Ten Important Considerations for Ovarian Cancer Screening
Abstract
:1. Introduction
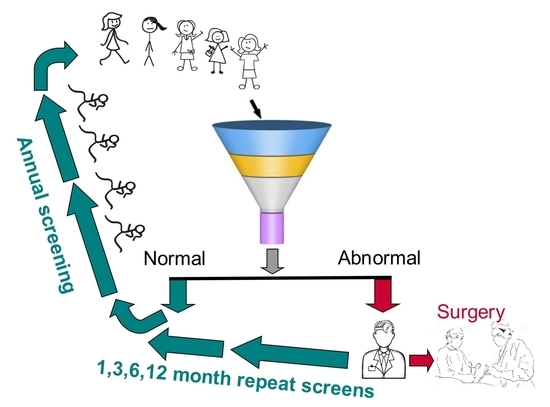
2. The Bare-Bones Basics of Screening
3. Collecting Evidence to Examine Screening Effectiveness—Perspective Analysis for a Prospective Screening Trial
3.1. Consideration 1
Deciding on the Number of Individuals to Be Screened
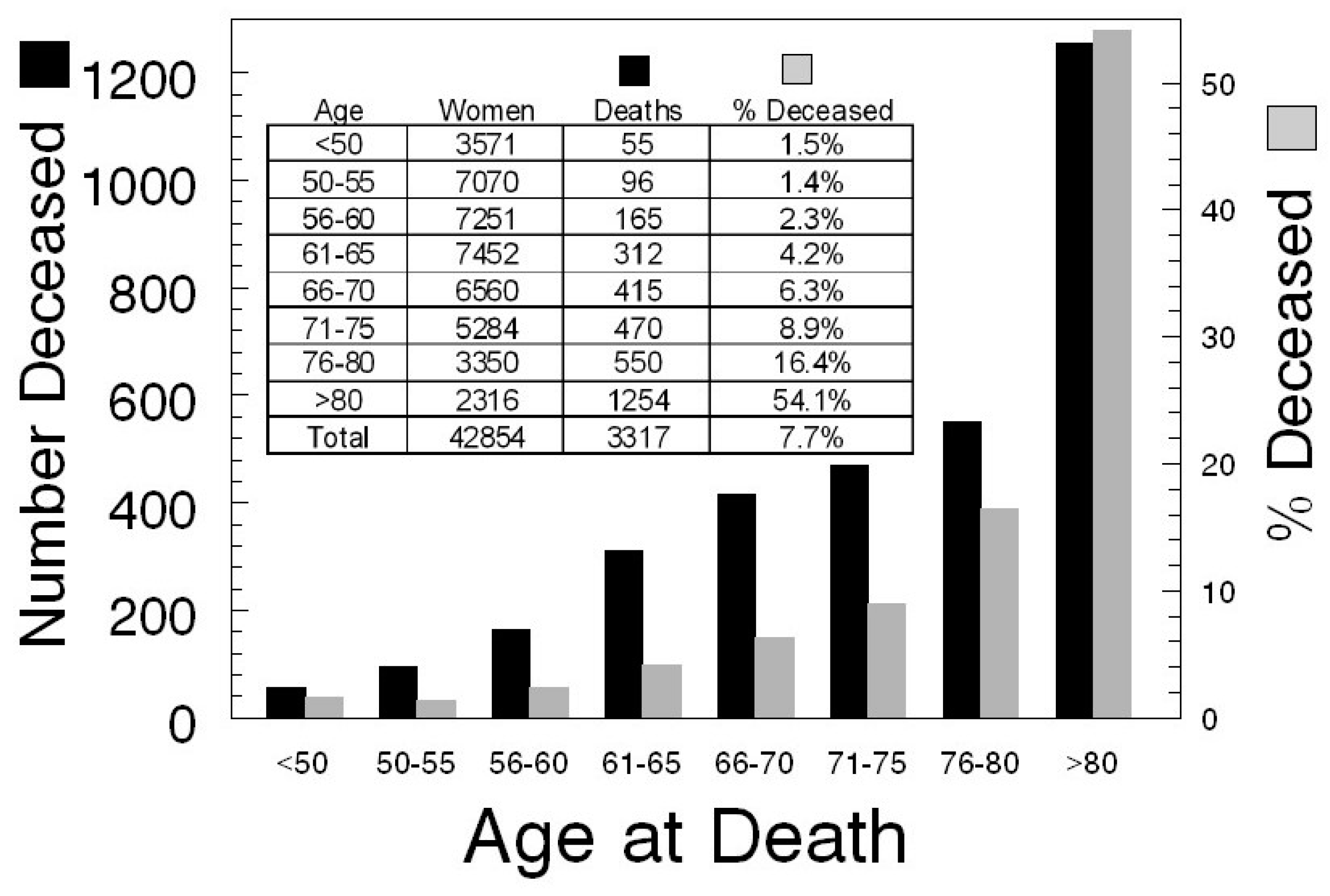
3.2. Consideration 2
Anticipating Screening Group Reductions due to Death
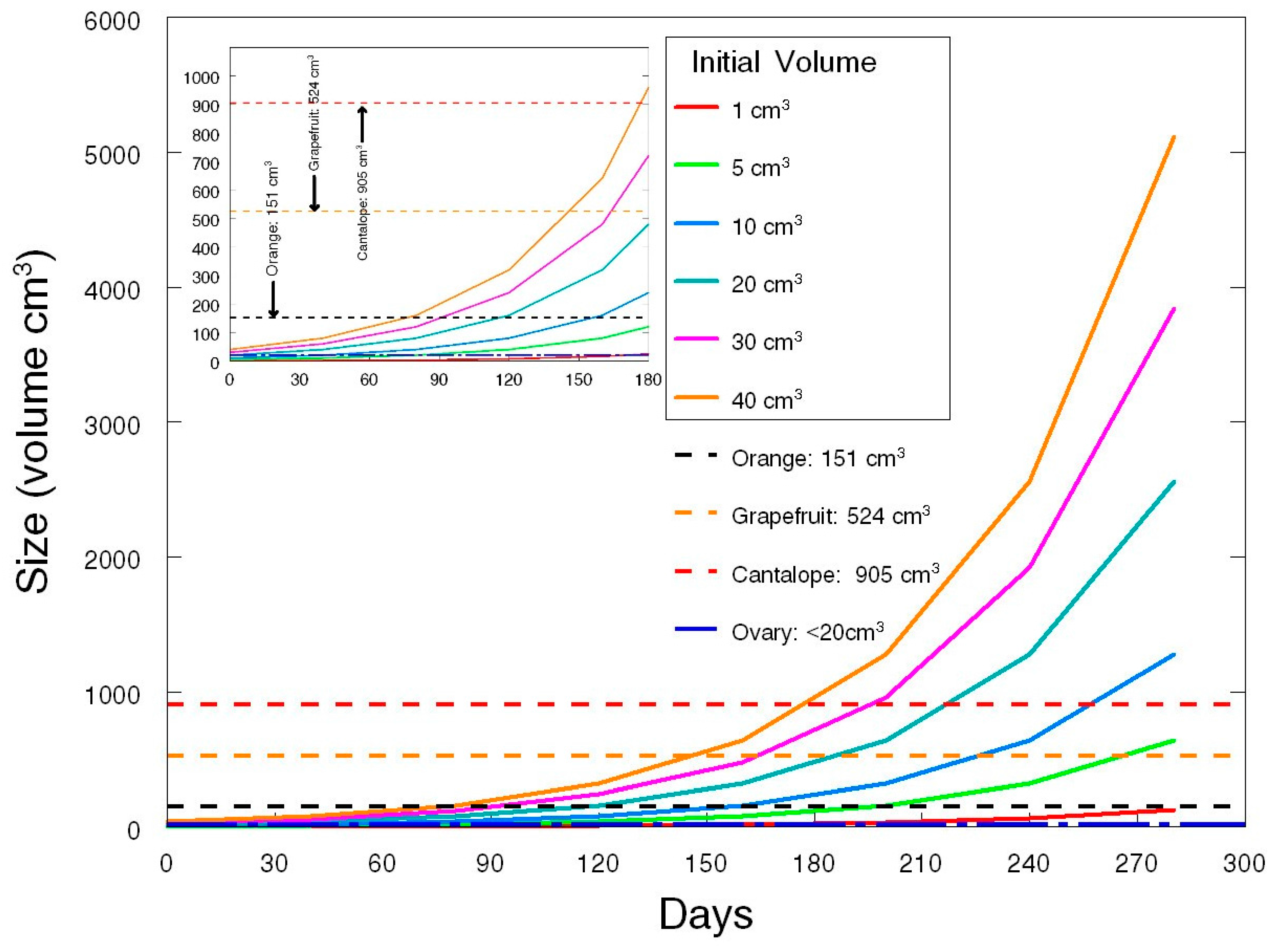
3.3. Consideration 3
Deciding on the Duration and Frequency of Screening
3.4. Consideration 4
Deciding on an Appropriate Follow-Up Period after Screening
3.5. Consideration 5
Deciding on Time to Surgery When Malignancy Is Suspected
3.6. Consideration 6
Deciding on How Screen-Detected Ovarian Cancers Are Treated and by Whom
3.7. Consideration 7
Deciding on How to Treat the Data of Enrolled Participants
3.8. Consideration 8
Deciding on the Most Appropriate Way to Assign Disease-Specific Death
3.9. Consideration 9
Deciding How to Avoid Biases Caused by Enrollments that Attract Participants with Late-Stage Disease Who Are either Symptomatic or Disposed by Factors that Are Genetic, Environmental or Social
3.10. Consideration 10
Deciding Whether the Screening Tool or a Screening Process Is Being Tested
4. Conclusions
Conflicts of Interest
References
- Handford, M. Where’s Waldo? Candlewick Press: Somerville, MA, USA, 2012. [Google Scholar]
- Daoud, E.; Bodor, G. CA-125 concentrations in malignant and nonmalignant disease. Clin. Chem. 1991, 37, 1968–1974. [Google Scholar] [PubMed]
- Nagell, J.R., Jr.; Miller, R.W.; Desimone, C.P.; Ueland, F.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E. Revised standards for statistical evidence. Proc. Natl. Acad. Sci. USA 2013, 110, 19313–19317. [Google Scholar] [CrossRef] [PubMed]
- Gaudart, J.; Huiart, L.; Milligan, P.J.; Thiebaut, R.; Giorgi, R. Reproducibility issues in science, is p value really the only answer? Proc. Natl. Acad. Sci. USA 2014, 111, E1934. [Google Scholar] [CrossRef] [PubMed]
