The Role of PET/CT Molecular Imaging in the Diagnosis of Recurrence and Surveillance of Patients Treated for Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Factors and Patterns of Recurrence of Non-Small Cell Lung Cancer
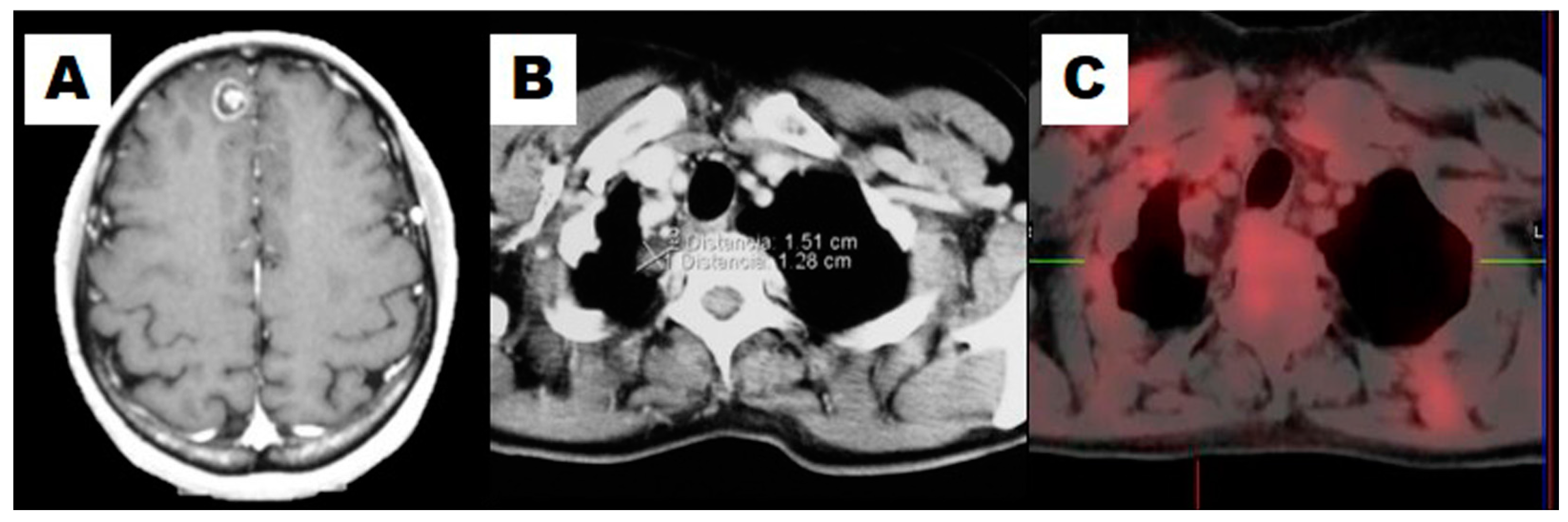
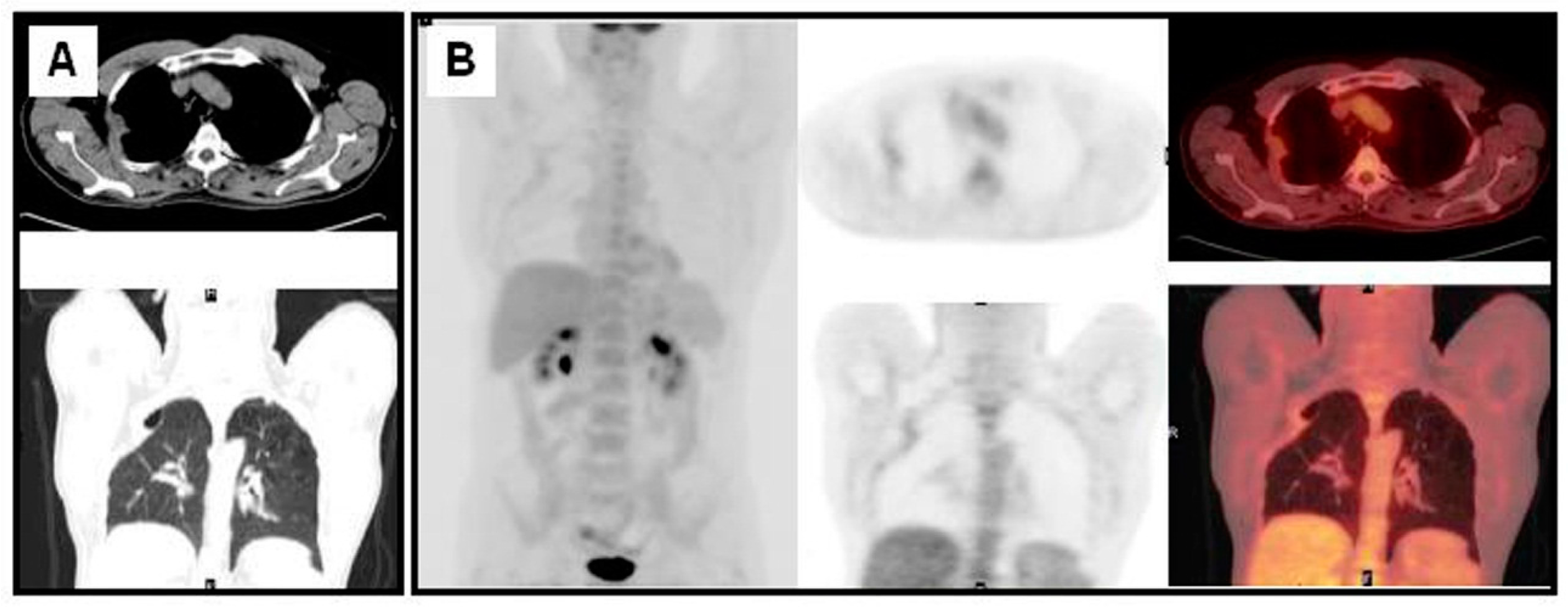
3. The Diagnosis of NSCLC Recurrence
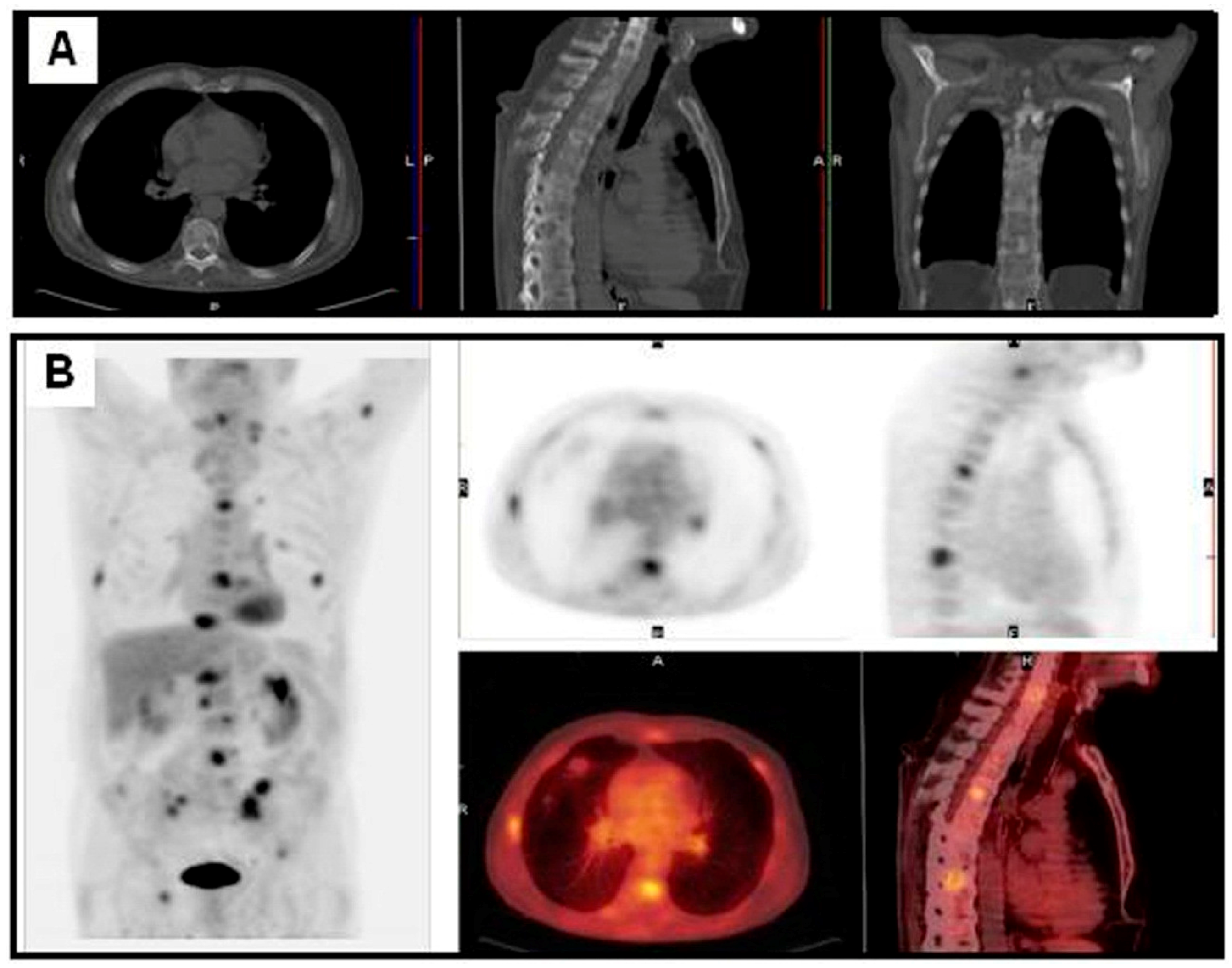
4. The Surveillance of NSCLC Recurrence
5. Future Perspectives
Conflicts of Interest
References
- Pinilla, I.; Gómez León, N. The usefulness of PET/CT in lung cancer. Radiologia 2009, 51, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Israel, O.; Kuten, A. Early detection of cancer recurrence: 18F-FDG PET/CT can make a difference in diagnosis and patient care. J. Nucl. Med. 2007, 48 (Suppl. S1), S28–S35. [Google Scholar]
- Kanzaki, R.; Higashiyama, M.; Fujiwara, A.; Tokunaga, T.; Maeda, J.; Okami, J.; Nishimura, K.; Kodama, K. Long-term results of surgical resection for pulmonary metastasis from renal cell carcinoma: A 25-year single-institution experience. Eur. J. Cardiothorac. Surg. 2011, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Varlotto, J.M.; Medford-Davis, L.N.; Recht, A.; Flickinger, J.C.; Schaefer, E.; DeCamp, M.M. Failure rates and patterns of recurrence in patients with resected N1 non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Carnio, S.; Novello, S.; Papotti, M.; Loiacono, M.; Scagliotti, G.V. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: Tumor based approaches including gene signatures. Transl. Lung Cancer Res. 2013, 2, 372–381. [Google Scholar] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [PubMed]
- Boyd, J.A.; Hubbs, J.; Kim, D.W.; Hollis, D.; Marks, L.B.; Kelsey, C.R. Timing of local and distant failure in resected lung cancer: Implications for reported rates of local failure. J. Thorac. Oncol. 2010, 5, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.R. A perspective on DNA microarrays in pathology research and practice. Am. J. Pathol. 