1. Introduction
Circular code is a mathematical structure of genes and genomes. This concept initially found for genes is extended for genomes (non-coding regions of eukaryotes) according to recent results. A circular code is a set of words such that any motif from , called motif, allows it to retrieve, maintain, and synchronize the original (construction) frame.
The circular code
identified in the genes of bacteria, eukaryotes, plasmids, and viruses [
1,
2] contains the 20 following trinucleotides
which allows it to both retrieve the reading frame with a window of 13 nucleotides (Figure 3 in [
3]) and to code the 12 following amino acids
The current genetic code is not circular. Thus, it cannot retrieve the reading frame. The loss during evolution of this circular code property on the 4-letter alphabet required a complex translation mechanism using 20 amino acids and proteins in current genomes.
motifs from Equation (1) are identified in (i) genes “universally” [
1,
4]; (ii) tRNAs of prokaryotes and eukaryotes [
3,
5]; (iii) rRNAs of prokaryotes (16S) and eukaryotes (18S), in particular in the ribosome decoding center where the universally conserved nucleotides G530, A1492, and A1493 are included in the
motifs [
3,
6,
7]; and (iv) genomes (non-coding regions of eukaryotes) [
4,
8].
The
motifs of maximal cardinality 20 (composition) in genes with the properties of the circular code,
and complementary allow the two reading frames and the four shifted frames to be retrieved by pairing between DNAs-DNAs, DNAs-mRNAs, mRNAs-rRNAs, mRNAs-tRNAs, and rRNAs-tRNAs, as shown with a 3D visualization of the
motifs in the ribosome [
3,
6,
7].
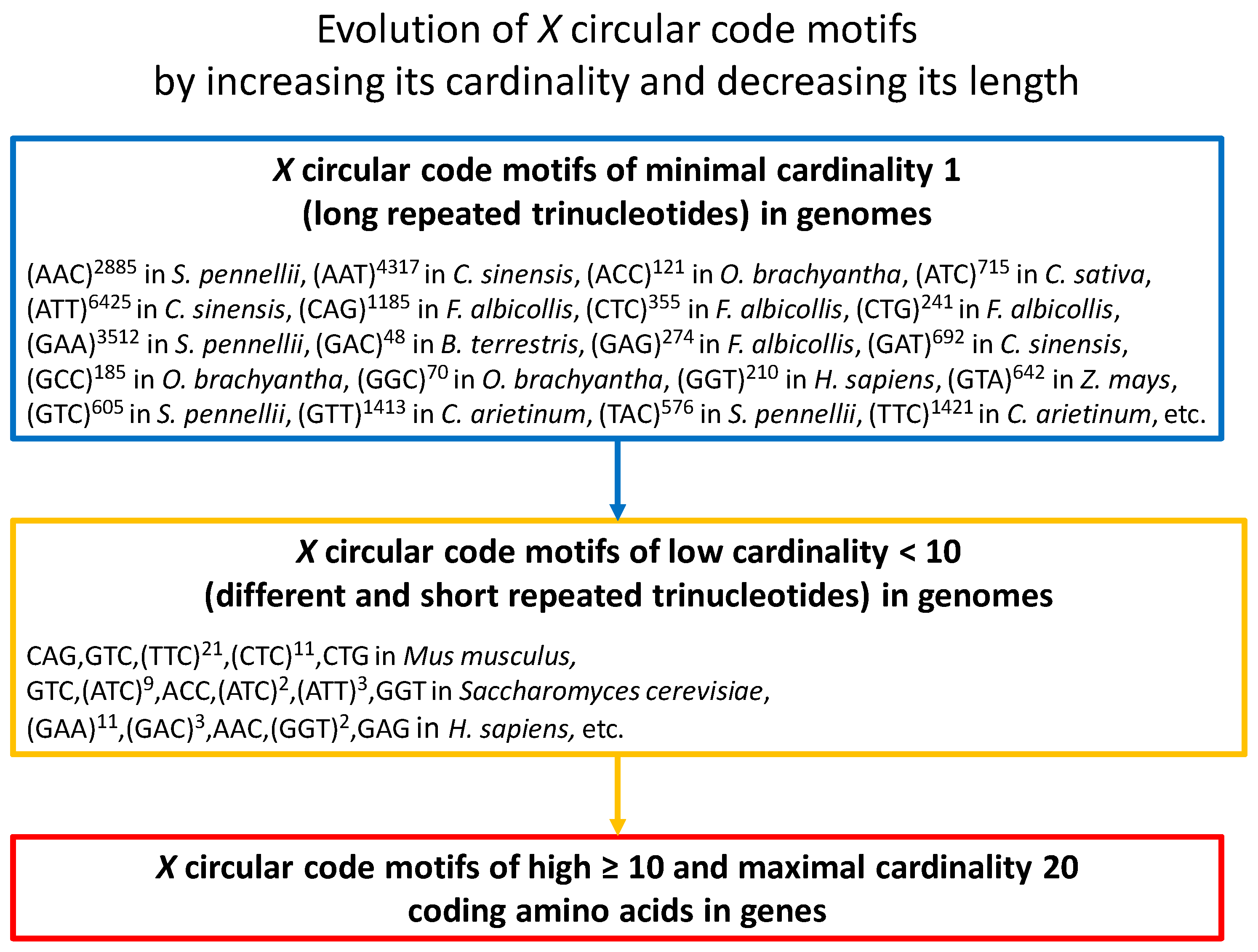
The
motifs in genomes have a different structure compared to the
motifs in genes [
8]. Indeed, their cardinality is not maximal (less than 10 for an order of magnitude), their size is longer, and their structure contains repeated trinucleotides. Furthermore, the
motifs of minimal cardinality 1 generated with the 20 repeated trinucleotides
where
(Equation (1)) are very common in the genomes of eukaryotes (e.g., [
8,
9,
10]). Their length
can be very large (e.g.,
, see
Figure 1). The repeated trinucleotides are very unstable with mutation rates up to 100,000 times higher than the genomic average mutation rate. Mutation in repeats increases its evolutionary stability.
A model of evolution of the
