Diagnostics Strategies with Electrochemical Affinity Biosensors Using Carbon Nanomaterials as Electrode Modifiers
Abstract
:1. Introduction
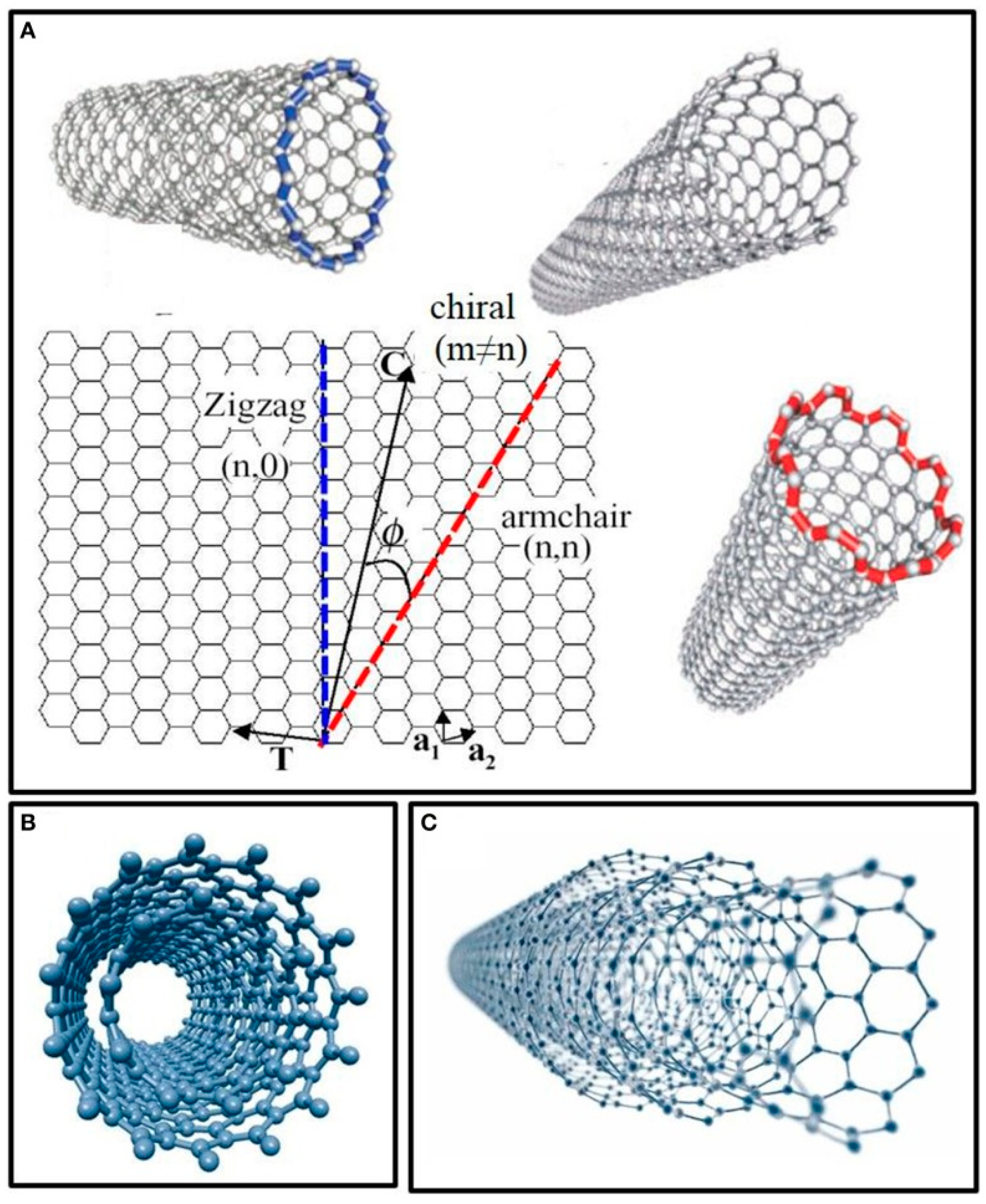
2. Carbon Nanomaterials as Electrode Modifiers for the Preparation of Electrochemical Biosensors
3. Electrochemical DNA Sensors Involving Carbon Nanomaterials as Electrode Modifiers
3.1. Electrochemical DNA Sensors Using CNTs
3.2. Electrochemical DNA Sensors Using Graphene
4. Electrochemical Immunosensors Involving Carbon Nanomaterials as Electrode Modifiers
4.1. Electrochemical Immunosensorsusing CNTs
4.2. Electrochemical Immunosensors Using Graphene
5. General Conclusions and Future Prospects
Acknowledgments
Conflicts of Interest
References
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Catanante, G.; Marty, J.L. Current trends in nanomaterial-based amperometric biosensors. Sensors 2014, 14, 23439–23461. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiong, E.H.; Zhang, X.; Zhang, X.H.; Chen, J.H. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today 2014, 9, 197–211. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Carbon nanomaterials based electrochemical sensors/biosensors for the sensitive detection of pharmaceutical and biological compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.D.; Piro, B.; Noël, V.; Reisberg, S.; Pham, M.-C. Applications of carbon nanotubes to electrochemical DNA sensors: A new strategy to make direct and selective hybridization detection from SWNTs. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Mejri, N.; Gomgnimbou, M.; Sola, C.; Korri-Youssoufi, H. E-DNA sensor of Mycobacterium tuberculosis based on electrochemical assembly of nanomaterials (MWCNTs/PPy/PAMAM). Anal. Chem. 2015, 87, 9257–9264. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Yan, S.; Wang, M.; Liu, P.; Dong, Y.; Zhang, C. Single-walled carbon nanotubes–carboxyl-functionalized graphene oxide-based electrochemical DNA biosensor for thermolabile hemolysin gene detection. Anal. Methods 2015, 7, 5303–5310. [Google Scholar] [CrossRef]
- Wu, L.; Ji, H.; Sun, H.; Ding, C.; Ren, J.; Qu, X. Label-free ratiometric electrochemical detection of the mutated apolipoprotein E gene associated with Alzheimer’s disease. Chem. Commun. 2016, 52, 12080–12083. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.P. Graphene–inorganic nanocomposites. RSC Adv. 2012, 2, 64–98. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Wang, W.; Lu, Y.; Xi, J.; Zheng, W.; Wu, F.; Ao, H.; Li, G. Electrochemical DNA sensor for Staphylococcus aureus nuc gene sequence with zirconia and graphene modified electrode. J. Solid State Electrochem. 2015, 19, 2431–2438. [Google Scholar] [CrossRef]
- Zribi, B.; Roy, E.; Pallandre, A.; Chebil, S.; Koubaa, M.; Mejri, N.; Magdinier Gomez, H.; Sola, C.; Korri-Youssoufi, H.; Haghiri-Gosnet, A.-M. A microfluidic electrochemical biosensor based on multiwall carbon nanotube/ferrocene for genomic DNA detection of Mycobacterium tuberculosis in clinical isolates. Biomicrofluidics 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lillehoj, P.B.; Ho, C.-M. DNA diagnostics: Nanotechnology-enhanced electrochemical detection of nucleic acids. Pediatr. Res. 2010, 67, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Graphene-DNA electrochemical sensor for the sensitive detection of BRCA1 gene. Sens. Actuators B Chem. 2014, 204, 777–782. [Google Scholar] [CrossRef]