2007, 171, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, M.; Sun, X.; Li, W.; Xing, L.; Yu, J. Prognostic Value of 18F-FDG PET/CT in Surgical Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0146195. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tsuboi, M.; Sakamaki, K.; Nishii, T.; Yamamoto, T.; Nagashima, T.; Ando, K.; Ishikawa, Y.; Woo, T.; Adachi, H.; et al. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2016, 49, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Prediction of recurrence after complete resection in patients with NSCLC. Anticancer Res. 2012, 32, 3953–3960. [Google Scholar] [PubMed]
- Henzler, T.; Goldstraw, P.; Wenz, F.; Pirker, R.; Weder, W.; Apfaltrer, P.; Meyer, M.; Buesing, K.; Crino, L.; Fennell, D.; et al. Perspectives of novel imaging techniques for staging, therapy response assessment, and monitoring of surveillance in lung cancer: Summary of the Dresden 2013 Post WCLC-IASLC State-of-the-Art Imaging Workshop. J. Thorac. Oncol. 2015, 10, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Lassen, U.; Mortensen, J.; Larsen, S.; Loft, A.; Bertelsen, A.; Ravn, J.; Clementsen, P.; Høgholm, A.; Larsen, K.; et al. Preoperative staging of lung cancer with combined PET-CT. N. Engl. J. Med. 2009, 361, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Mortensen, J.; Hansen, H.; Vilmann, P.; Larsen, S.S.; Loft, A.; Bertelsen, A.K.; Ravn, J.; Clementsen, P.; Høegholm, A.; et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: A randomised trial. Thorax 2011, 66, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Bonilla, J.F.; Quirce, R.; Martínez-Rodríguez, I.; Banzo, I.; Rubio-Vassallo, A.S.; Del Castillo-Matos, R.; Ortega-Nava, F.; Martínez-Amador, N.; Ibáñez-Bravo, S.; Carril, J.M. Diagnosis of recurrence and assessment of post-recurrence survival in patients with extracranial non-small cell lung cancer evaluated by 18F-FDG PET/CT. Lung Cancer 2013, 81, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Husmann, L.; Stolzmann, P. Staging, Restaging and Response Evaluation of Non-Small-Cell Lung Cancer. In Diseases of the Chest and Heart; Springer: Milano, Italy, 2015; pp. 183–188. [Google Scholar]