- Gelmana, A.; Rober, C.P. Revised evidence for statistical standards. Proc. Natl. Acad. Sci. USA 2014, 111, E1933. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, H. Theory of Probability; Oxford University Press Inc.: New York, NY, USA, 1961. [Google Scholar]
- Pericchi, L.; Pereira, C.A.; Pérez, M.E. Adaptive revised standards for statistical evidence. Proc. Natl. Acad. Sci. USA 2014, 111, E1935. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Stokes, L. Sample size calculation for a hypothesis test. JAMA 2014, 312, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality—The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J. Ovarian cancer screening effectiveness: A realization from the UK Collaborative Trial of Ovarian Cancer Screening. Women Health 2016, 12, 5–475. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; Ueland, F.R.; Miller, R.W.; Ubellacker, J.M.; Desimone, C.P.; Elder, J.; Hoff, J.; Baldwin, L.; Kryscio, R.J.; Nagell, J.R., Jr. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet. Gynecol. 2013, 122 Pt 1, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nagell, J.R., Jr.; Miller, R.W. Evaluation and Management of Ultrasonographically Detected Ovarian Tumors in Asymptomatic Women. Obstet. Gynecol. 2016, 127, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Miller, M.C.; Disilvestro, P.; Landrum, L.M.; Gajewski, W.; Ball, J.J.; Skates, S.J. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet. Gynecol. 2011, 118, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.W.; Pavlik, E.J.; Long, A.; Miller, R.W.; DeSimone, C.P.; Hoff, J.T.; Ueland, W.R.; Kryscio, R.J.; van Nagell, J.R., Jr.; Ueland, F.R. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol. Oncol. 2014, 135, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Han, L.Y.; Karavasilis, V.; Hagen, T.V.; Nicum, S.; Thomas, K.; Harrison, M.; Papadopoulos, P.; Blake, P.; Barton, D.P.; Gore, M.; et al. Doubling time of serum CA125 is an independent prognostic factor for survival in patients with ovarian cancer relapsing after first-line