- Tosar, J.P.; Braňas, G.; Laíz, J. Electrochemical DNA hybridization sensors applied to real and complex biological samples. Biosens. Bioelectron. 2010, 26, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ferreira, D.S.; Nascimento, G.A.; Souza, E.V.M.; Souto-Maior, M.A.; Arruda, M.S.; Zanforlin, D.M.L.; Ekert, M.H.F.; Bruneska, D.; Lima-Filho, J.L. Electrochemical DNA biosensor for the detection of human papillomavirus E6 gene inserted in recombinant plasmid. Anal. Chim. Acta 2013, 804, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ren, J.; Qu, X. Target-responsive DNA-capped nanocontainer used for fabricating universal detector and performing logic operations. Nucleic Acids Res. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Li, J. Electrochemical DNA Sensors: From nanoconstruction to biosensing. Curr. Org. Chem. 2011, 15, 506–517. [Google Scholar] [CrossRef]
- Pilehvar, S.; Rather, J.A.; Dardenne, F.; Robbens, J.; Blust, R.; Wael, K.D. Carbon nanotubes based electrochemical aptasensing platform for the detection of hydroxylated polychlorinated biphenyl in human blood serum. Biosens. Bioelectron. 2014, 54, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerface 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Collinge, J. Prion diseases of humans and animals: Their causes and molecular basis. Annu. Rev. Neurosci. 2011, 24, 519–550. [Google Scholar] [CrossRef] [PubMed]
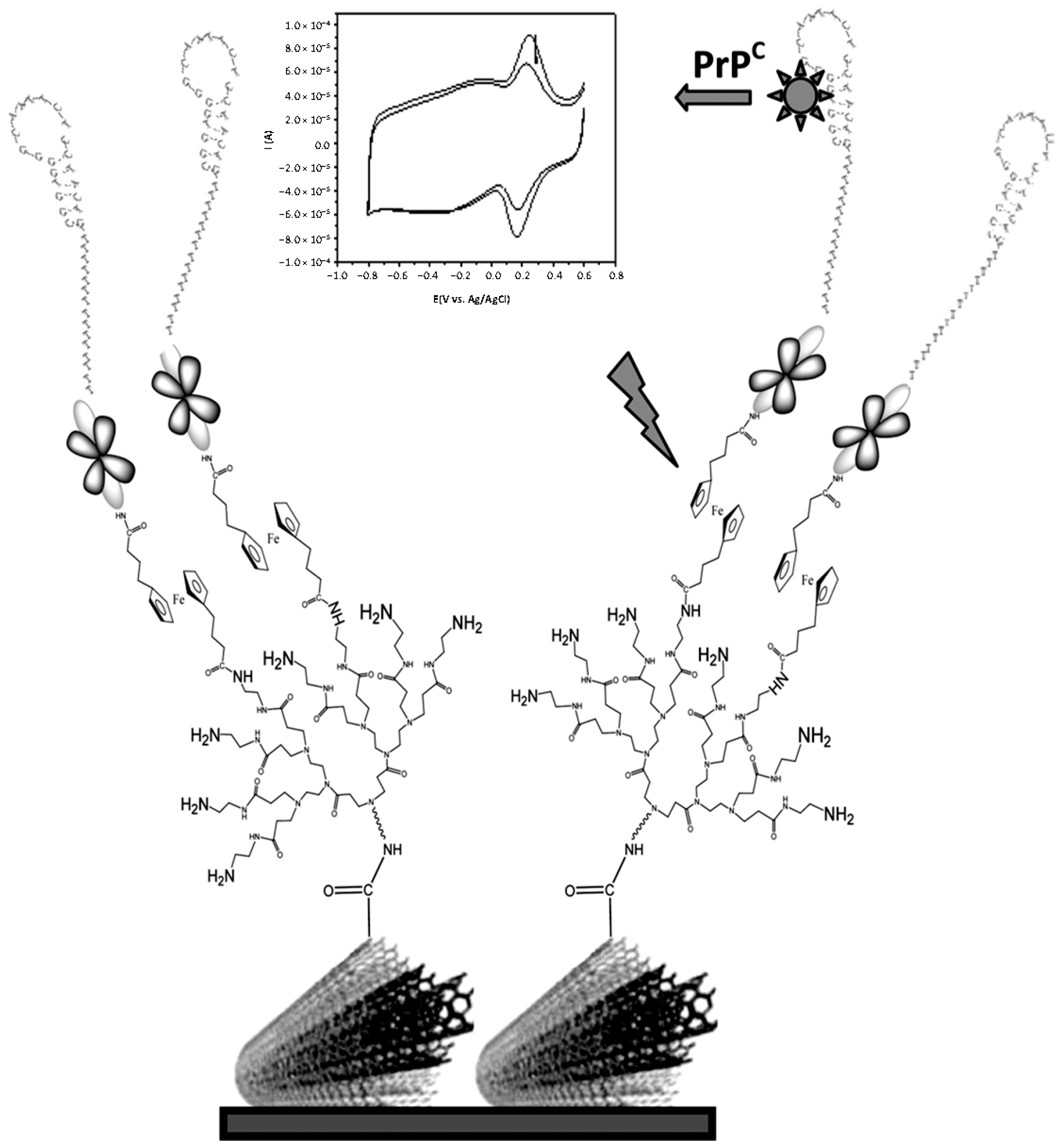
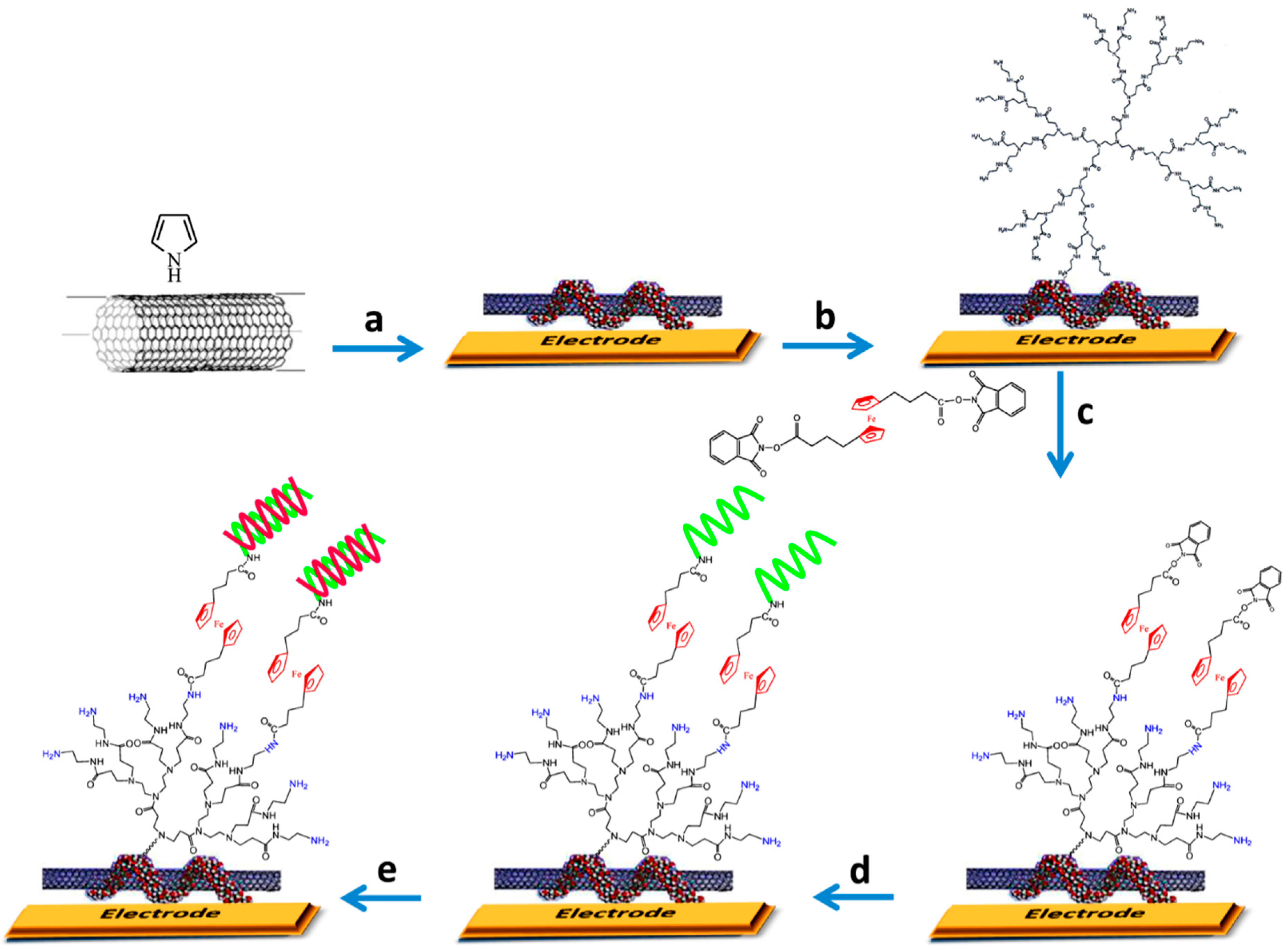
- Miodek, A.; Castillo, G.; Hianik, T.; Korri-Youssoufi, H. Electrochemical aptasensor of human cellular prion based on multiwalled carbon nanotubes modified with dendrimers: A platform for connecting redox markers and aptamers. Anal. Chem. 2013, 85, 7704–7712. [Google Scholar] [CrossRef] [PubMed]
- Zari, N.; Amine, A.; Ennaji, M.M. Label-free DNA biosensor for electrochemical detection of short DNA sequences related to human papilloma virus. Anal. Lett. 2009, 42, 519–535. [Google Scholar] [CrossRef]
- Piro, B.; Kapella, A.; Le, V.H.; Anquetin, G.; Zhang, Q.D.; Reisberg, S.; Noel, V.; Tran, L.D.; Duc, H.T.; Pham, M.C. Towards the development of human papillomavirus infection by a reagentless electrochemical peptide biosensor. Electrochim. Acta 2011, 56, 10688–10693. [Google Scholar] [CrossRef]
- Ali, R.; Al-Achkar, K.; Al-Mariri, A.; Safi, M. Role of polymerase chainreaction (PCR) in the detection of antibiotic-resistant Staphylococcus aureus. Egypt. J. Med. Hum. Gen. 2014, 15, 293–298. [Google Scholar] [CrossRef]
- Vremera, T.; Iancu, L.S.; Logigan, C.; Năstase, E.; Miftode, E.; Luncă, C.; Dorneau, O. Optimization of triplex real time PCR for detecting Staphylococcus aureus mecA, pvl and nuc genes. Roum. Arch. Microbiol. Immunol. 2011, 70, 69–73. [Google Scholar] [PubMed]
- Broberg, C.A.; Calder, T.J.; Orth, K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 2011, 13, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Yeung, P.S.M.; Boor, K.J. Epidemiology, pathogenesis, and prevention of foodborne Vibrio parahaemolyticus infections. Foodborne Pathog. Dis. 2004, 1, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Okon, S.L. Nanomaterial-based electrochemical immunosensors for clinically significant biomarkers. Materials 2014, 7, 4669–4709. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Kaushik, A.; Tracy, K.; Bhansali, S.; Pala, N. Zinc oxide nanostructures for electrochemical cortisol biosensing. In Proceedings of the SPIE 9107, Smart Biomedical and Physiological Sensor Technology XI, 91070U, Baltimore, MD, USA, 22 May 2014.
