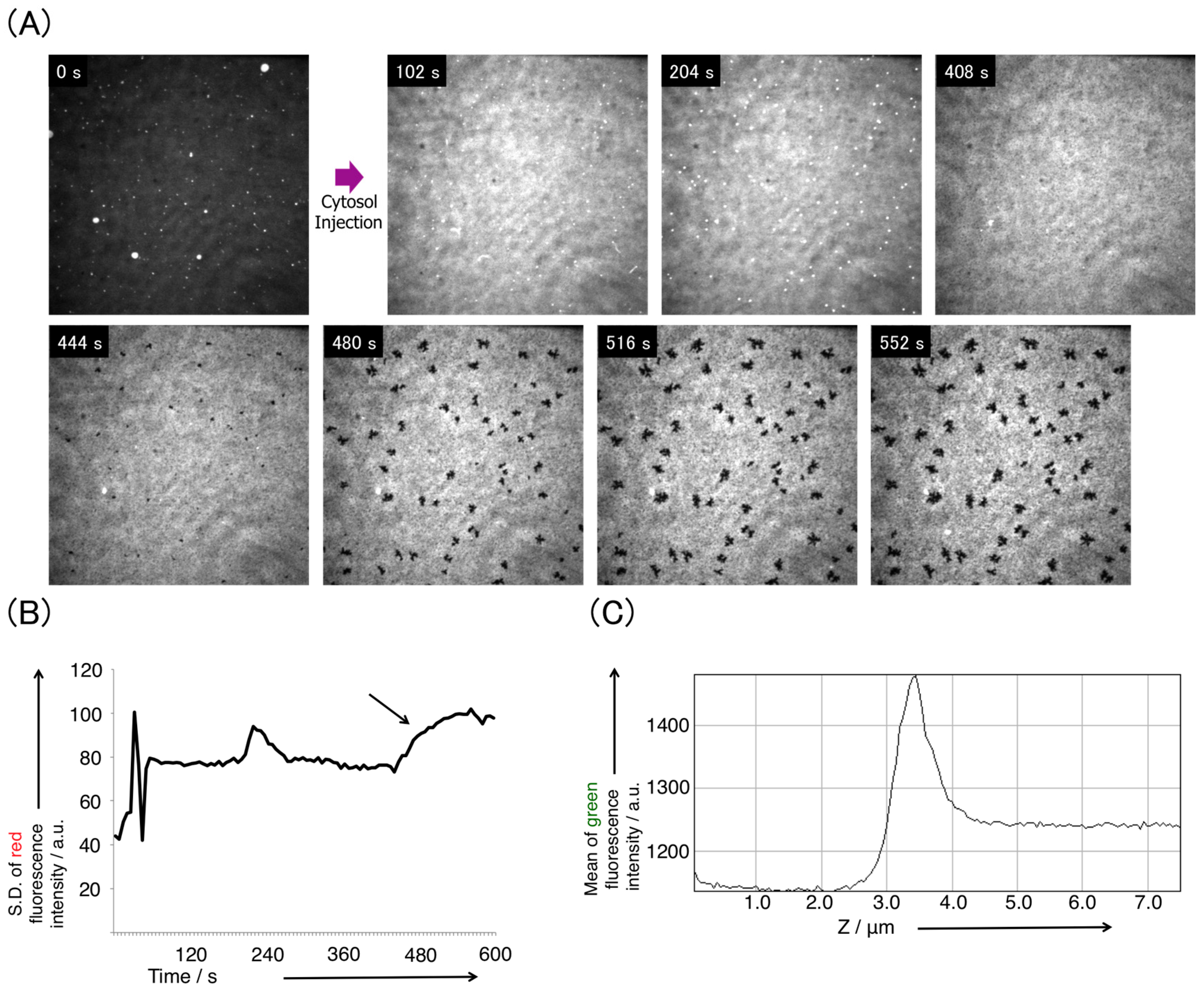
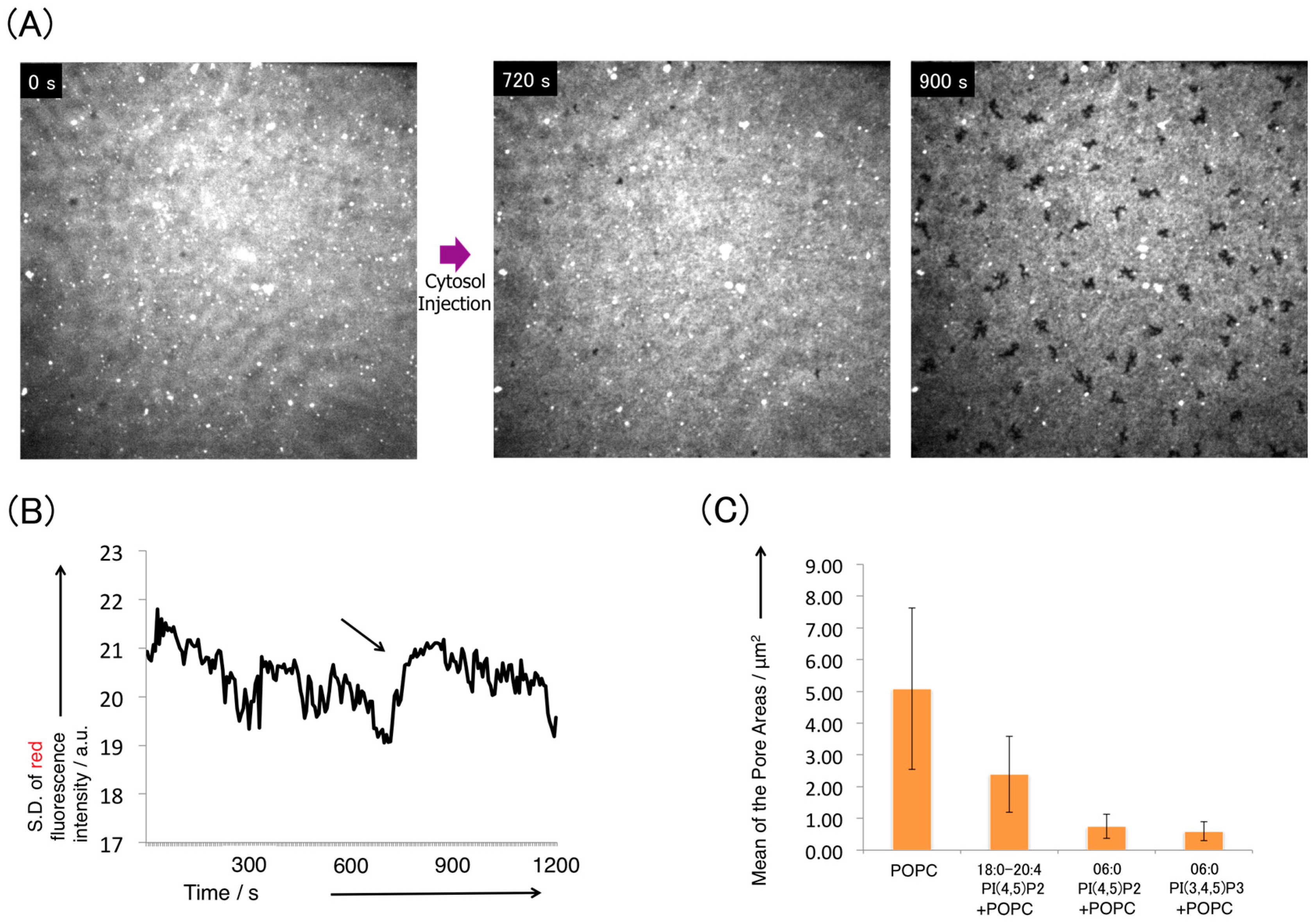
3.2. Pore Formation on the SLB Membrane after Injection of Cytosol Extract
3.3. Disturbance of Pore Formation in the SLB Membrane by Phosphatidylinositides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Upadhyaya, A.; Chabot, J.R.; Andreeva, A.; Samadani, A.; Oudenaarden, A. Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 4521–4526. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Kuribayashi, K.; Takeuchi, S. Artificial flagellates: Analysis of advancing motions of biflagellate micro-objects. Appl. Phys. Lett. 2010, 96, 083701. [Google Scholar] [CrossRef]
- Nishigami, Y.; Ichikawa, M.; Kazama, T.; Kobayashi, R.; Shimmen, T.; Yoshikawa, K.; Sonobe, S. Reconstruction of active regular motion in amoeba extract: Dynamic cooperation between sol and gel states. PLoS ONE 2010, 8, e70317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Toyota, T. Autonomous buckling of micrometer-sized lipid-protein membrane patches constructed by Dictyostelium discoideum. J. Biol. Eng. 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Mennicke, U.; Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 2002, 18, 8172–8177. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.; Deleu, M.; Brasseur, R.; Dufrêne, Y.F. Atomic force microscopy of supported lipid bilayers. Nat. Protoc. 2008, 3, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Facci, P. Unraveling lipid/protein interaction in model lipid bilayers by atomic force microscopy. J. Mol. Recognit. 2011, 24, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Seeger, H.M.; Cerbo, A.D.; Caramaschi, T.; Facci, P. Mechanical properties of mushroom and brush poly (ethylene glycol)-phospholipid membranes. Soft Matter 2011, 7, 7054–7064. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N. Spectrum of Membrane Morphological Responses to Antibacterial Fatty Acids and Related Surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Thid, D.; Benkoski, J.J.; Svedhem, S.; Kasemo, B.; Gold, J. DHA-Induced Changes of Supported Lipid Membrane Morphology. Langmuir 2007, 23, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Staykova, M.; Arroyo, M.; Rahimi, M.; Stone, H.A. Confined Bilayers Passively Regulate Shape and Stress. Phys. Rev. Lett. 2013, 110, 028101. