Detecting Breast Cancer with a Dual-Modality Device
Abstract
:1. Introduction
2. Materials and Methods
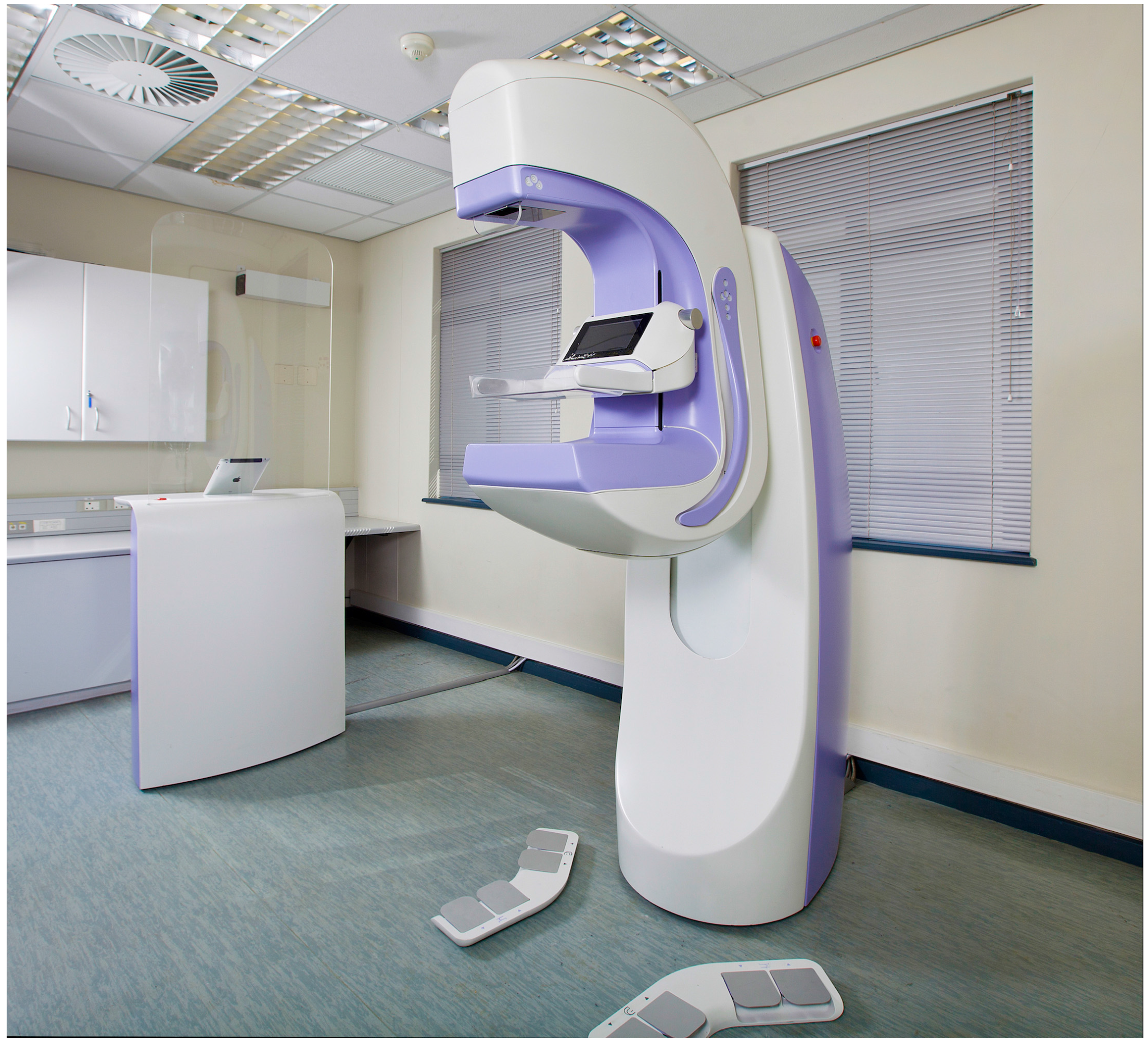
2.1. Dual-Modality Aceso System
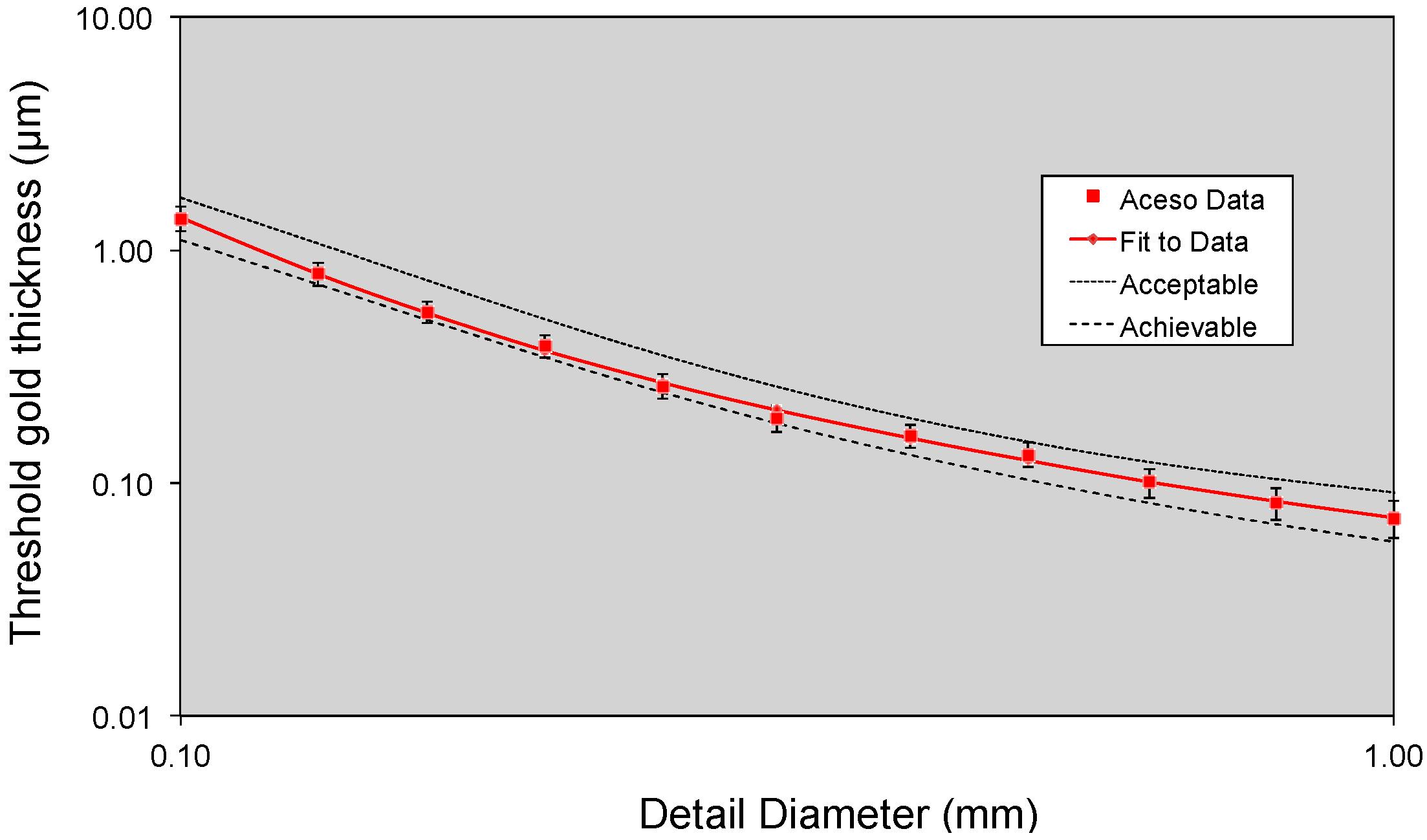
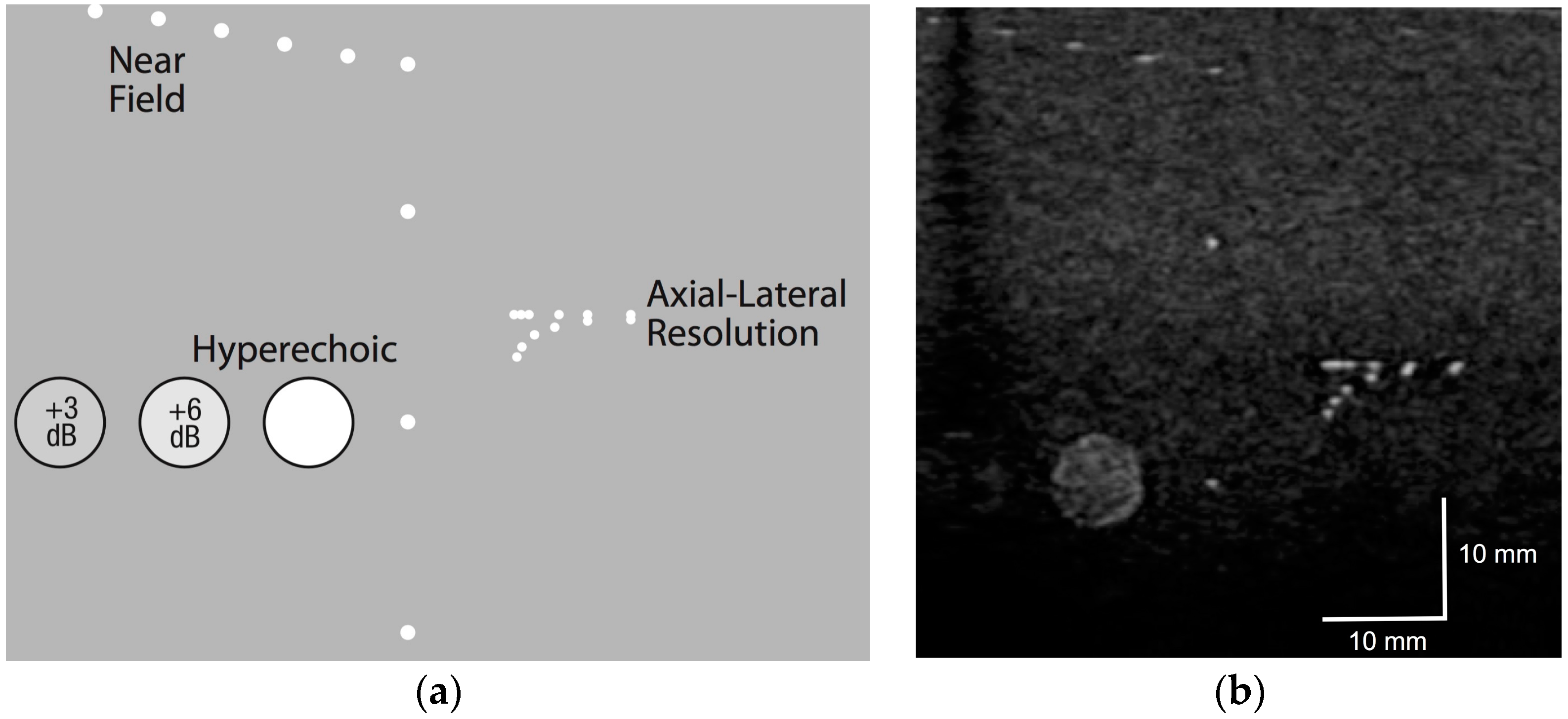
2.2. Phantom Testing
2.3. Human Subjects
3. Results
3.1. Phantom Testing
3.2. All Human Subjects
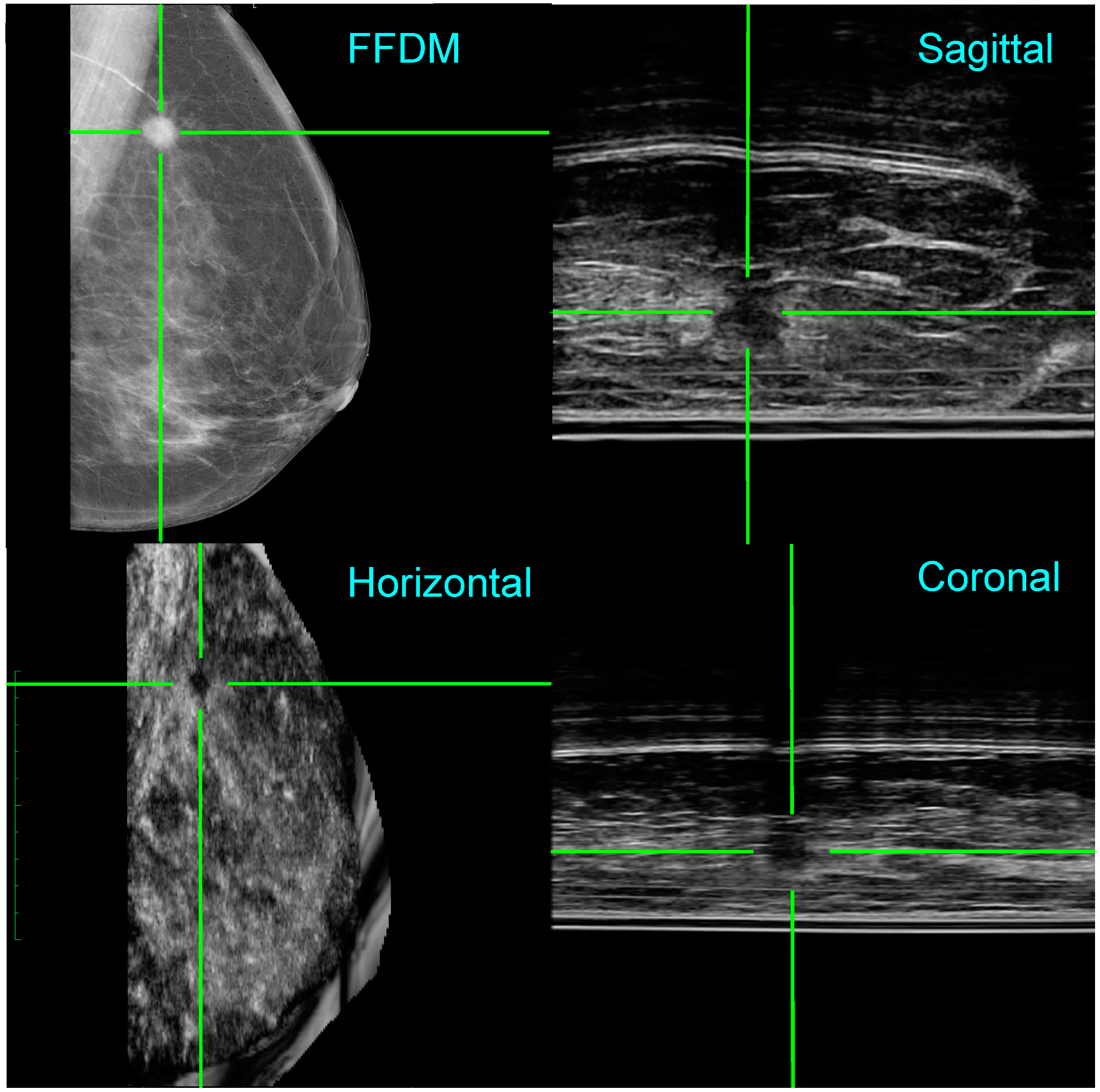
3.3. Two Clinical Examples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moseley, T.W. Digital mammography and digital breast tomosynthesis. Clin. Obstet. Gynecol. 2016, 59, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Kopans, D.B. Annual screening mammography beginning at age 40 saves the most lives. OBG Manag. 2015, 27, 15–18. [Google Scholar]
- Miller, A.B.; Wall, C.; Baines, C.J.; Sun, P.; To, T.; Narod, S.A. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: Randomised screening trial. Br. Med. J. 2014, 348, g366. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.L. New developments in medical imaging to detect breast cancer. Contin. Med. Educ. 2011, 29, 122–125. [Google Scholar]