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical magnetoimmunosensor for the ultrasensitive determination of interleukin-6 in saliva and urine using poly-HRP streptavidin conjugates as labels for signal amplification. Anal. Bioanal. Chem. 2014, 406, 6363–6371. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, G.; Serafín, V.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for the determination of total ghrelin hormone in saliva. Electroanalsis 2015, 27, 1119–1126. [Google Scholar] [CrossRef]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Barderas, R.; Torrente-Rodríguez, R.M.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Amperometric magnetoimmunosensor for ErbB2 breast cancer bio-marker determination in human serum, cell lysates and intact breast cancer cells. Biosens. Bioelectron. 2015, 70, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Yadav, P.; Singh, A.; Kaur, S.; Ramirez-Vick, J.; Chandra, P.; Arora, K.; Singh, S.P. CD 59 targeted ultrasensitive electrochemical immunosensor for fast and noninvasive diagnosis of oral cancer. Electroanalsis 2016, 28, 2565–2574. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas-Montiel, V.; Pedrero, M.; Fernández-Aceñero, M.J.; Barderas, R.; Pingarrón, J.M. Rapid endoglin determination in serum samples using anamperometric magneto-actuated disposable immunosensing platform. J. Pharm. Biomed. Anal. 2016, 129, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer proteins biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Brault, D.; Gligorov, J.; Antoine, M.; Neumann, R.; Lotz, J.-P.; Capeau, J. Evaluation of the quantitative analytical methods real-time PCR for HER-2 gene quantification and ELISA of serum HER-2 protein and comparison with fluorescence in situ hybridization and immunohistochemistry for determining HER-2 status in breast cancer patients. Clin. Chem. 2005, 51, 1093–1101. [Google Scholar] [PubMed]
- Saber, R.; Karimi, Z.; Shamsipur, M. A novel antibody–antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode. Anal. Chim. Acta 2015, 874, 66–74. [Google Scholar]
- Peng, N.-J.; Liou, W.-S.; Liu, R.-S.; Hu, C.; Tsay, D.-G.; Liu, C.B. Early detection of recurrent ovarian cancer in patients with low-level increases in serum CA-125 levels by 2-[F-18] fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography. Cancer Biother. Radiopharm. 2011, 26, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.B.; Singh, V.; Vanjari, S.R.K.; Singh, S.G. One step biofunctionalized electrospun multiwalled carbon nanotubes embedded zinc oxide nanowire interface for highly sensitive detection of carcinoma antigen-125. Biosens. Bioelectron. 2017, 88, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Panini, N.V.; Messina, G.A.; Salinas, E.; Fernandez, H.; Raba, J. Integrated microfluidic systems with an immunosensor modified with carbon nanotubes for detection of prostate specific antigen (PSA) in human serum samples. Biosens. Bioelectron. 