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, G.; Arribas-Layton, N.; Hillebrandt, H.; Sackmann, E.; Wagner, P. Electrical properties of supported lipid bilayer membranes. J. Phys. Chem. B 2002, 106, 4245–4254. [Google Scholar] [CrossRef]
- Loose, M.; Fischer-Friedrich, E.; Ries, J.; Kruse, K.; Schwille, P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science 2008, 320, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Chalmeau, J.; Monina, N.; Shin, J.; Vieu, C.; Noireaux, V. α-Hemolysin pore formation into a supported phospholipid bilayer using cell-free expression. Biochim. Biophy. Acta 2011, 1808, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gallop, J.L.; Rambani, K.; Kirschner, M.W. Self-assembly of filopodia-like structures on supported lipid bilayers. Science 2010, 329, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, D.; Ishihara, S.; Oonuki, T.; Honda-Kitahara, M.; Kaneko, K.; Sawai, S. Phase geometries of two-dimensional excitable waves govern self-organized morphodynamics of amoeboid cells. Proc. Natl. Acad. Sci. USA 2013, 110, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Ashworth, J.M. Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 1970, 119, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Watarai, H.; Inagaki, Y.; Kubota, N.; Fuju, K.; Nagafune, J.; Yamaguchi, Y.; Kadoya, T. Proteomic approach to the identification of cell membrane proteins. Electrophoresis 2000, 21, 460–464. [Google Scholar] [CrossRef]
- Gregor, T.; Fujimoto, K.; Masaki, N.; Sawai, S. The onset of collective behavior in social amoebae. Science 2010, 328, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, J.; Cremer, P.S. Sub-100 nm patterning of supported bilayers by nanoshaving lithography. J. Am. Chem. Soc. 2008, 130, 2718–2719. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.; Islam, M.T.; Orr, B.G., Jr.; Baker, J.R.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Devreotes, P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 2002, 109, 599–610. [Google Scholar] [CrossRef]