motifs in genes and genomes can be proposed according to the previous works and the recent results [
8]. It proposes that the
motifs of maximal cardinality 20 in genes have evolved from the
motifs of minimal cardinality 1 (repeated trinucleotides) in genomes (
Figure 1). An
motif of minimal cardinality 1 which is unstable, mutates into an
motif of low cardinality
containing thus different repeated trinucleotides of short lengths. This evolutionary process continues by increasing the cardinality and decreasing the length of the
motifs up to generate the
motifs of high
and maximal cardinality 20 coding the 12 amino acids (Equation (2)) in genes. The
motifs of high cardinality have acquired the protein coding function in addition to the reading frame retrieval. This model suggests that the property of reading frame retrieval has preceded the protein coding function.
Since 1996, all the statistical analyses studying the preferential occurrence of trinucleotides among the three frames were done at the gene population level (kingdoms, taxonomic groups, genomes). We extend here the method from [
1] at the gene level. This new approach is important as all the genes, i.e., of large and small lengths, are now considered with the same weight in the statistical definition for searching the circular code
. As a consequence, the concept of circular code, in particular the reading frame retrieval, is directly associated to each gene. Thus, at the gene level, the circular code
is searched here in the genes of bacteria, archaea, eukaryotes, plasmids, viruses, and eukaryotic organelles, i.e., mitochondria and chloroplasts. Finally, genes of double-stranded DNA and RNA viruses, and single-stranded DNA and RNA viruses are also analysed with this approach in order to assign a genetic information unit (DNA or RNA, double-stranded or single-stranded) to the circular code
.
2. Method
2.1. Definitions
We recall a few definitions without detailed explanations (i.e., without figures and examples) for understanding the main properties of the trinucleotide circular code
identified in genes [
1,
2].
Notation 1. Let us denote the nucleotide 4-letter alphabet where A stands for Adenine, C stands for Cytosine, G stands for Guanine, and T stands for Thymine. The trinucleotide set over is denoted by . The set of non-empty words (words, respectively) over is denoted by (, respectively).
