Selection of Prebiotic Molecules in Amphiphilic Environments
Abstract
:1. Introduction
- (a)
- It is constantly supplied with a huge amount and a huge variety of hydrothermally formed compounds from geological sources.
- (b)
- It is well protected against potentially destructive influences such as variable weather conditions or UV radiation.
- (c)
- It provides constant conditions over long periods of time (such as thousands of years), hereby allowing for undisturbed molecular evolution.
- (d)
- It generates structures which are highly selective for amphiphilic compounds such as lipids or peptides composed of hydrophilic and hydrophobic amino acids in a suitable sequence.
- (e)
- The system has access to the surface allowing the products of the molecular and structural evolution to spread out into new environments, especially on and near the terrestrial surface.
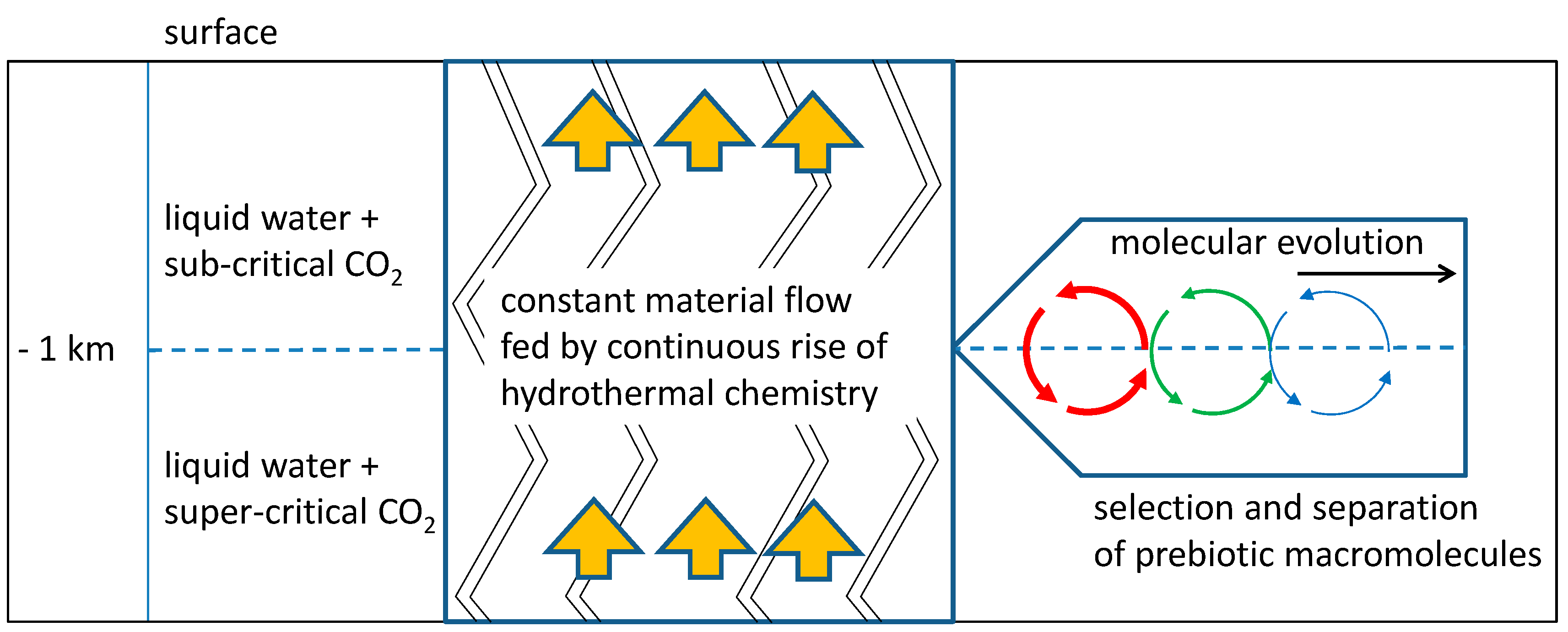
2. Tectonic Fault Zones—A Description of the Environment
3. Vesicle Formation
4. Accumulation and Selection of Molecules in Vesicle Membranes
- (1)
- Hydrophilic amino acids and primarily hydrophilic oligopeptides (left in Figure 3) will primarily remain dissolved in the aqueous phase. Here, they are part of a series of connected condensation–hydrolysis equilibria where oligomers with increasing chain length occur in decreasing concentration. Regarding the long-term contribution of this fraction of molecules, it can be considered as a material reservoir for amphiphilic amino acids. Regarding the formation of longer chains, this fraction suffers from the water problem [9].
