Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer
Abstract
:1. Introduction
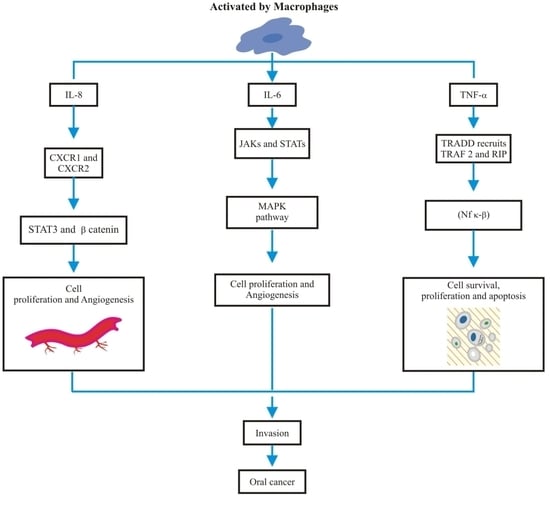
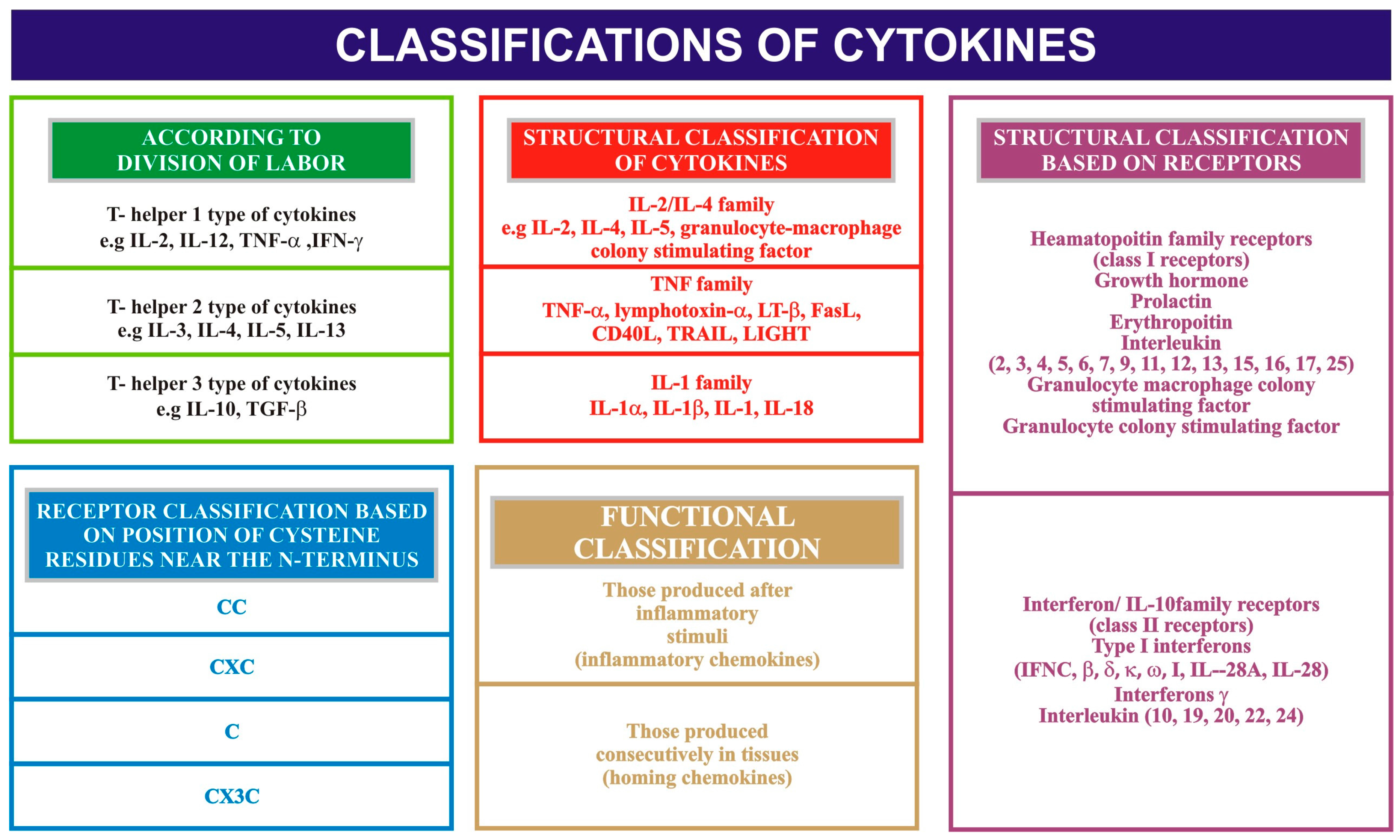
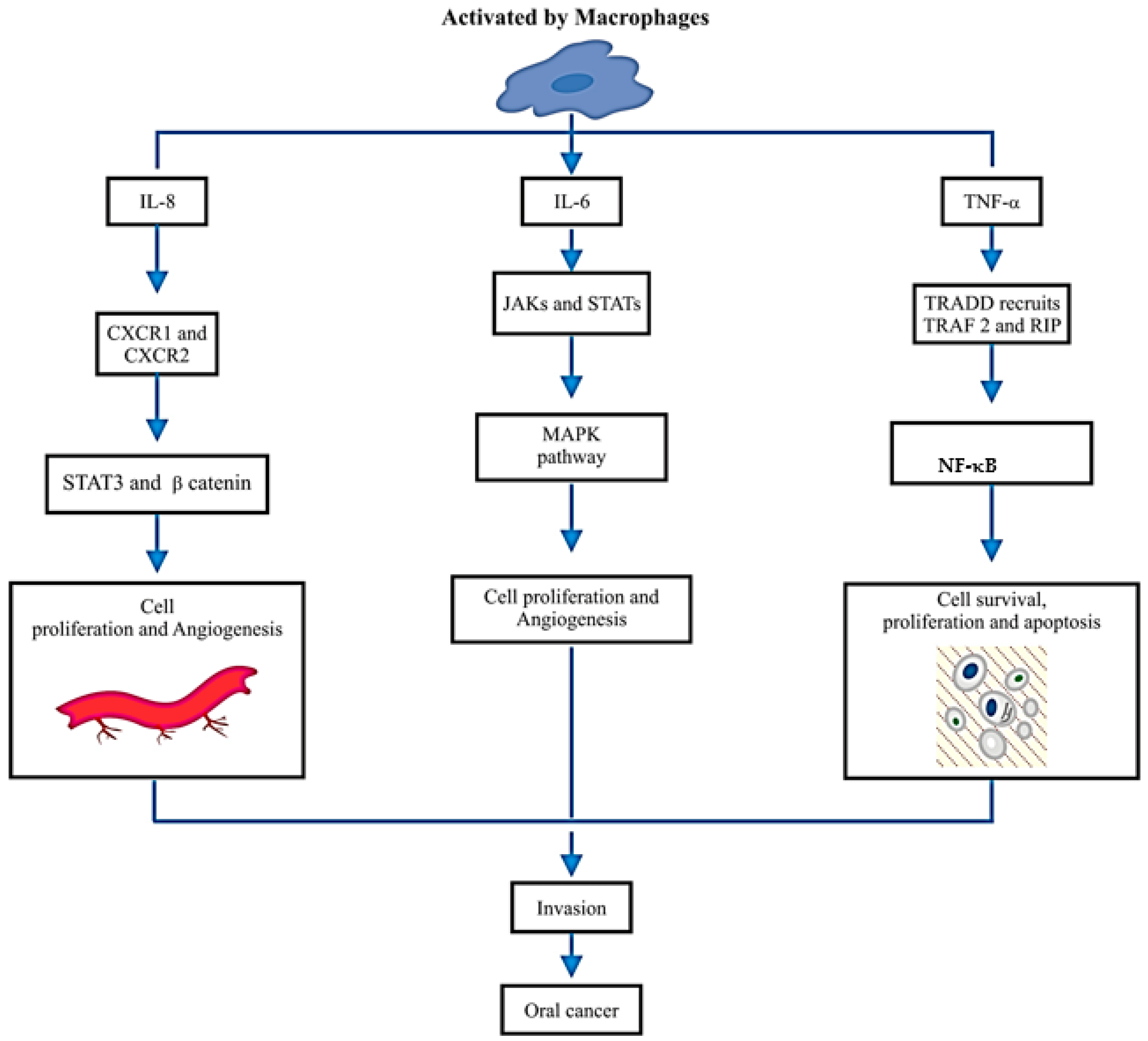
2. Salivary IL-8, IL-6 and TNF-α
3. Salivary IL-8, IL-6 and TNF-α Role in Oral Cancer Diagnosis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baig, M.S.; Bhutto, R.A.; Muhammad, S.; Siddiqui, M.I. Epidemiology of oral cancer in Southern Punjab, Pakistan. Pak. J. Med. Heal. Sci. 2015, 9, 1269–1271. [Google Scholar]
- Ford, P.J.; Farah, C.S. Early detection and diagnosis of oral cancer: Strategies for improvement. J. Cancer Policy 2013, 1, e2–e7. [Google Scholar] [CrossRef]
- Kalavrezos, N.; Scully, C. Mouth Cancer for Clinicians Part 7: Cancer Diagnosis and Pre-treatment Preparation. Dent. Update 2016, 43, 50–54, 57–60 & 63–65. [Google Scholar] [PubMed]
- Zhang, Y.; Sun, J.; Lin, C.-C.; Abemayor, E.; Wang, M.B.; Wong, D.T.W. The emerging landscape of salivary diagnostics. Periodontol 2000 2016, 70, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Gstaiger, M.; Aebersold, R. Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nat. Rev. Genet. 2009, 10, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M.S. Human Gingival Crevicular Fluids (GCF) proteomics: An dverview. Dent. J. 2017, 5, 12. [Google Scholar] [CrossRef]
- Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of salivary proteomics in Oral Squamous Cell Carcinoma (OSCC) detection: An update. Proteomes 2016, 4, E41. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016, 17, E846. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.; Rehman, R.; Rehman, I. Advances of proteomic sciences in dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Punyani, S.R.; Sathawane, R.S. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin. Oral Investig. 2013, 17, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Scarano, E.; Fiorita, A.; Picciotti, P.M.; Passali, G.C.; Calò, L.; Cabras, T.; Inzitari, R.; Fanali, C.; Messana, I.; Castagnola, M.; et al. Proteomics of saliva: Personal experience. Acta Otorhinolaryngol. Ital. 2010, 30, 125–130. [Google Scholar] [PubMed]
- Xiao, H.; Wong, D.T. Proteomics and its applications for biomarker discovery in human saliva. Bioinformation 2011, 5, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Köhler, H.; Topf, H.-G.; Rupprecht, T.; Rauh, M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J. Pharm. Biomed. Anal. 2008, 47, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Rehan, F.; Rabia Sannam Khan, B.; Khurshid, Z.; Mohammed Sohail Memon, M.; Naqvi, S.; Muhammad Sohail Zafar, B. Analysis of resting mouth salivary flow rate and salivary pH of tobacco chewers and smokers. J. Pak. Dent. Assoc. 2016, 25, 158–163. [Google Scholar]