Notation 2. Genes have three frames . By convention here, the reading frame is set up by a start trinucleotide , and the frames and are the reading frame shifted by one and two nucleotides in the direction (to the right), respectively.
Two biological maps are involved in gene coding.
Definition 1. According to the complementary property of the DNA double helix, the nucleotide complementarity map is defined by , , , and . According to the complementary and antiparallel properties of the DNA double helix, the trinucleotide complementarity map is defined by for all . By extension to a trinucleotide set , the set complementarity map , being the set of all subsets of , is defined by , e.g., .
Definition 2. The trinucleotide circular permutation map is defined by for all . denotes the 2nd iterate of . By extension to a trinucleotide set , the set circular permutation map is defined by , e.g., and .
Definition 3. A set is a code if, for each , , the condition implies and for .
Definition 4. Any non-empty subset of the code is a code and called trinucleotide code .
Definition 5. A trinucleotide code is self-complementary if, for each , , i.e., .
Definition 6. A trinucleotide code is circular if, for each , , , , the conditions and imply , (empty word), and for .
The proofs to decide whether a code is circular or not are based on the flower automaton [
2], the necklace 5
LDCN (Letter Diletter Continued Necklace) [
11], the necklace
LDCCN (Letter Diletter Continued Closed Necklace) with
[
12], and the graph theory [
13].
Definition 7. A trinucleotide circular code is self-complementary if , , and are trinucleotide circular codes such that (self-complementary), , and ( and are complementary).
The trinucleotide set
(Equation (1)) coding the reading frame (
) in genes is a maximal (20 trinucleotides)
self-complementary trinucleotide circular code [
2] where the circular code
coding the frame
contains the 20 following trinucleotides
and the circular code
coding the frame
contains the 20 following trinucleotides
The trinucleotide circular codes
and
are related by the permutation map, i.e.,
and
, and by the complementary map, i.e.,
and
[
14].
Several classes of methods were developed for identifying the circular code
in genes over the last 20 years: frequency methods [
2,
15,
16], correlation function [
17], covering capability function [
18], and occurrence probability of a complementary/permutation (CP) trinucleotide set at the gene population level [
1].
The class of the 216
self-complementary trinucleotide circular codes (Definition 7; [
2]; list given in Tables 4a, 5a, and 6a in [
19]; [
20]) is included in a larger class of codes
by relaxing the circularity property which was defined in [
1]:
Definition 8. A trinucleotide code is self-complementary if (self-complementary), , and ( and are complementary) where and .
The statistical approach developed analyses the self-complementary codes (Definition 8) for searching the particular circular code .
2.2. Gene Kingdoms
Gene kingdoms
of bacteria
, archaea
, plasmids
, eukaryotes
, chromosomes of eukaryotes
, mitochondria
, chloroplasts
, viruses
, and its five taxonomic double-stranded DNA viruses
, double-stranded RNA viruses
, single-stranded DNA viruses
, single-stranded RNA viruses
, and retro-transcribing viruses
are obtained from the GenBank database (
http://www.ncbi.nlm.nih.gov/genome/browse/, May 2016) (
Table 1). Computer tests exclude genes when (i) their nucleotides do not belong to the alphabet
; (ii) they do not begin with a start trinucleotide
; (iii) they do not end with a stop trinucleotide
; and (iv) their lengths are not modulo 3. In order to have an order of magnitude of data acquisition (details in
Table 1), the kingdom of bacteria
contains 15,735,053 genes and 5,222,267,667 trinucleotides (7,851,762 genes and 2,481,566,882 trinucleotides in [
1]), i.e., a trinucleotide increase of about 110%, and the kingdom of eukaryotes
contains 4,356,391 genes and 2,406,844,838 trinucleotides (1,662,579 genes and 824,825,761 trinucleotides in [
1]), i.e., a trinucleotide increase of about 192%. The gene kingdoms
,
,
,
, and
have gene and trinucleotide data that are significantly lower (less than 1 million trinucleotides) than the other gene kingdoms (
Table 1).