- (2)
- Hydrophobic amino acids and primarily hydrophobic oligopeptides (right in Figure 3) will have a higher tendency to dissolve in the scCO2 phase. Even though its solubility in scCO2 may be very small in terms of numbers, the huge amount of carbon dioxide passing by at the water interface can carry a substantial amount over time. With the high mobility of the supercritical medium, these fractions are likely to be separated and to end up at positions where carbon dioxide is undergoing transition towards the gaseous state. The removal of this fraction is important as it prevents the formation of an insoluble hydrophobic residue which otherwise could block further reactions. Without a given selectivity, this product mixture is likely to gain in complexity and to lead to a dead end which has been referred to as the asphalt problem by Steven Benner [9].
- (3)
- Peptides formed from a combination of hydrophilic and hydrophobic amino acids may develop a tendency to integrate into the bilayer membranes of existing vesicles (center in Figure 3). This requires a stretch of hydrophobic amino acids which is long enough to fit into the aliphatic chain region of the bilayer together with at least one hydrophilic part which reaches into the polar head group region. It would be incorporated in the bilayer membrane by the hydrophobic effect: any dislocation of the peptide towards the inside or the outside of the vesicle would result in a steep increase of the free energy of the system. In this state, the peptide is partially protected against hydrolysis as the access of water molecules to the peptide bonds is hampered.
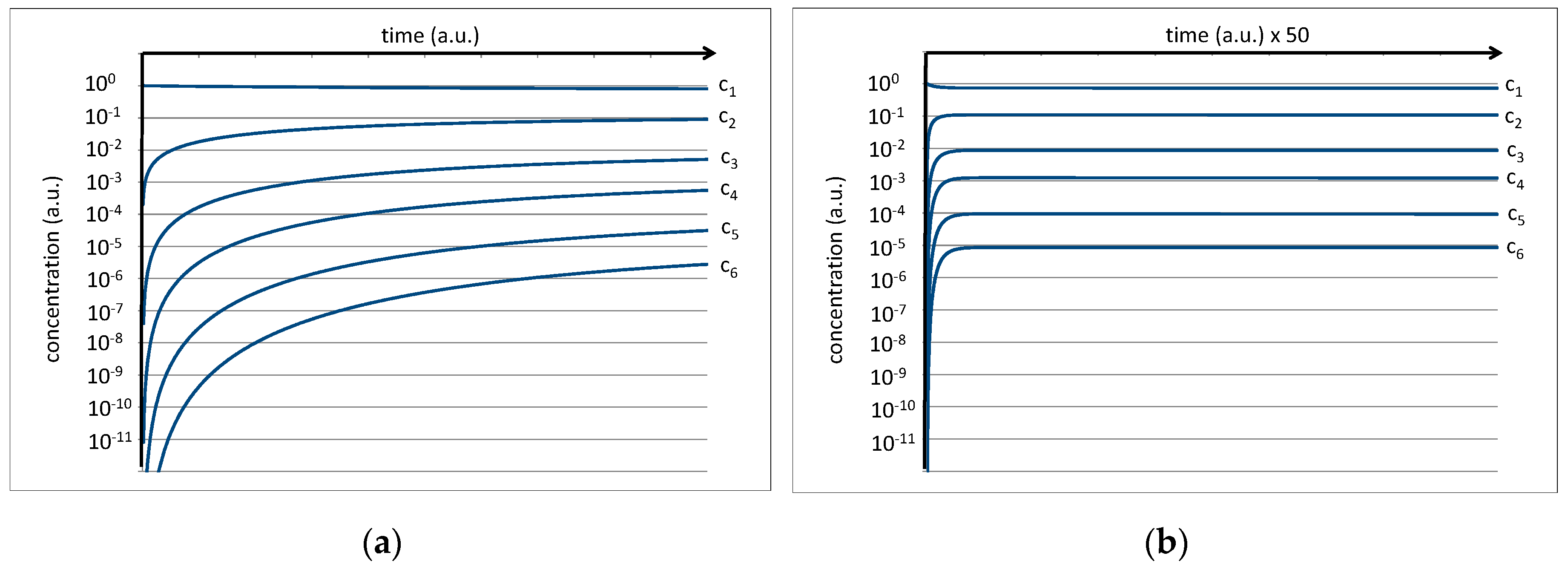
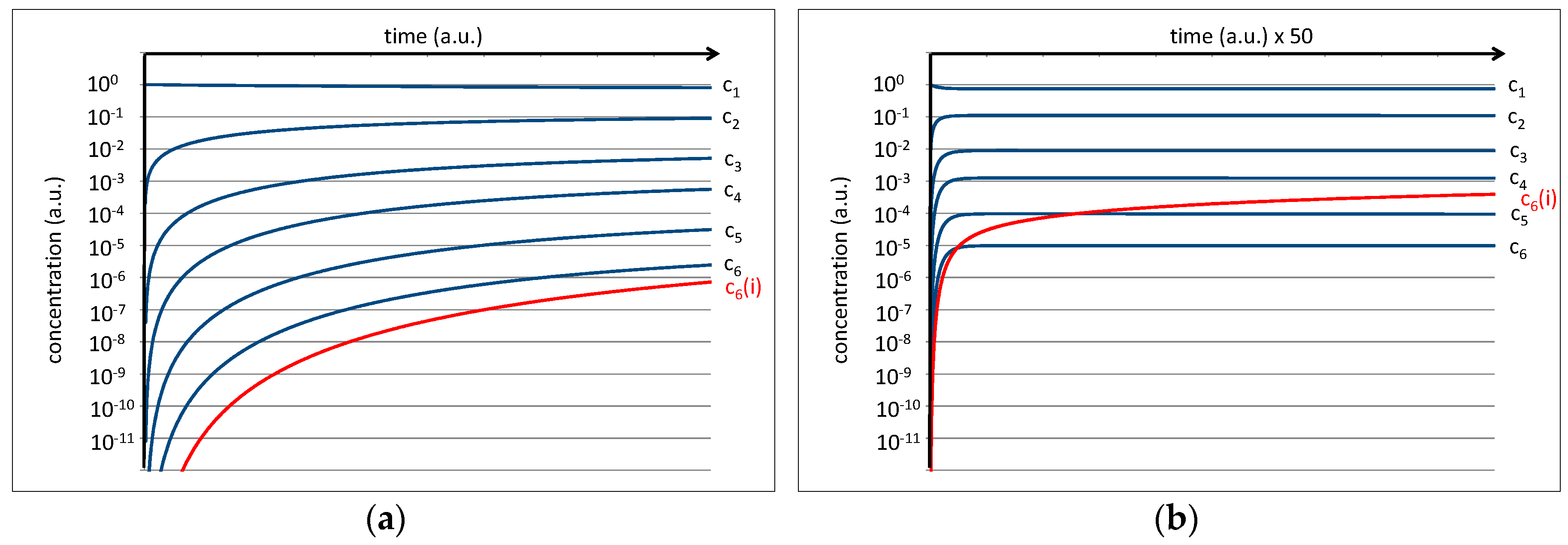
5. Numeric Simulation of Peptide Formation and the Accumulation Process
6. Possible Mechanisms of Vesicle-Induced Molecular Evolution
- (1)
- Integration. In analogy to biological counterparts, this effect could also be named a “parasitic” behavior as the advantage is limited to the peptide itself. Amphiphilic peptides with an amphiphilicity profile fitting into the bilayer cross section integrate into the membrane. Thereby, they gain an individual selective advantage as they are partially protected against hydrolysis and as they become less easily eluted from the vesicle zone. For this selection criterion, the effect on the stability of the vesicle is negligible.