- Bollineni, V.R.; Kramer, G.M.; Jansma, E.P.; Liu, Y.; Oyen, W.J. A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 2016, 55, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Srirajaskanthan, R.; Kayani, I.; Quigley, A.M.; Soh, J.; Caplin, M.E.; Bomanji, J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 2010, 51, 875–882. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, G.; Früh, M.; Reck, M.; Baumann, P.; Klepetko, W.; Felip, E. ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. S5), v116–v119. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, D.; Ohno, Y.; Matsumoto, K.; Aoyama, N.; Onishi, Y.; Koyama, H.; Nogami, M.; Yoshikawa, T.; Matsumoto, S.; Sugimura, K. Detection of bone metastases in non-small cell lung cancer patients: Comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J. Magn. Reson. Imaging 2009, 30, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Colt, H.G.; Murgu, S.D.; Korst, R.J.; Slatore, C.G.; Unger, M.; Quadrelli, S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e437S–e454S. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.; De Ruysscher, D.; Eberhardt, W.E.; Lim, E.; Senan, S.; Felip, E.; Peters, S. ESMO Guidelines Working Group. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi89–vi98. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Kalff, V.; MacManus, M.P.; Ware, R.E.; McKenzie, A.F.; Matthews, J.P.; Ball, D.L. The utility of 18F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: Impact on management and prognostic stratification. J. Nucl. Med. 2001, 42, 1605–1613. [Google Scholar] [PubMed]
- Keidar, Z.; Haim, N.; Guralnik, L.; Wollner, M.; Bar-Shalom, R.; Ben-Nun, A.; Israel, O. PET/CT using 18F-FDG in suspected lung cancer recurrence: Diagnostic value and impact on patient management. J. Nucl. Med. 2004, 45, 1640–1646. [Google Scholar] [PubMed]