2008, 23, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Kavosi, B.; Fathi, F.; Hallaj, R. Highly sensitive immunosensing of prostate-specific antigen based on ionic liquid–carbon nanotubes modified electrode: Application as cancer biomarker for prostate biopsies. Biosens. Bioelectron. 2013, 42, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Amani, K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens. Bioelectron. 2014, 52, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, W.; Zhang, X.; Wang, S. Electrochemical immunosensor for the prostate specific antigen detection based on carbon nanotube and gold nanoparticle amplification strategy. Microchim. Acta 2015, 182, 1855–1861. [Google Scholar] [CrossRef]
- Altintas, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators B Chem. 2013, 188, 988–998. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, W.; Li, Q.; Luo, C.; Yan, L.; Zhang, D.; Yin, Y.; Ding, S.; Ju, H. Electrochemical immunosensor for competitive detection of neuron specific enolase using functional carbon nanotubes and gold nanoprobe. Talanta 2012, 93, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabelamplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wei, Z.; Zhang, H.; Shao, M. Sensitive immunosensor for the label-free determination of tumor marker based on carbon nanotubes/mesoporous silica and graphene modified electrode. Biosens. Bioelectron. 2013, 41, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Z.F.; Yan, F.; Ju, H.X. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Wu, Q.; Chen, H.; Chen, Z.; Lin, X. One step electrochemically deposited nanocomposite film of chitosan—carbon nanotubes—gold nanoparticles for carcinoembryonic antigen immunosensor application. Talanta 2011, 85, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, R.; Chai, R.Y.; Chen, S. Reagentlessamperometric cancer antigen 15-3 immunosensor based on enzyme-mediated direct electrochemistry. Biosens. Bioelectron. 2010, 25, 2548–2552. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, C.; Shu, G.; Wang, Y.; Liu, X. The enzyme electrocatalytic immunosensor based on functional composite nanofibers for sensitive detection of tumor suppressor protein p53. J. Electroanal. Chem. 2015, 756, 101–107. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Electrochemical biosensors for the determination of cardiovascular markers: A review. Electroanalsis 2014, 26, 1132–1153. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Henry, P.A.; Raut, A.S.; Ubnoske, S.M.; Parker, C.B.; Glass, J.T. Enhanced electron transfer kinetics through hybrid graphene carbon nanotubes films. Electrochem. Commun. 2014, 48, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Singal, S.; Srivastava, A.K.; Gahtori, B. Immunoassay for troponin I using a glassy carbon electrode modified with a hybrid film consisting of graphene and multiwalled carbon nanotubes and decorated with platinum nanoparticles. Microchim. Acta 2016, 183, 1375–1384. [Google Scholar] [CrossRef]
- Gomes-Filho, S.L.; Dias, A.C.; Silva, M.M.; Silva, B.V.; Dutra, R.F. A carbon nanotube-based electrochemical immunosensor for cardiac troponin T. Microchem. J. 2013, 109, 10–15. [Google Scholar] [CrossRef]