- (2)
- Stabilization. In this case, amphiphilic peptides are being protected by vesicles but in turn also stabilize the vesicle structure. Again, in analogy, one could categorize this mutual advantage as “symbiotic”. Very efficient candidates for species causing vesicle stabilization could be peptides consisting of a long hydrophobic part with two hydrophilic ends. In this case, the peptide could fully integrate into the bilayer and connect the two hydrophilic layers of the membrane in analogy to a bola-amphiphile [25]. Such bola-amphiphiles lead to a significantly higher thermal stability of the surrounding bilayer membrane and occur in thermophilic organisms. With increased stability, the vesicles gain an advantage from hosting the peptide molecules while the latter in turn enjoy an increased degree of protection. Altogether, this symbiotic effect would act more efficiently than the parasitic integration effect alone.
- (3)
- Function. This part of the possible evolution process is the most speculative, but also the most exciting one. The potential is obvious: peptides with new functions beyond a simple mechanical stabilization may develop. A possible example may be an ion channel based on a trans-membrane polypeptide [26]. In the vesicle cycle, the vesicles are generated with a natural ionic concentration gradient across the membrane which destabilizes their structure by the resulting osmotic stress. An ion channel could cause a rapid relaxation of this gradient, leading to a longer vesicle lifetime and resulting in a corresponding selection advantage for the channel-forming peptide. Other selected functions could include the ability to bind to a specific surface, to adopt a specific charge, to selectively bind to other vesicles, or even to make the membrane permeable for specific molecules. More complex versions of the peptides could even make use of the concentration gradient as a source of free energy. In any case, such functional peptides could be seen as initial primitive predecessors of functional membrane proteins.
Author Contributions
Conflicts of Interest
References
- Dyson, F.J. Origins of Life; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Trainer, M.G. Atmospheric prebiotic chemistry and organic hazes. Curr. Org. Chem. 2013, 17, 1710–1723. [Google Scholar] [CrossRef] [PubMed]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic chemistry and atmospheric warming of early Earth by an active young sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Prebiotic organic synthesis under hydrothermal conditions: An overview. Adv. Space Res. 2004, 33, 88–94. [Google Scholar] [CrossRef]
- Matsuno, K. Hydrothermal reaction. In Encyclopedia of Astrobiology; Springer: Berlin, Germany, 2011; pp. 788–790. [Google Scholar]
- Lawless, J.G. Amino acids in the Murchison meteorite. Geochim. Cosmochim. Acta 1973, 37, 2207–2212. [Google Scholar] [CrossRef]
- Guillemin, J.C.; Bouyahyi, M.; Riague, E.H. Prebiotic, planetary and interstellar chemistry starting from compounds detected in the interstellar medium. Adv. Space Res. 2004, 33, 81–87. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.J.; Carrigan, M.A. Asphalt, water, and the prebiotic synthesis of ribose, ribonucleotides, and RNA. Acc. Chem. Res. 2011, 45, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of life in deep-reaching tectonic faults. Orig. Life Evol. Biosph. 2012, 42, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Schreiber, U.; Dávila, M.J. Periodic vesicle formation in tectonic fault zones: An ideal scenario for molecular evolution. Orig. Life Evol. Biosph. 2015, 45, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 1994, 58, 2099–2106. [Google Scholar] [CrossRef]