2.3. Preferential Frame of a Trinucleotide in a Gene
The method developed in [
1] for identifying the circular code
in genes determined the preferential frame of trinucleotides at the gene population level (kingdoms, taxonomic groups, genomes), i.e., after summing the trinucleotide frequencies of all genes in a kingdom. We extend this method at the gene level, i.e., the preferential frame of trinucleotides among the three frames is determined for each gene. There is no sum of trinucleotide frequencies of all genes in a kingdom. Thus, all the genes, i.e., of large and small lengths, have the same weight in respect to the preferential frame.
Consider a gene kingdom
listed in
Table 1. Let
be the occurrence frequency of a trinucleotide
in a frame
of a gene
belonging to a kingdom
. Thus, there are
trinucleotide occurrence frequencies
in the three frames
of a gene
. Then, the preferential frame
of a trinucleotide
in a gene
is the frame of maximal occurrence frequency
among the three frames
of
The three frequencies of a given trinucleotide are computed in the three frames 0, 1, and 2 of a gene. Then, the preferential frame of the trinucleotide in this gene is the frame associated to its highest trinucleotide frequency.
Remark 1. In [
1]
, the three occurrence frequencies of a trinucleotide in the three frames computed in a gene kingdom , always have different values, thus a unique preferential frame can be assigned to the trinucleotide. At the gene level, particularly for genes of small lengths, a trinucleotide may have an identical occurrence frequency in two or three frames . In this case, two or three preferential frames are assigned to the trinucleotide . If a trinucleotide is absent in a gene , mainly for genes of very small lengths, then no preferential frame is attributed to . The indicator function
is 1 if the preferential frame
of a trinucleotide
is equal to the frame
of a gene
, and 0 otherwise
where
is defined in Equation (5).
2.4. Number of Preferential Frames of a Trinucleotide in a Gene Kingdom
The number
of preferential frames of a trinucleotide
for each frame
in a gene kingdom
is simply obtained by summing for all genes in
where
is defined in Equation (6).
2.5. Occurrence Probability of a Complementary/Permutation Trinucleotide Set in a Gene Kingdom
In order to study the self-complementary codes (Definition 8) including the class of circular codes, and in particular the circular code , Equation (7) for a trinucleotide is expanded to a set of six trinucleotides involving the complementarity map and the permutation map simultaneously, precisely with in frame 0, in frame 1, in frame 2, and . is called a complementary and permutation (CP) trinucleotide set and is completely defined by the trinucleotide .
Remark 2. and (proof obvious).
When the trinucleotide
is given then the trinucleotide
is also known. Thus, there are
CP trinucleotide sets noted
where
with
in frame 0,
in frame 1, and
in frame 2. A maximal (20 trinucleotides)
self-complementary code
is identified with the first 10 values of the numbers
(defined below). Precisely, the code
has 20 trinucleotides
in frame 0, 20 trinucleotides
in frame 1, and 20 trinucleotides
in frame 2 with
(self-complementary),
, and
(
and
are complementary). There are
self-complementary trinucleotide codes, and among them only 216 are circular [
2,
20].
Notation 3. A CP trinucleotide set belongs to the self-complementary trinucleotide circular code , i.e., , if , i.e., if the trinucleotide and its complementary trinucleotide belong to . Ten CP trinucleotide sets among 30 belong to the circular code , i.e., such that 10 sets with and .
Notation 4. In order to facilitate the reading of Table 2, the 30 CP trinucleotide sets are presented in the following way (i) the first 10 sets belong to the circular code (with , and ) and are in lexicographical order with respect to the trinucleotide (in bold), and (ii) the 20 remaining sets are in lexicographical order with respect to the trinucleotide (in italics). The occurrence number
of a CP trinucleotide set
in a gene kingdom
is equal to
where
is defined in Equation (7).
In order to normalize the numbers
which depend on the numbers of genes in a kingdom
, we simply define the occurrence probability
of a CP trinucleotide set
in a gene kingdom
as follows
where
is defined in Equation (8).
The parameter gives the rank of the values among the 30 CP trinucleotide sets , the 1st rank being associated to the highest value of and the 30th rank, to the lowest value of .