- Marcus, C.; Paidpally, V.; Antoniou, A.; Zaheer, A.; Wahl, R.L.; Subramaniam, R.M. 18F-FDG PET/CT and lung cancer: Value of fourth and subsequent posttherapy follow-up scans for patient management. J. Nucl. Med. 2015, 56, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Dunphy, M.; Naidoo, J.; Travis, W.D.; Hellmann, M.; Woo, K.; Downey, R.; Rusch, V.; Ginsberg, M.S.; Azzoli, C.G. Adaptive Neoadjuvant Chemotherapy Guided by 18F-FDG PET in Resectable Non-Small Cell Lung Cancers: The NEOSCAN Trial. J. Thorac. Oncol. 2016, 11, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Iwano, S.; Kishimoto, M.; Ito, S.; Kato, K.; Naganawa, S. Correlation between FDG-PET/CT findings and solid type non-small cell cancer prognostic factors: Are there differences between adenocarcinoma and squamous cell carcinoma? Ann. Nucl. Med. 2015, 29, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Goodgame, B.; Viswanathan, A.; Zoole, J.; Gao, F.; Miller, C.R.; Subramanian, J.; Meyers, B.F.; Patterson, A.G.; Govindan, R. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J. Thorac. 2009, 4, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Varlotto, J.M.; Yao, A.N.; DeCamp, M.M.; Ramakrishna, S.; Recht, A.; Flickinger, J.; Andrei, A.; Reed, M.F.; Toth, J.W.; Fizgerald, T.J.; et al. Nodal stage of surgically resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jo, E.J.; Eom, J.S.; Mok, J.H.; Kim, M.H.; Lee, K.; Kim, K.U.; Park, H.K.; Lee, C.H.; Kim, Y.D.; et al. Predictors of Recurrence after Curative Resection in Patients with Early-Stage Non-Small Cell Lung Cancer. Tuberc. Respir. Dis. 2015, 78, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Shimada, Y.; Saji, H.; Kato, Y.; Yoshida, K.; Matsubayashi, J.; Nagase, S.; Kakihana, M.; Kajiwara, N.; Ohira, T.; et al. Prognostic Factors for Survival after Recurrence in Patients With Completely Resected Lung Adenocarcinoma: Important Roles of Epidermal Growth Factor Receptor Mutation Status and the Current Staging System. Clin. Lung Cancer 2015, 16, e213–e221. [Google Scholar] [CrossRef] [PubMed]