- Andersson, E.M.; Holm, N.G. The stability of some selected amino acids under attempted redox constrained hydrothermal conditions. Life Evol. Biosph. 2000, 30, 9–23. [Google Scholar] [CrossRef]
- Kitadai, N. Energetics of amino acid synthesis in alkaline hydrothermal environments. Orig. Life Evol. Biosph. 2015, 45, 377–409. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.T.; Mayo, A.L.; Gilfillan, S.; Dutson, S.J.; Harris, R.A.; Shipton, Z.K.; Tingey, T.G. Enhanced fracture permeability and accompanying fluid flow in the footwall of a normal fault: The Hurricane fault at Pah Tempe hot springs, Washington County, Utah. Geol. Soc. Am. Bull. 2009, 121, 236–246. [Google Scholar] [CrossRef]
- Rushdie, A.I.; Simoneit, B.R. Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig. Life Evol. Biosph. 2006, 36, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Kümpel, H.J.; Grecksch, G.; Lehmann, K.; Rebscher, D.; Schulze, K.C. Studies of in situ pore pressure fluctuations. Oil Gas Sci. Technol. 1999, 54, 679–688. [Google Scholar] [CrossRef]
- Tuck, A. The role of atmospheric aerosols in the origin of life. Surv. Geophys. 2002, 23, 379–409. [Google Scholar] [CrossRef]
- Dobson, C.M.; Allison, B.G.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Nat. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Imai, E.I.; Honda, H.; Hatori, K.; Matsuno, K. Prebiotic oligomerization on or inside lipid vesicles in hydrothermal environments. Orig. Life Evol. Biosph. 2002, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, R.; Imai, E.I.; Honda, H.; Hatori, K.; Matsuno, K. Evolving lipid vesicles in prebiotic hydrothermal environments. Orig. Life Evol. Biosph. 2005, 35, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L.; Walde, P.; Oberholzer, T. Lipid vesicles as possible intermediates in the origin of life. Curr. Opin. Colloid Interface Sci. 1999, 4, 33–39. [Google Scholar] [CrossRef]
- Williams, L.L.; Rubin, J.B.; Edwards, H.W. Calculation of Hansen solubility parameter values for a range of pressure and temperature conditions, including the supercritical fluid region. Ind. Eng. Chem. Res. 2004, 43, 4967–4972. [Google Scholar] [CrossRef]
- Lemke, K.H.; Rosenbauer, R.J.; Bird, D.K. Peptide synthesis in early Earth hydrothermal systems. Astrobiology 2009, 9, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrhop, J.H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2937. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S. Peptide ion channels: Design and creation of function. Biopolymers 1998, 47, 75–81. [Google Scholar] [CrossRef]
- Deamer, D.W. The first living systems: A bioenergetics perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [PubMed]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Walde, P. From self-assembled vesicles to protocells. Cold Spring Harb. Perspect. Biol. 2010, 2, a002170. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Blosser, M.C.; Stottrup, B.L.; Tavakley, R.; Deamer, D.W.; Keller, S.L. Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc. Nat. Acad. Sci. USA 2013, 110, 13272–13276. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Blosser, M.C. A self-assembled aggregate composed of a fatty acid membrane and the building blocks of biological polymers provides a first step in the emergence of protocells. Life 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, C.; Schreiber, U.; Dávila, M.J. Selection of Prebiotic Molecules in Amphiphilic Environments. Life 2017, 7, 3. https://doi.org/10.3390/life7010003
Mayer C, Schreiber U, Dávila MJ. Selection of Prebiotic Molecules in Amphiphilic Environments. Life. 2017; 7(1):3. https://doi.org/10.3390/life7010003
Chicago/Turabian StyleMayer, Christian, Ulrich Schreiber, and María J. Dávila. 2017. "Selection of Prebiotic Molecules in Amphiphilic Environments" Life 7, no. 1: 3. https://doi.org/10.3390/life7010003