- Silva, B.V.M.; Cavalcanti, I.T.; Silva, M.M.S.; Dutra, R.F. A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta 2013, 117, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Loria, V.; Dato, I.; Graziani, F. Myeloperoxidase: A new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediat. Inflamm. 2008, 2008, 135625. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Li, Y.; Zhou, M.; Xie, G. A new immunosensor for serum myeloperoxidase based on self-assembly of multi-walled carbon nanotubes/thionine/gold nanoparticles-chitosan. Adv. Mater. Res. 2012, 343, 1207–1211. [Google Scholar] [CrossRef]
- Liu, B.; Lu, L.; Li, Q.; Xie, G. Disposable electrochemical immunosensor for myeloperoxidase based on the indium tin oxide electrode modified with an ionic liquid composite film containing gold nanoparticles, poly(o-phenylenediamine) and carbon nanotubes. Microchim. Acta 2011, 173, 513–520. [Google Scholar] [CrossRef]
- Prakash, M.D.; Singh, S.G.; Sharma, C.S.; Krishna, V.S.R. Electrochemical detection of cardiac biomarkers utilizing electrospun multiwalled carbon nanotubes embedded. Electroanalsis 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Cabral-Miranda, G.; Yamashiro-Kanashiro, E.H.G.; Gidlunda, M.; Sales, M.G.F. Specific label-free and real-time detection of oxidized low density lipoprotein (oxLDL) using an immunosensor with three monoclonal antibodies. Mater. Chem. B 2014, 2, 477–484. [Google Scholar] [CrossRef]
- Moselhy, S.S.; Demerdash, S.H. Serum free l-carnitine in association with myoglobin as a diagnostic marker of acute myocardial infarction. Clin. Biochem. 2009, 42, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Pal, M.; Kuzikov, A.V.; Bulko, T.; Suprun, E.V.; Shumyantseva, V.V. Impedimetric immunosensor for detection of cardiovascular disorder risk biomarker. Mater. Sci. Eng. C 2016, 68, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.B.; Quan, P.D. The neuroimmune guidance cue netrin-1: A new therapeutic target in cardiovascular disease. Am. J. Cardiovasc. Dis. 2013, 3, 129–134. [Google Scholar]
- Xu, W.; He, J.; Gao, L.; Zhang, J.; Yu, C. Immunoassay for netrin 1 via a glassy carbon electrode modified with multi-walled carbon nanotubes, thionine and gold nanoparticles. Microchim. Acta 2015, 182, 2115–2122. [Google Scholar] [CrossRef]
- Jernberg, T.; James, S.; Lindahl, B.; Stridsberg, M.; Venge, P.; Wallentin, L. NT-proBNP in unstable coronary artery disease—Experiences from the FAST, GUSTO IV and FRISC II trials. Eur. J. Heart Fail. 2004, 6, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yi, W.-J.; Lian, W.-B.; Yuan, R.; Chai, Y.-Q.; Chen, A.; Hu, C.-M. Ultrasensitive electrochemical strategy for NT-proBNP detection with gold nanochains and horseradish peroxidase complex amplification. Biosens. Bioelectron. 2011, 26, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators B Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.-W. 3D label-free prostate specific antigen (PSA) immunosensor based on graphene–gold composites. Biosens. Bioelectron. 2015, 63, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Li, X.; Li, R.; Du, B.; Wei, Q. Electrochemical immunosensor for detecting typical bladder cancer biomarker based on reduced graphene oxide-tetraethylenepentamine and trimetallic Au Pd Pt nanoparticles. Talanta 2015, 143, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, G.; Xiao, C.; Xue, Y.; Yang, A.; Ren, H.; Lu, W.; Zhao, H.; Li, X.; Yuan, Z. Sensitive electrochemical immunosensor for α-fetoprotein based on graphene/SnO2/Au nanocomposite. Biosens. Bioelectron. 