- Glubb, D.M.; Paré-Brunet, L.; Jantus-Lewintre, E.; Jiang, C.; Crona, D.; Etheridge, A.S.; Mirza, O.; Zhang, W.; Seiser, E.L.; Rzyman, W.; et al. Functional FLT1 Genetic Variation is a Prognostic Factor for Recurrence in Stage I–III Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Hur, J.; Moon, Y.W.; Hong, S.R.; Suh, Y.J.; Kim, Y.J.; Im, D.J.; Hong, Y.J.; Lee, H.J.; Kim, Y.J.; et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer 2016, 16, 224. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, C.; Garcia-Velloso, M.J.; Lozano, M.D.; Labiano, T.; Vigil Diaz, C.; Lopez-Picazo, J.M.; Gurpide, A.; Zulueta, J.J.; Richter Echevarria, J.A.; Perez Gracia, J.L. Role of [¹⁸F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, D.; Ohno, Y.; Koyama, H.; Nogami, M.; Onishi, Y.; Matsumoto, K.; Matsumoto, S.; Yoshikawa, T.; Sugimura, K. Integrated FDG-PET/CT vs. standard radiological examinations: Comparison of capability for assessment of postoperative recurrence in non-small cell lung cancer patients. Eur. J. Radiol. 2010, 74, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Sudarski, S.; Henzler, T.; Schoenberg, S.O. Post-therapeutic positron emission tomography/computed tomography for early detection of non-small cell lung cancer recurrence. Transl. Lung Cancer Res. 2013, 2, 295–303. [Google Scholar] [PubMed]
- Onishi, Y.; Ohno, Y.; Koyama, H.; Nogami, M.; Takenaka, D.; Matsumoto, K.; Yoshikawa, T.; Matsumoto, S.; Maniwa, Y.; Nishimura, Y.; et al. Non-small cell carcinoma: Comparison of postoperative intra- and extrathoracic recurrence assessment capability of qualitatively and/or quantitatively assessed FDG-PET/CT and standard radiological examinations. Eur. J. Radiol. 2011, 79, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Thureau, S.; Dubray, B.; Modzelewski, R.; Thiberville, L.; Gardin, I.; Vera, P. Areas of high 18F-FDG uptake on preradiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for non-small cell lung cancer. J. Nucl. Med. 2015, 56, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Li, W.; He, X.; Xu, L. Radiofrequency ablation of advanced lung tumors: Imaging features, local control, and follow-up protocol. Int. J. Clin. Exp. Med. 2015, 8, 18137–18143. [Google Scholar] [PubMed]