2.6. A Statistical Test to Evaluate the Significance of the Obtained Ranks
In order to evaluate the statistical significance of the ranks of the probabilities (Equation (9)) of the 30 CP trinucleotide sets in a given kingdom , we derive confidence intervals for . If the confidence interval for two probabilities do not overlap, then their associated ranks are assumed to be valid (in the population). The confidence interval for two probabilities is evaluated by using the classical 2-sample z-test which is briefly recalled here.
Let
and
be the populations associated to the CP trinucleotide sets
and
of probabilities
and
, respectively. The probabilities
and
of
and
are observed in a given gene kingdom
(sample) of size
(defined from Equation (8)). The tests carried out in
Section 3 are applied on large samples (the size of the smallest sample analysed being
with the archaea
). Thus, the assumptions of normality for the variables and of the homogeneity for the variances in the two populations are not needed. The equality
is tested against the alternative
if they are not equal. Under
and with large samples (
),
(always verified in the tests carried out in
Section 3), and
and
are independent events (realistic hypothesis with kingdoms
of large sizes), then
The
z-value and the
p-value are given for each statistical test carried out in
Section 3.
2.7. Explained Example of the Statistical Approach Developed
As an example, we explain the definition of the occurrence probability
(Equation (9)) which takes the value of 6.1% (see
Table 2) with the CP trinucleotide set
with
in frame 0,
in frame 1 and
in frame 2 in the gene kingdom of bacteria
(
Table 1).
The occurrence frequencies of the 64 trinucleotides are computed in the three frames of each gene belonging to . Then, the preferential frame of each trinucleotide for each gene in is determined according to Equation (5). For example, with the trinucleotide in a gene of , if the frequency of AAC in frame (reading frame) is greater than the two frequencies and of AAC in frames and , i.e., , then the preferential frame of AAC in is 0, i.e., .
The indicator function of each trinucleotide for each gene in is obtained from Equation (6). With the previous example of AAC in the gene of , the indicator function is equal to for the frame and for the frames and .
The number of preferential frames of each trinucleotide for each frame in is computed according to Equation (7). With the previous example of AAC in , the following numbers are obtained: for the frame , for the frame , and for the frame . Thus, the preferential frame of AAC in is 0.
The occurrence number of the 30 CP trinucleotide sets in is determined according to Equation (8). With in , the following numbers are obtained: for the frame , and for the frame , and and for the frame . Then, the occurrence number of in is equal to .
Finally, the occurrence probability of the 30 CP trinucleotide sets in is deduced from Equation (9). With in , the occurrence probability of in is equal to .
4. Conclusions
The “universal” occurrence in genes of a same set
of 20 trinucleotides, which has in addition the mathematical property to be a circular code, must be confirmed by several statistical approaches and various gene data analyses at different levels: kingdom, taxonomic group, genome, and gene. All the previous approaches have studied and identified the circular code
at the gene population level (kingdom, taxonomic group, and genome) [
1,
2,
15,
16,
17,
21]. The statistical approach at the gene level developed here, for the first time since 1996, analyses the preferential occurrence of trinucleotides among the three frames of each gene. This new methodology allows all genes, i.e., of large and small lengths, to be considered with the same weight. As a consequence, the concept of circular code, in particular the reading frame retrieval, is directly associated to each gene. Thus,
motifs from the circular code
at different locations in a gene may assist the ribosome to maintain and synchronize the reading frame. The number, the cardinality, and the length of
motifs in genes may be associated to the length, the function, and the ancestry of genes. This research work is currently under investigation.
At the gene level, the circular code is strengthened in the genes of bacteria, eukaryotes, plasmids, and viruses, and is now also identified in the genes of archaea. In addition to eukaryotic genomes, it is also found in the genes of eukaryotic chromosomes. The genes of mitochondria and chloroplasts contain a subset of the circular code . It should be stressed that some mitochondrial and chloroplast genes lack the stop codon and are excluded from this data acquisition. Such a statistical bias may prevent a proper detection of preferential frames for some trinucleotides in the genes of eukaryotic organelles. The circular code is searched in the large class of self-complementary trinucleotide codes which contains in particular the 216 maximal self-complementary circular codes. Thus, for a basic order of magnitude, the probability to retrieve the same circular code in four independent gene kingdoms (bacteria , plasmids , eukaryotes , double-stranded DNA viruses ) is equal to .