2015, 71, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-S.; Zhu, X.-F.; Xu, J.-K.; Lu, L.-M.; Wang, W.-M.; Yang, T.-T.; Xing, H.-K.; Yu, Y.-F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Li, S.; Turner, A.P. Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3. Biosens. Bioelectron. 2013, 43, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Sun, M.; Liu, W.; Li, S.; Wang, X.; Chu, C.; Yan, M.; Yu, J. Disposable electrochemical immunosensor based on peroxidase-like magnetic silica–graphene oxide composites for detection of cancer antigen 153. Sens. Actuators B Chem. 2014, 192, 317–326. [Google Scholar] [CrossRef]
- Afsharan, H.; Khalilzadeh, B.; Tajalli, H.; Mollabashi, M.; Navaeipour, F.; Rashidi, M.-R. A sandwich type immunosensor for ultrasensitive electrochemical quantification of p53 protein based on gold nanoparticles/graphene oxide. Electrochim. Acta 2016, 188, 153–164. [Google Scholar] [CrossRef]
- Diaconu, I.; Cristea, C.; Hârceagă, V.; Marrazza, G.; Berindan-Neagoe, I.; Săndulescu, R. Electrochemical immunosensors in breast and ovarian cancer. Clin. Chim. Acta 2013, 425, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yánez-Sedeño, P.; Pingarrón, J.M. Electrochemical bioaffinity sensors for salivary biomarkers detection. TrAC Trends Anal. Chem. 2017, 86, 14–24. [Google Scholar] [CrossRef]
- Nagler, R.; Bahar, G.; Shpitzer, T.; Feinmesser, R. Concomitant analysis of salivary tumor markers—A newdiagnostic tool for oral cancer. Clin. Cancer Res. 2006, 12, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qi, M.; Zhang, Y.; Cao, C.; Goldys, E.M. Nanocomposites of gold nanoparticles and graphene oxide towards an stable label-free electrochemical immunosensor for detection of cardiac marker troponin-I. Anal. Chim. Acta 2016, 909, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.H.; Ghodsi, E.; Abdollahi, S.; Nadri, S. Porous graphene oxide nanostructure as an excellent scaffold for label-free electrochemical biosensor: Detection of cardiac troponin I. Mater. Sci. Eng. C 2016, 69, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Singal, S.; Srivastava, A.K.; Biradar, A.M.; Mulchandani, A. Rajesh, Pt nanoparticles-chemical vapor deposited graphene composite based immunosensor for the detection of human cardiac troponin I. Sens. Actuators B Chem. 2014, 205, 363–370. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.-H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef] [PubMed]
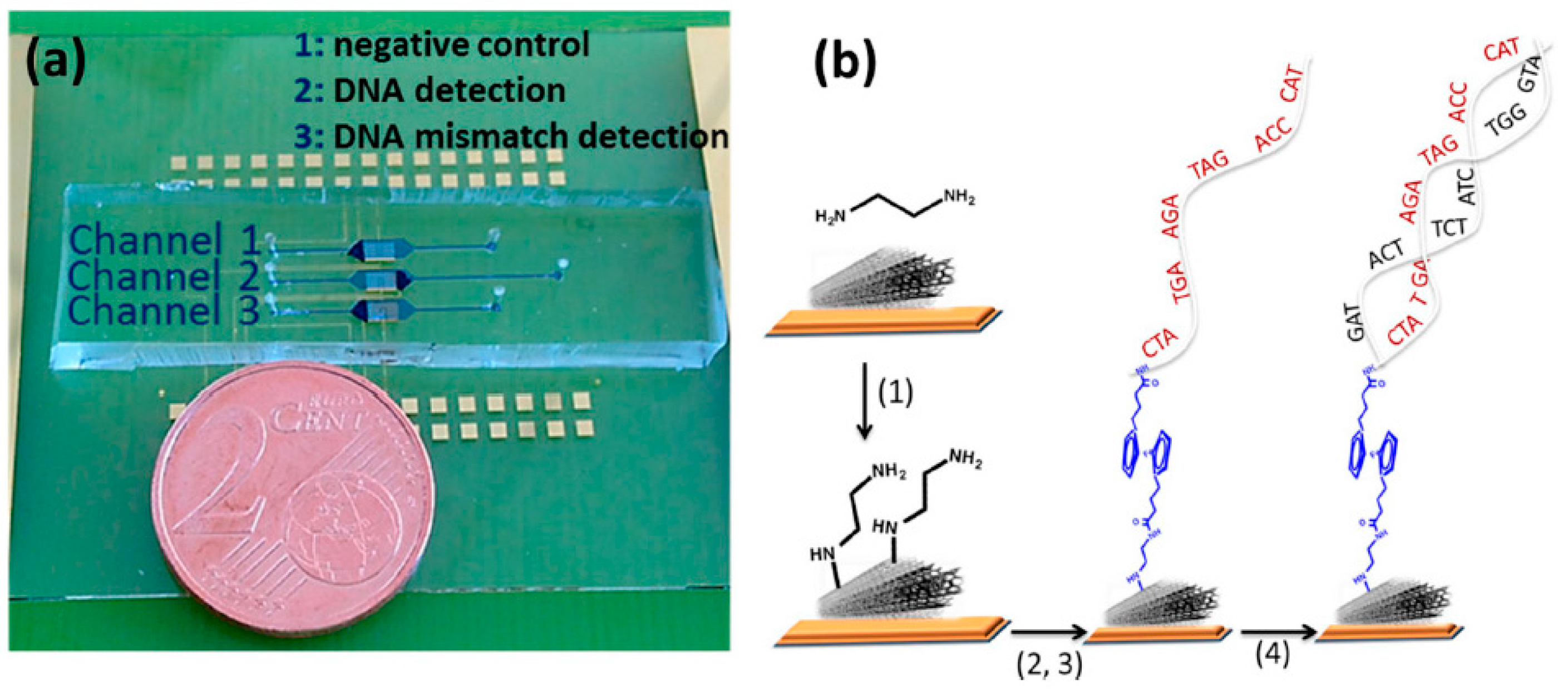
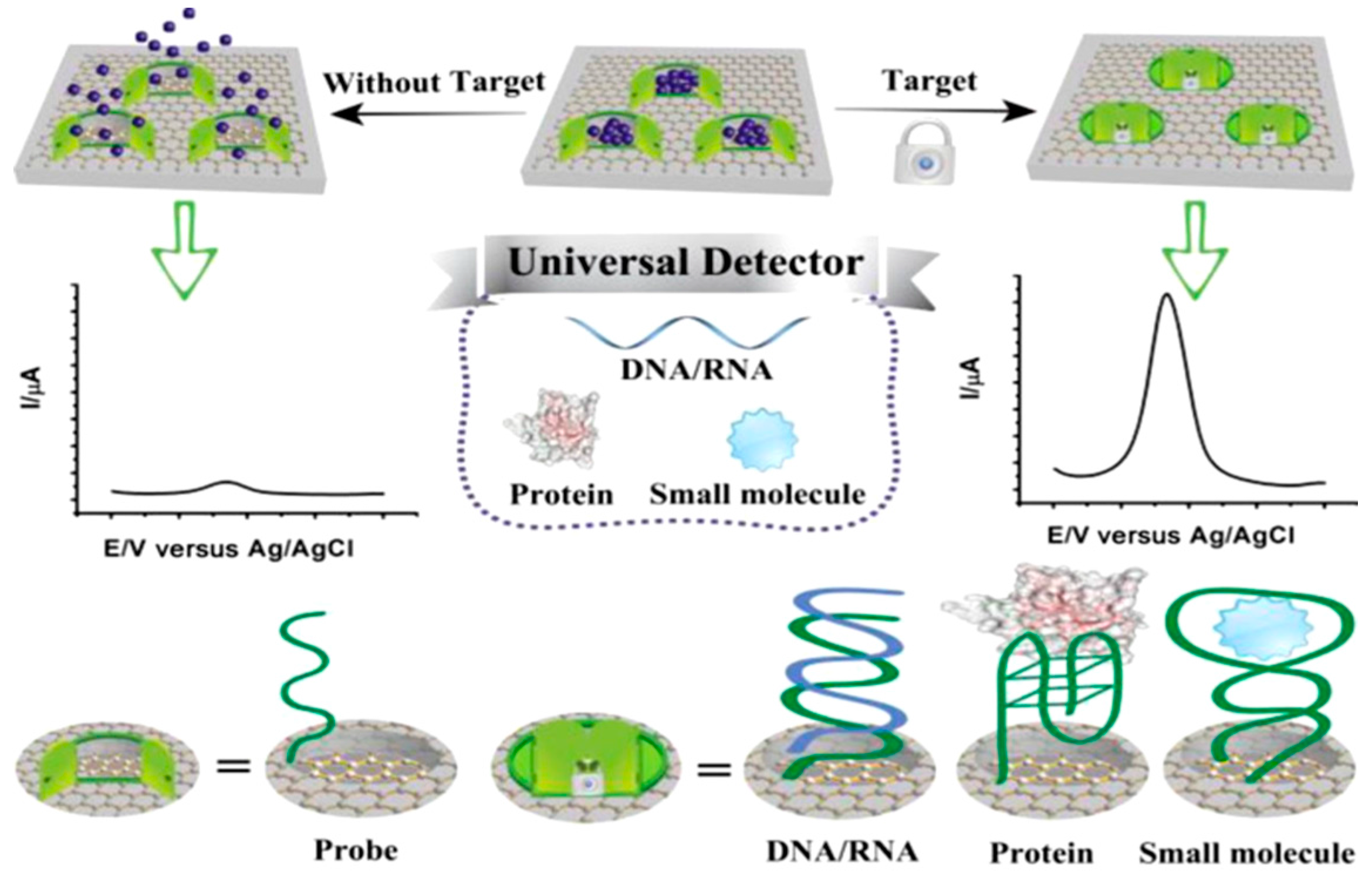
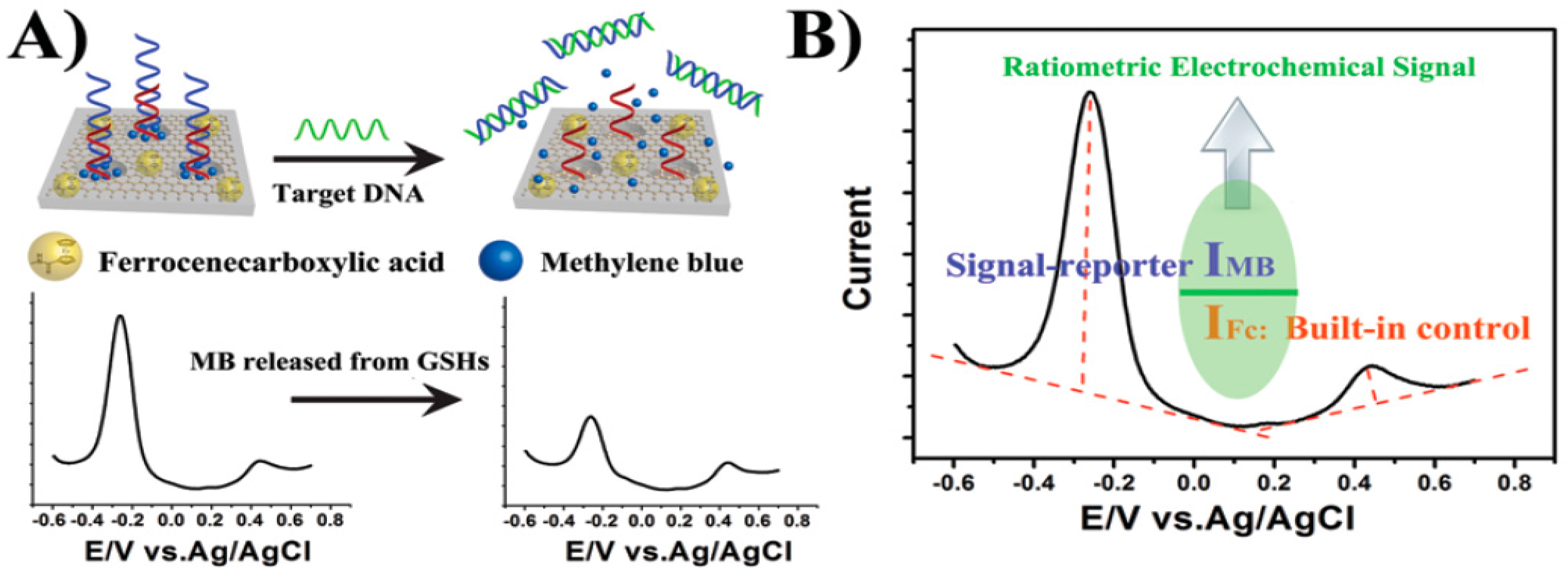
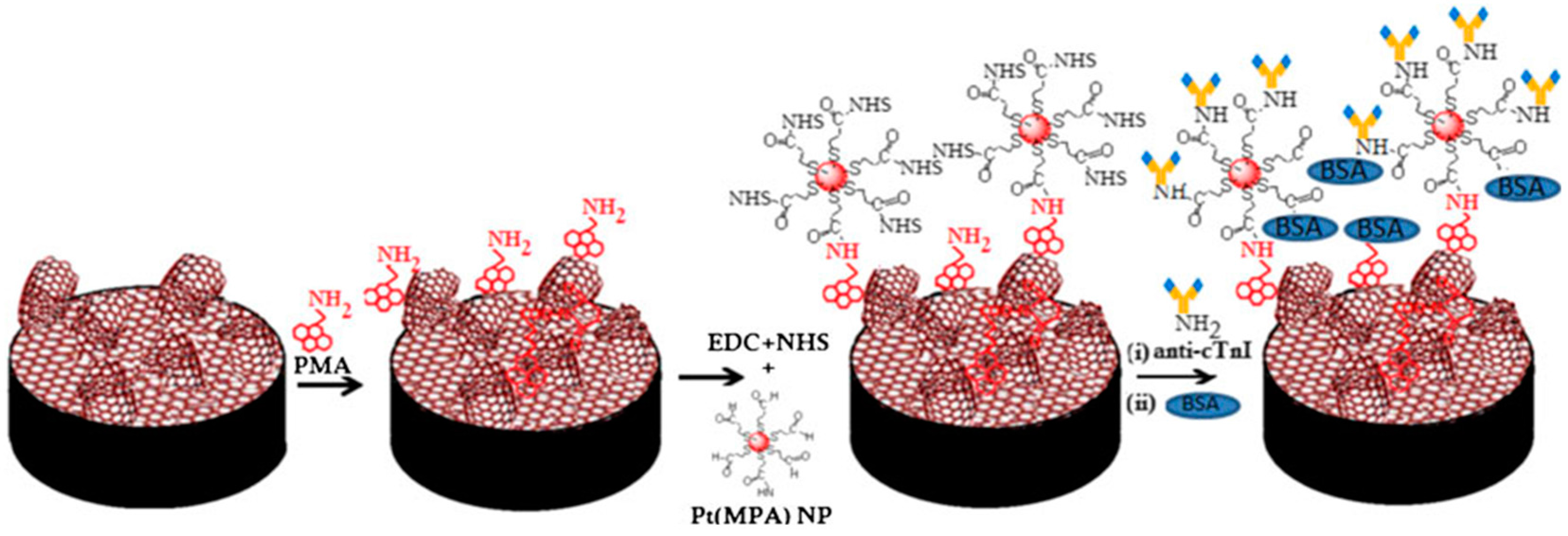
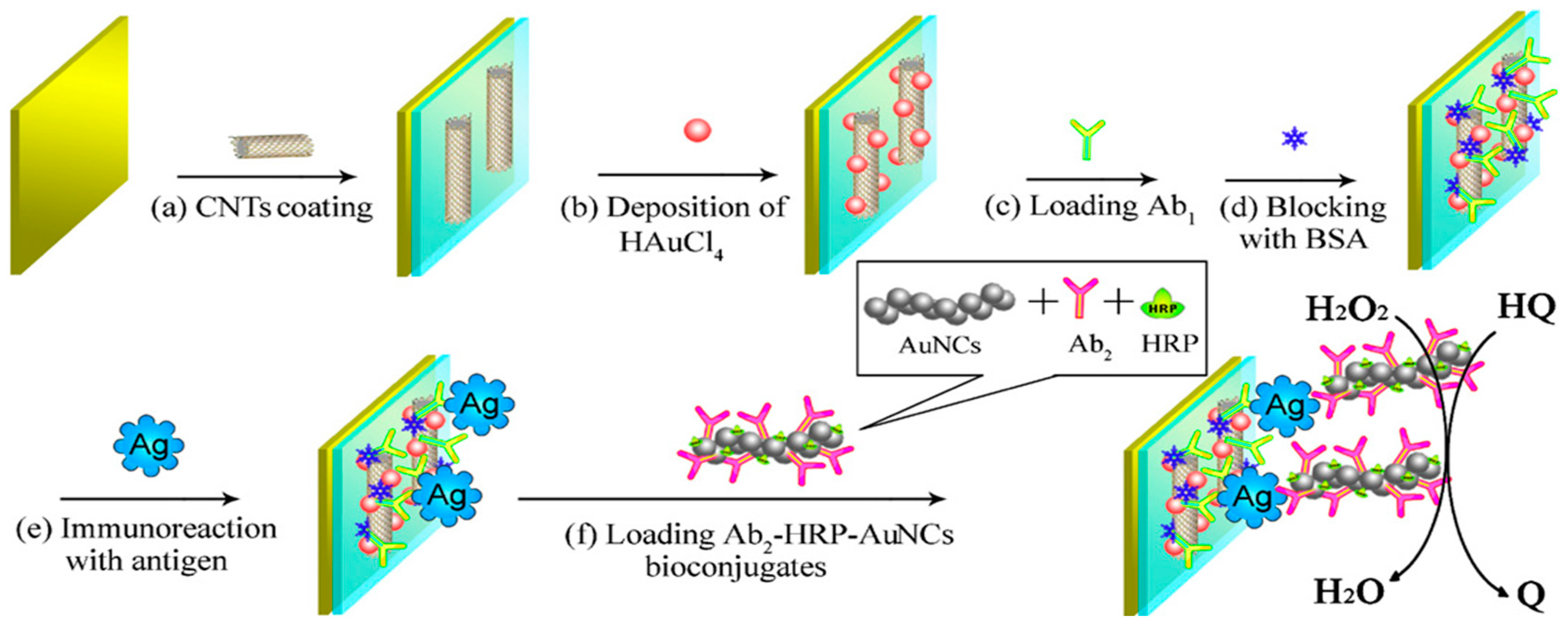
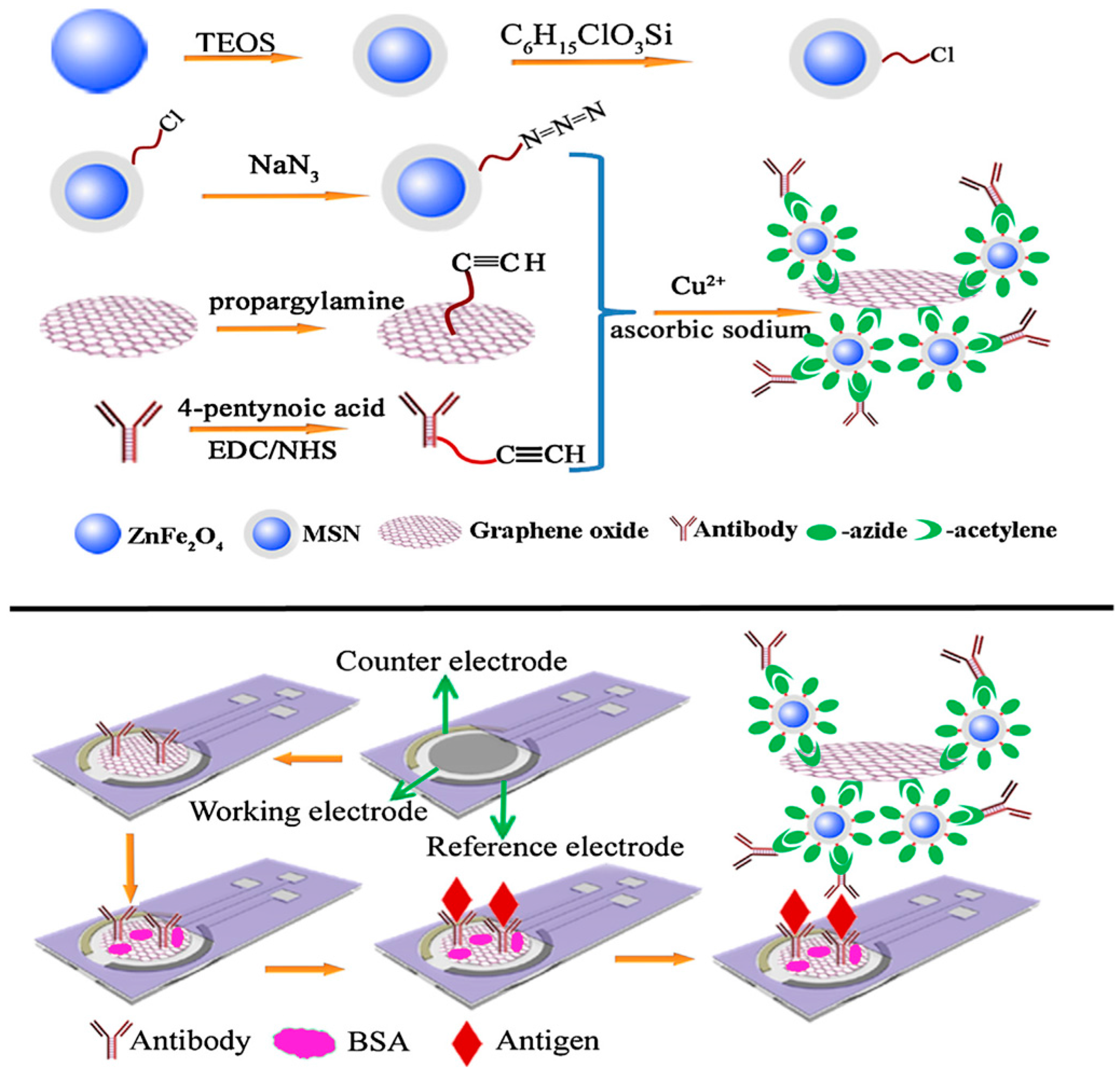

| Electrode | Type of Carbon Nanomaterial | Analyte | Sample | Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Au electrode | PAMAM G4-MWCNTs | human cellular prions protein (PrPC) | spiked blood plasma | CV | 1 pM–10 μM | 0.5 pM | [26] |
| Au electrode | MWCNTs-PPy-PAMAM G4 | rpoB gene of Mycobacterium tuberculosis | real PCR samples | SWV | 1 fM–10 pM (15 nts synthetic target), 1–100 fM (75 nts synthetic targets) | 0.3 fM (for both target DNAs) | [8] |
| Au electrode | MWCNTs | Hepatitis C viral DNA and Mycobacterium tuberculosis genomic DNA | Mycobacterium tuberculosis (H37Rv) rpoB allele in DNA extracted from clinical isolates | EIS | 0.1 fM–1 pM (synthetic oligonucleotide from Hepatitis C virus) | 7 fM | [16] |