- Everitt, S.J.; Ball, D.L.; Hicks, R.J.; Callahan, J.; Plumridge, N.; Collins, M.; Herschtal, A.; Binns, D.; Kron, T.; Schneider, M.; et al. Differential 18F-FDG and 18F-FLT Uptake on Serial PET/CT Imaging Before and During Definitive Chemoradiation for Non-Small Cell Lung Cancer. J. Nucl. Med. 2014, 55, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Okamoto, T.; Fukuyama, S.; Maehara, Y. Therapeutic strategy for postoperative recurrence in patients with non-small cell lung cancer. World J. Clin. Oncol. 2014, 5, 1048–1054. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameters | ||||
|---|---|---|---|---|
| Lymphatic permeation | Pleural Invasion | Vessel invasion | ||
| Intratumoral vascular invasion | Nodal involvement | Incomplete MLNs dissection | ||
| Biochemical and Molecular Parameters | ||||
| High CEA MIB-1 expression MACC1 expression CK19 mRNA IGF1R | Histological Dedifferentiation Methylation (promoter regions p16 and CDH13) CXCR7 expression MicroRNA expression | KRAS Ki-67 TS expression EGFR mutations | ||
| 18F-FDG PET or PET/CT findings in primary tumour Intensity of uptake (SUVm) Metabolic tumor volume (MTV) | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Bonilla, J.F.; Quirce, R.; Martínez-Rodríguez, I.; De Arcocha-Torres, M.; Carril, J.M.; Banzo, I. The Role of PET/CT Molecular Imaging in the Diagnosis of Recurrence and Surveillance of Patients Treated for Non-Small Cell Lung Cancer. Diagnostics 2016, 6, 36. https://doi.org/10.3390/diagnostics6040036
Jiménez-Bonilla JF, Quirce R, Martínez-Rodríguez I, De Arcocha-Torres M, Carril JM, Banzo I. The Role of PET/CT Molecular Imaging in the Diagnosis of Recurrence and Surveillance of Patients Treated for Non-Small Cell Lung Cancer. Diagnostics. 2016; 6(4):36. https://doi.org/10.3390/diagnostics6040036
Chicago/Turabian StyleJiménez-Bonilla, Julio Francisco, Remedios Quirce, I. Martínez-Rodríguez, María De Arcocha-Torres, José Manuel Carril, and Ignacio Banzo. 2016. "The Role of PET/CT Molecular Imaging in the Diagnosis of Recurrence and Surveillance of Patients Treated for Non-Small Cell Lung Cancer" Diagnostics 6, no. 4: 36. https://doi.org/10.3390/diagnostics6040036