- Khurshid, Z.; Haq, J.A.; Khan, R.S.; Zafar, M.S.; Altaf, M.; Najeeb, S. Human saliva and its role in oral & systemic health. J. Pak. Dent. Assoc. 2016, 25, 170–174. [Google Scholar]
- Collins, T.S.; Lee, L.F.; Ting, J.P. Paclitaxel up-regulates interleukin-8 synthesis in human lung carcinoma through an NF-κB-and AP-1-dependent mechanism. Cancer Immunol. Immunother 2000, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 2016, 6, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Unus, S.; Narasimham; Gunasekaran, N.; Krishnan, R.; Lakshmi, P.; Ramabadran, S. Role of cytokines in oral malignancies. SRM J. Res. Dent. Sci. 2014, 5, 274–279. [Google Scholar] [CrossRef]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- De Larco, J.E.; Wuertz, B.R.K.; Manivel, J.C.; Furcht, L.T. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001, 61, 2857–2861. [Google Scholar] [PubMed]
- Panneer Selvam, N.; Sadaksharam, J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac. J. Clin. Oncol. 2015, 11, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Juretić, M.; Cerović, R.; Belušić-Gobić, M.; Brekalo Pršo, I.; Kqiku, L.; Špalj, S.; Pezelj-Ribarić, S. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol. 2013, 59, 99–102. [Google Scholar]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Karsan, A. Signaling pathways mediated by tumor necrosis factor α. Histol. Histopathol. 2000, 15, 1303–1325. [Google Scholar] [PubMed]
- St John, M.A.R.; Li, Y.; Zhou, X.; Denny, P.; Ho, C.-M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Sinha, U.; et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2004, 130, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. At the crossroads of inflammation and cancer. Cell 2004, 118, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Khyani, I.A.M.; Qureshi, M.A.; Farooq, M.U.; Mirza, T. Molecular diagnosis of oral pre-malignant lesions & oral squamous cell carcinoma in saliva-a breakthrough in pakistan. Inter. J. Endorsing Health Sci. Res. 2014, 2, 108–116. [Google Scholar]
- Hamad, A.-W.R.; Gaphor, S.M.; Shawagfeh, M.T.; Al-talabani, N.G. Study of serum and salivary levels of proinflammatory cytokines, potential biomarkers in the diagnosis of oral Squamous cell carcinoma. Acad. J. Cancer Res. 2011, 4, 47–55. [Google Scholar]