chemotherapy. Eur. J. Cancer 2010, 46, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Willemse, P.H.; Aalders, J.G.; de Bruyn, H.W.; Mulder, N.H.; Sleijfer, D.T.; de Vries, E.G. CA-125 in ovarian cancer: Relation between half-life, doubling time and survival. Eur. J. Cancer 1991, 27, 993–995. [Google Scholar] [CrossRef]
- Engelen, M.J.; Kos, H.E.; Willemse, P.H.; Aalders, J.G.; de Vries, E.G.; Schaapveld, M.; Otter, R.; van der Zee, A.G. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer 2006, 106, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Earle, C.C.; Schrag, D.; Neville, B.A.; Yabroff, K.R.; Topor, M.; Fahey, A.; Trimble, E.L.; Bodurka, D.C.; Bristow, R.E.; Carney, M.; et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J. Natl. Cancer Inst. 2006, 98, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Zahurak, M.L.; Diaz-Montes, T.P.; Giuntoli, R.L.; Armstrong, D.K. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol. Oncol. 2009, 115, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Palis, B.E.; Chi, D.S.; Cliby, W.A. The National Cancer Database report on advanced-stage epithelial ovarian cancer: Impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol. Oncol. 2010, 118, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Chang, J.; Ziogas, A.; Anton-Culver, H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet. Gynecol. 2013, 121, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Chang, J.; Ziogas, A.; Randall, L.M.; Anton-Culver, H. High-volume ovarian cancer care: Survival impact and disparities in access for advanced-stage disease. Gynecol. Oncol. 2014, 132, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cliby, W.A.; Powell, M.A.; Al-Hammadi, N.; Chen, L.; Philip, M.J.; Roland, P.Y.; Mutch, D.G.; Bristow, R.E. Ovarian cancer in the United States: Contemporary patterns of care associated with improved survival. Gynecol. Oncol. 