- Hatta, I.; Murata, M. Biological Membrane Dynamics Series: New biophysics; Kyoritsushuppan: Tokyo, Japan, 2000. [Google Scholar]
- Zou, L.N. Myelin figures: The buckling and flow of wet soap. Phys. Rev. E 2009, 79, 061502. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, J. Phagocytosis and macropinocytosis in Dictyostelium: Phosphoinositide-based processes, biochemically distinct. Traffic 2001, 2, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; D’Arrigot, A.; Stenmark, H. Phosphoinositides in membrane traffic. Curr. Opin. Cell Biol. 1999, 11, 460–465. [Google Scholar] [CrossRef]
- Singh, S.S.; Chauhan, A.; Murakami, N.; Chauhan, V.P.S. Profilin and gelsolin stimulate phosphatidylinositol 3-kinase activity. Biochemistry 1996, 35, 16544–16549. [Google Scholar] [CrossRef] [PubMed]
- Chubb, J.R.; Wilkins, A.; Thomas, G.M.; Insall, R.H. The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J. Cell Sci. 2000, 113, 709–719. [Google Scholar] [PubMed]
- Dormann, D.; Weijer, G.; Dowler, S.; Weijer, C.J. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 2004, 117, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.M.; Lemieux, M.G.; Knecht, D.A.; Insall, R.H. PIP3-dependent macropinocytosis is incompatible with chemotaxis. J. Cell Biol. 2014, 204, 497–505. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Toyota, T. Micrometer-Scale Membrane Transition of Supported Lipid Bilayer Membrane Reconstituted with Cytosol of Dictyostelium discoideum. Life 2017, 7, 11. https://doi.org/10.3390/life7010011
Takahashi K, Toyota T. Micrometer-Scale Membrane Transition of Supported Lipid Bilayer Membrane Reconstituted with Cytosol of Dictyostelium discoideum. Life. 2017; 7(1):11. https://doi.org/10.3390/life7010011
Chicago/Turabian StyleTakahashi, Kei, and Taro Toyota. 2017. "Micrometer-Scale Membrane Transition of Supported Lipid Bilayer Membrane Reconstituted with Cytosol of Dictyostelium discoideum" Life 7, no. 1: 11. https://doi.org/10.3390/life7010011