- SahebJamee, M.; Eslami, M.; AtarbashiMoghadam, F.; Sarafnejad, A. Salivary concentration of TNFα, IL1 α, IL6, and IL8 in oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E292–E295. [Google Scholar] [PubMed]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171. [Google Scholar] [CrossRef] [PubMed]
- Brailo, V.; Vučićević-Boras, V.; Cekić-Arambašin, A.; Alajbeg, I.Ž.; Milenović, A.; Lukač, J. The significance of salivary interleukin 6 and tumor necrosis factor α in patients with oral leukoplakia. Oral Oncol. 2006, 42, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Khyani, I.A.M.; Qureshi, M.A.; Mirza, T. Salivary diagnosis of oral pre-neoplastic and oral squamous cell carcinoma in etiologically distinct population of Pakistan. Pak. J. Med. Sci. 2015, 31, 1104–1109. [Google Scholar] [PubMed]

| Refrences | Patients | Sample Type | Sample Collection Time | Type of the Study | Analysing Tools Name | Outcome of the Study | p Value |
|---|---|---|---|---|---|---|---|
| [30] | Patients with newly diagnosed T1 or T2 oral cavity or oropharyngeal histologically confirmed squamous cell carcinoma were recruited for the study. | WMUS | Not mentioned | ECS | PCR, ELISA | Findings indicate that IL-8 in saliva and IL-6 in serum hold promise as biomarkers for OSCC. A saliva-based test could be a cost-effective adjunctive tool in the diagnosis and follow-up of patients with OSCC. | Interleukin-8 was detected at higher concentrations in saliva (p < 01) and IL-6 was detected at higher concentrations in serum of patients with OSCC (p < 0.01). |
| [35] | 54 oral lichen planus, 50 oral leukoplakia, 51 oral submucous fibrosis, and 50 healthy controls. | WMUS | 9:00 and 10:00 a.m. | ECS | ELISA | Salivary and serum IL-8, IL-6, and TNF-α levels might act as diagnostic markers for the detection of oral precancer. | The levels of serum and salivary TNF-α, IL-6, and IL-8 were statistically significantly increased in oral leukoplakia, sub-mucous fibrosis, and lichen planus in contrast to normal healthy subjects (p < 0.05). Serum and salivary correlation analysis revealed strong and highly significant correlations for TNF-α, IL-6, and IL-8 in all groups (r = 0.72–0.82, p < 0.05). |
| [34] | Nine patients with oral squamous cell carcinomas and healthy controls. | WMUS | 9:00 and 11:00 a.m. | ECS | ELISA | Results shows that more studies are needed to accept the utility of these cytokines in predicting or diagnosis of oral squamous cell carcinoma or evaluation of treatment. | The concentration of salivary tumor necrosis factor α, interleukin-1α and 8 in case group was higher than control group, but it was not statistically significant (p > 0.05). |