In the genes of the bacterial, eukaryotic, and plasmid kingdoms, 14 among the 47 studied gene taxonomic groups (about 30%) have variant
codes [
1], i.e., trinucleotide codes which differ from
. Seven variant
codes are identified. However, all have at least 16 trinucleotides of
. Two variant
codes
(according to the notation in [
1]) in cyanobacteria and plasmids of cyanobacteria, and
in birds, are self-complementary, without permuted trinucleotides, but are non-circular. Five variant
codes
in
Deinococcus, plasmids of chloroflexi and
Deinococcus, mammals, and kinetoplasts,
in elusimicrobia and apicomplexans,
in fishes,
in insects, and
in basidiomycetes and plasmids of spirochaetes, are
self-complementary circular. Thus, two variant
codes
and
are not circular and do not belong to the set of the 216 maximal
self-complementary circular codes [
2] having the strong mathematical structure of the dihedral group [
20]. The reason could be related to the gene data or to a biological property which remains to be identified. All these variant
codes in the genes are identified at the taxonomic group level. However, as the circular code
is now also identified at the gene level, variant
codes may also be associated with genes belonging to the same genome but with different protein coding functions. This interesting and open problem should be investigated in the future.
A probability measure of the reading frame retrieval (
) of each trinucleotide of
has been introduced in [
22] and [
23] (
Section 2.2 and 1st row of
Table 1). The
probability
of the circular code
, i.e., the average
probability of the 20 trinucleotides of
, is equal to
(Result 5 in [
22]; 1st row of Table 1 in [
23]). This
measure can be applied to the non-maximal
self-complementary circular codes, precisely to the excluded trinucleotides
of archaea (Equation (11)),
of mitochondria (Equation (15)),
of chloroplasts (Equation (16)),
of viruses (Equation (17)), and
of single-stranded DNA viruses (Equation (22)). The computation leads to
,
,
,
, and
. Archaeal genes miss two trinucleotides of
which have the lowest
values. In contrast, mitochondrial, chloroplast, and viral genes miss trinucleotides of
with high
values. Thus, the genes in reduced genomes are more flexible in translation, allowing overlap coding by frameshifting in agreement with [
24] (and the cited references). However, it should be stressed that this result may vary with the increase of gene data of eukaryotic organelles in the future. The circular code
(20 trinucleotides) with the functions of reading frame retrieval and maintenance in regular RNA transcription, may also have, through its bijective transformation codes, the same functions in nucleotide exchanging RNA transcription in mitochondrial genes [
23]. Indeed, as the mitochondrial gamma polymerase has bacterial origins (e.g., [
25]), mitochondrial polymerization and its associated bijective transformations might use the circular code
. However at the translational level, the ribosome might follow the non-maximal
self-complementary circular code
observed in mitochondrial genes (Equation (15)). A similar explanation could be applied to the chloroplast genes which have also bacterial origins (cyanobacteria).
By a study of viral genes, the circular code is found in DNA genomes, RNA genomes, double-stranded genomes, and single-stranded genomes. Thus, the reading frame retrieval property of could operate for translating DNA and RNA genes, in particular for the “primitive” RNA genes. The property of could be involved for translating the two shifted frames in DNA and RNA genes, in particular for optimizing the genomes of small sizes. The complementarity property of is naturally associated to the double-stranded DNA and RNA genomes. It could also be used to pair single-stranded DNA genomes between them and single-stranded RNA genomes between them. Thus, the and complementary properties of could be involved for translating the three frames (reading frame and its two shifted frames) in one strand and the three frames in the complementary strand of DNA and RNA genes.
In summary, this new statistical approach at the gene level which is applied to massive gene data identifies the maximal
self-complementary trinucleotide circular code
in the genes of bacteria, archaea, eukaryotes, plasmids, and viruses, which may be involved in translation coding [
3].