| GCE | GO | cancer-related BRCA1 gene | - | chronoamperometry | 1 fM–1 nM | 1 fM | [18] |
| GCE | graphene/AuNR/PT | HPV DNA | serum samples | DPV | 1.0 × 10−13–1.0 × 10−10 M | 4.03 × 10−14 M | [10] |
| CILE | ZrO2/graphene | S. aureus thermonuclease nuc gene | PCR samples | DPV | 1.0 × 10−13–1.0 × 10−6 mol·L−1 | 3.23 × 10−14 M | [15] |
| GCE | SWCNTs/CFGO | Vibrio parahaemolyticus thermolabile hemolysin (tlh) gene | - | DPV | 1 × 10−6–1 × 10−13 mol·L−1 | 7.27 × 10−14 M | [11] |
| GCE | graphene-MSGNs | DNA sequences correlated to Alzheimer, thrombin, ATP | - | DPV | - | - | [21] |
| GCE | GSHs | mutated apolipoprotein E gene associated with Alzheimer’s disease | - | DPV | - | - | [12] |
| Electrode | Analyte | Immunoassay | Technique | Linear Range | LOD | Sample | Reference |
|---|---|---|---|---|---|---|---|
| Cancer biomarkers | |||||||
| AuNPs-HDT-AuNPs/MW-CILE | HER2 | Direct with immobilized anti-HER | EIS | 10–110 ng·mL−1 | 7.4 ng·mL−1 | serum | [43] |
| MWCNTs-ZnONF/GCE | CA-125 | Direct with immobilized anti-CA-125. [Fe(CN)6]3−/4− as the redox probe | DPV | 0.001–1000 U·mL−1 | 0.00113 U·mL−1 | spiked serum | [45] |
| THI/MWCNTs/IL/GCE | PSA | Sandwich with immobilized anti-PSA and HRP-Ab2 | DPV | 0.2–1.0 ng·mL−1 1–40 ng·mL−1 | 20 pg·mL−1 | prostate tissue, serum | [47] |
| MWCNTs/IL/Chit/GCE | PSA | Direct (EIS) with immobilized anti-PSA-AuNPs-PAMAM, or sandwich (DPV) with HRP-Ab2 | EIS DPV | up to 25 ng·mL−1 up to 80 ng·mL−1 | 0.5 pg·mL−1 1 pg·mL−1 | serum | [48] |
| MWCNTs/DAH/GCE | PSA | Sandwich with immobilized anti-PSA and Ab2-AuNPs-FcH | DPV | 0.01–100 ng·mL−1 | 5.4 pg·mL−1 | spiked human serum | [49] |
| SWCNTs/GCE | NSE | Indirect competitive with immobilized NSE, anti-NSE, and AP-anti-IgG/AuNPs | DPV | 0.1–2000 ng·mL−1 | 0.033 ng·mL−1 | clinical serum specimens | [51] |
| SWCNTs/Nafion/Fe(OH)x/PG | IL-6 | Sandwich with immobilized anti-IL6 and multi-HRP-MWCNTs-labeled-Ab2 | amperom | 0.5–30 pg·mL−1 | 0.5 pg·mL−1 | calf serum | [52] |
| SWCNTs/TMCS-MPS/graphene/GCE | AFP | Direct with immobilized anti-AFP. FCA as the redox probe | DPV | 0.1–100 ng·mL−1 | 0.06 ng·mL−1 | serum | [53] |
| Au/PDDA/MWCNTs/(PSS/PDDA/MWCNTs)2/GCE | CEA | Direct with immobilized anti-CEA. [Fe(CN)6]3−/4− as the redox probe | CV | 0.1–2.0; 2.0–160 ng·mL−1 | 0.06 ng·mL−1 | serum | [55] |
| pTHI/PA6/MWCNTs/GCE | p53 | Sandwich with immobilized anti-p53 and HRP-Ab2 | DPV | 0.002–2 ng·mL−1 | <1 pg·mL−1 | - | [57] |
| Cardiovascular biomarkers | |||||||
| PtNPs(MPA)/G-MWCNTs | cTn1 | Direct with immobilized anti-cTn1 | EIS | 0.001–10 ng·mL−1 | 1.0 pg·mL−1 | human serum | [61] |
| SU-8/MWCNTs/GCE | Mb, cTn1, CK-MB | Direct with immobilized anti-Mb, or anti-cTnI, or anti-CK-MB | EIS | 1–50 (Mb); 0.1–10 (cTnI); 10–10,000 (CK-MB) ng·mL−1 | 0.1 (Mb; cTnI); 1 ng·mL−1 (CK-MB) | - | [67] |
| CNTs/PEI/AuE | cTnT | Sandwich with immobilized anti-cTnT and Ab2-HRP | amperom. | 0.1–10 ng·mL−1 | 0.033 ng·mL−1 | human serum | [62] |
| NH2-MWCNTs/SPE | cTnT | Direct with immobilized anti-cTnT. [Fe(CN)6]3−/4− as the redox probe | DPV | 0.0025–0.5 ng·mL−1 | 0.0035 ng·mL−1 | serum | [63] |
| MWCNTs/SPE | Mb | Direct with immobilized anti-Mb | EIS | 0.1–90 ng·mL−1 | 0.08 ng·mL−1 | serum | [70] |