- Kelly, K.M.; Dean, J.; Comulada, W.S.; Lee, S.-J. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 2010, 20, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; O’Meara, E.S.; Kerlikowske, K.; Balch, S.; Miglioretti, D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: An analysis of registry data. Ann. Intern. Med. 2016, 164, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dempsey, P.J. Diagnostic breast ultrasound: Current status and future directions. Ultrasound Clin. 2009, 4, 117–133. [Google Scholar] [CrossRef]
- Dempsey, P.J. The history of breast ultrasound. J. Ultrasound Med. 2004, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Bohm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. J. Am. Med. Assoc. 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Nothacker, M.; Duda, V.; Hahn, M.; Warm, M.; Degenhardt, F.; Madjar, H.; Weinbrenner, S.; Albert, U.S. Early detection of breast cancer: Benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer 2009, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Calabrese, M.; Mariscotti, G.; Durando, M.; Tosto, S.; Monetti, F.; Airaldi, S.; Bignotti, B.; Nori, J.; Bagni, A.; et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: Interim report of a prospective comparative trial. J. Clin. Oncol. 2016, 34, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A. Current status of supplemental screening in dense breasts. J. Clin. Oncol. 2016, 34, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J.; Jong, R.A. Adjunctive ultrasonography in breast cancer screening. Lancet 2016, 387, 313–314. [Google Scholar] [CrossRef]
- Wilczek, B.; Wilczek, H.E.; Rasouliyan, L.; Leifland, K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radiol. 2016, 85, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Sak, M.; Duric, N.; Littrup, P.; Bey-Knight, L.; Ali, H.; Vallieres, P.; Sherman, M.E.; Gierach, G.L. Using speed of sound imaging to characterize breast density. Ultrasound Med. Biol. 2017, 43, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Tiu, C.M.; Chen, J.; Chang, R.F. Automated full-field breast ultrasonography: The past and the present. J. Med. Ultrasound 2007, 15, 31–44. [Google Scholar] [CrossRef]
- Arleo, E.K.; Saleh, M.; Ionescu, D.; Drotman, M.; Min, R.J.; Hentel, K. Recall rate of screening ultrasound with automated breast volumetric scanning (ABVS) in women with dense breasts: A first quarter experience. Clin. Imaging 2014, 38, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, V.; Giuliano, C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging 2013, 37, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.L.; Evans, M.D. Diagnosing breast cancer: An opportunity for innovative engineering. S. Afr. Med. J. 2012, 102, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.L.; Douglas, T.S.; Said-Hartley, Q.; Baasch, R.V.; Boonzaier, J.A.; Goemans, B.C.; Harverson, J.; Mingay, M.W.; Omar, S.; Smith, R.V.; et al. Testing a dual-modality system that combines full-field digital mammography and automated breast ultrasound. Clin. Imaging 2016, 40, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Tesic, M.M.; Piccaro, M.F.; Munier, B. Full field digital mammography scanner. Eur. J. Radiol. 1999, 31, 2–17. [Google Scholar] [CrossRef]
- Young, K.C.; Alsager, A.; Oduko, J.M.; Bosmans, H.; Verbrugge, B.; Geertse, T.; van Engen, R. Evaluation of software for reading images of the CDMAM test object to assess digital mammography systems. In Proceedings of the Medical Imaging 2008: Physics of Medical Imaging, San Diego, CA, USA, 16 February 2008; Volume 6913.