| [12] | Oral pre-cancer and oral squamous cell carcinoma (OSCC) patients were compared with healthy controls. | WMUS | Not mentioned | ECS | ELISA | Results suggested that salivary IL-8 can be utilized as a potential biomarker for OSCC. Salivary IL- 8 was found to be non-conclusive for oral pre-malignancy in this preliminary study. | The levels of salivary IL-8 were found to be significantly elevated in patients with OSCC as compared to the pre-cancer group (p < 0.0001) and healthy controls (p < 0.0001). However, the difference in salivary IL-8 concentrations among the pre-cancer group and controls was not statistically significant. |
| [33] | 50 patients in total, with 30 diagnosed with OSCC and 20 healthy controls. | Serum and salivary analysis | 10 a.m.–1 p.m. | ECS | ELISA | Salivary IL-1α and Granulocyte macrophage colony stimulating factor GM-CSF was useful in the diagnosis of OSCC patients. Serum IL-6 was more useful in the diagnosis of OSCC patients than salivary IL-6. Serum and salivary IL-8 were very useful in the diagnosis of OSCC patients and for identifying between OSCC patients and the control group. | Serum IL-6 and IL-8 levels were detected at higher concentrations in patients with OSCC than in the control group (p < 0.001). |
| [37] | 105 cases total; A = PMD, B = OSSC, C = Healthy controls; 35 in each group | Salivary analysis | Not mentioned | ECS | ELISA | The values were found to be consistently raised in groups A and B. | Statistically significant association between the groups with regards to IL-8 levels. |
| [32] | 105 cases total; A = PMD, B = OSSC, C = Healthy controls; 35 in each group | Salivary analysis | Not mentioned | ECS | ELISA | The values were found to be consistently raised in groups A and B. | Statistically significant association between the groups with regards to IL-8 levels. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. https://doi.org/10.3390/diagnostics7020021
Sahibzada HA, Khurshid Z, Khan RS, Naseem M, Siddique KM, Mali M, Zafar MS. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics. 2017; 7(2):21. https://doi.org/10.3390/diagnostics7020021
Chicago/Turabian StyleSahibzada, Haafsa Arshad, Zohaib Khurshid, Rabia Sannam Khan, Mustafa Naseem, Khalid Mahmood Siddique, Maria Mali, and Muhammad Sohail Zafar. 2017. "Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer" Diagnostics 7, no. 2: 21. https://doi.org/10.3390/diagnostics7020021