| MWCNTs/THI/AuNPs/GCE | MPO | Direct with immobilized anti-MPO. THI as the redox probe. | CV | 2.5–125 ng·mL−1 | 1.425 ng·mL−1 | human serum | [65] |
| poPD-MWCNTs-IL/ITO | MPO | Direct with immobilized anti-MPO | amperom. | 0.2–23.4 ng·mL−1 23.4–300 ng·mL−1 | 0.05 ng·mL−1 | human serum | [66] |
| Cyst/SPAuE | oxLDL | Direct with immobilized anti-oxLDL | SWV EIS | 3–10.5 μg·mL−1 0.5–18.0 μg·mL−1 | 0.22 μg·mL−1 | serum | [68] |
| p-MWCNTs@Chit/Nafion/THI/Au NPs/GCE | netrin 1 | Direct with immobilized anti-netrin 1 | DPV | 0.09–1800 pg·mL−1 | 30 fg·mL−1 | serum | [72] |
| AuNPs-CNTs/AuE | NT-proBNP | Sandwich with immobilized anti-NT-proBNP and HRP-Ab2-AuNCs | CV | 0.02–100 ng·mL−1 | 6 pg·mL−1 | - | [74] |
| Electrode | Analyte | Immunoassay | Technique | Linear Range | LOD | Sample | Reference |
|---|---|---|---|---|---|---|---|
| Cancer biomarkers | |||||||
| Strept-AuNPs-SH-GO/GCE | p53 | Sandwich with immobilized Biotin-anti-p53 and HRP-Ab2 | DPV | 0.2–2 pmol·L−1 2–200 pmol·L−1 | 30 pmol L−1 | human serum | [83] |
| SnO2/AuNPs/rGO/GCE | AFP | Direct with immobilized anti-AFP. [Ru(NH3)6]3+ as the redox probe. | DPV | 0.02–50 ng·mL−1 | 0.01 ng·mL−1 | spiked serum | [79] |
| AuNPs/NB/rGO/GCE | CEA | Direct with immobilized anti-CEA. Nile Blue (NB) as the redox probe | DPV | 0.001–40 ng·mL−1 | 0.00045 ng·mL−1 | spiked serum | [80] |
| GO-SPCE | CA 153 | Sandwich with immobilized anti-CA153 and Ab2-ZnFe2O4/GO | DPV | 10−3–200 U·mL−1 | 2.8 × 10−4 U·mL−1 | spiked serum | [82] |
| 3D-rGO/AuNPs/ | PSA | Direct with immobilized anti-PSA. [Fe(CN)6]3−/4− as the redox probe | CV | up to 10 ng·mL−1 | 0.59 ng·mL−1 | - | [76] |
| APTES/ZrO2-rGO/ITO | CYFRA-21-1 | Direct with immobilized anti-CYFRA-21-1. [Fe(CN)6]3−/4− as the redox probe | DPV | 2–22 ng·mL−1 | 0.122 ng·mL−1 | saliva | [39] |
| Ag/Au–DN–graphene/GCE | CEA | Sandwich with immobilized anti-CEA and Ab2-Ag/Au–DN-graphene | Amperometry | 0.01–1200 ng·mL−1 | 8 pg·mL−1 | spiked human plasma | [75] |
| AuPdPtNPs/rGO/TEPA/GCE | NMP22 | Direct with immobilized anti-NMP22. [Fe(CN)6]3−/4− as the redox probe | DPV | 0.040–20 U·mL−1 | 0.01 U·mL−1 | urine | [78] |
| Cardiovascular biomarkers | |||||||
| PrGO/GCE | cTnI | Direct with immobilized anti-TnI | EIS | 0.1–10 ng·mL−1 | 0.07 ng·mL−1 | - | [88] |
| PtNPs(MPA)/EG/GCE | cTnI | Direct with immobilized anti-TnI | EIS | 0.01–10 ng·mL−1 | 4.2 pg·mL−1 | - | [89] |
| AuNPs-Ph-GO/GCE | cTnI | Direct with immobilized anti-cTnI-Fc-GO. [Fe(CN)6]3−/4− as the redox probe | SWV | 0.05–3 ng·mL−1 | 0.05 ng·mL−1 | spiked serum | [87] |
| PtMPA/GR-MWCNTs/GCE | cTnI | Direct with immobilized anti-TnI | EIS | 0.001–10 ng·mL−1 | 0.001 ng·mL−1 | - | [61] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Diagnostics Strategies with Electrochemical Affinity Biosensors Using Carbon Nanomaterials as Electrode Modifiers. Diagnostics 2017, 7, 2. https://doi.org/10.3390/diagnostics7010002
Campuzano S, Yáñez-Sedeño P, Pingarrón JM. Diagnostics Strategies with Electrochemical Affinity Biosensors Using Carbon Nanomaterials as Electrode Modifiers. Diagnostics. 2017; 7(1):2. https://doi.org/10.3390/diagnostics7010002
Chicago/Turabian StyleCampuzano, Susana, Paloma Yáñez-Sedeño, and José M. Pingarrón. 2017. "Diagnostics Strategies with Electrochemical Affinity Biosensors Using Carbon Nanomaterials as Electrode Modifiers" Diagnostics 7, no. 1: 2. https://doi.org/10.3390/diagnostics7010002