- European Reference Organisation for Quality Assured Breast Screening and Diagnostic Services (EUREF). Available online: http://www.euref.org (accessed on 9 January 2017).
- Guidance for Industry and FDA Staff—Class II Special Controls Guidance Document: Full Field Digital Mammography System. Available online: http://www.fda.gov/RegulatoryInformation/Guidances/ucm107552.htm (accessed on 9 January 2017).
- Dance, D.R.; Young, K.C.; van Engen, R.E. Further factors for the estimation of mean glandular dose using the United Kingdom, European and IAEA breast dosimetry protocols. Phys. Med. Biol. 2009, 54, 4361–4372. [Google Scholar] [CrossRef] [PubMed]
- Kuzmiak, C.M.; Cole, E.; Zeng, D.; Kim, E.; Koomen, M.; Lee, Y.; Pavic, D.; Pisano, E.D. Comparison of image acquisition and radiologist interpretation times in a diagnostic mammography center. Acad. Radiol. 2010, 17, 1168–1174. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Definition |
|---|---|
| Breast positioning | Assess coverage of the breast on cranio-caudal (CC) and medio-lateral oblique (MLO) views |
| Exposure | Assess visualization of the adipose and fibroglandular tissues and visualization of breast tissue underlying the pectoralis muscle |
| Breast compression | Assess overlapping breast structures, uniformity of exposure of fibroglandular tissues, adequacy of penetration of thicker portions, exposure of thinner areas, and motion unsharpness |
| Image contrast | Assess differentiation of subtle tissue density differences |
| Sharpness | Assess the edges of fine linear structures, tissue borders, and benign calcifications |
| Tissue visibility | Assess the tissue visibility on the skin line |
| Noise | Assess noise obscuring breast structures or suggestive of structures not actually present |
| Artifacts | Assess artifacts due to image processing, detector failure, and other factors external to the breast |
| Image quality | Assess the overall clinical image quality |
| Parameter | Mean Value | |
|---|---|---|
| Breast positioning | Cranio-caudal | –0.16 |
| Medio-lateral oblique | –0.40 | |
| Exposure | Adipose | 0.00 |
| Fibroglandular | 0.00 | |
| Pectoralis | –0.40 | |
| Breast compression | 0.00 | |
| Image contrast | –0.12 | |
| Sharpness | –0.12 | |
| Tissue visibility | –0.08 | |
| Noise | 0.00 | |
| Artifacts | –0.24 | |
| Image quality | –0.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padia, K.; Douglas, T.S.; Cairncross, L.L.; Baasch, R.V.; Vaughan, C.L. Detecting Breast Cancer with a Dual-Modality Device. Diagnostics 2017, 7, 17. https://doi.org/10.3390/diagnostics7010017
Padia K, Douglas TS, Cairncross LL, Baasch RV, Vaughan CL. Detecting Breast Cancer with a Dual-Modality Device. Diagnostics. 2017; 7(1):17. https://doi.org/10.3390/diagnostics7010017
Chicago/Turabian StylePadia, Kamila, Tania S. Douglas, Lydia L. Cairncross, Roland V. Baasch, and Christopher L. Vaughan. 2017. "Detecting Breast Cancer with a Dual-Modality Device" Diagnostics 7, no. 1: 17. https://doi.org/10.3390/diagnostics7010017