2015, 136, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Chang, J.; Ziogas, A.; Campos, B.; Chavez, L.R.; Anton-Culver, H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J. Am. Coll. Surg. 2015, 220, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, T.H.; Suh, D.H.; Kim, J.W.; Kim, H.S.; Chung, H.H.; Park, N.H.; Song, Y.S.; Kang, S.B. Impact of guideline adherence on patient outcomes in early-stage epithelial ovarian cancer. Eur. J. Surg. Oncol. 2015, 41, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, F.; Heintz, A.P.; Witteveen, P.O.; van der Heiden-van der Loo, M.; Coebergh, J.W.; van der Graaf, Y. Specialized care and survival of ovarian cancer patients in The Netherlands: Nationwide cohort study. J. Natl. Cancer. Inst. 2008, 100, 399–406. [Google Scholar] [CrossRef] [PubMed]
- UK Collaborative Trial of Ovarian Cancer Screening. Available online: http://www.isrctn.com/ISRCTN22488978 (accessed on 11 April 2017).
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Clinical fficacy of Ovarian Cancer Screening. J. Cancer 2016, 7, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Supplimentary Appenidix to Reference 9: Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A Randomised Controlled Trial. Available online: http://www.thelancet.com/cms/attachment/2049825434/2058773146/mmc1.pdf (accessed on 11 April 2017).
- Miller, A.B.; Yurgalevitch, S.; Weissfeld, J.L. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin. Trials 2000, 231 (Suppl. 6), 400S–406S. [Google Scholar] [CrossRef]
- Pavlik, E.J.; Nagell, J.R., Jr. Early detection of ovarian tumors using ultrasound. Women Health 2013, 9, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, A.; Richards, J.L.; Glanz, J.; Salmon, D.A.; Omer, S.B. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine 2013, 31, 4293–4304. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Brown, D.R.; Carmody, D.P.; Fogarty, S. HPV Vaccination Completion and Compliance with Recommended Dosing Intervals Among Female and Male Adolescents in an Inner-City Community Health Center. J. Community Health 2015, 40, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.N.; Lindsay, F.S.M.; Philpott, S.; Manchanda, R.; Burnell, M.; Badman, P.; Hadwin, R.; Rizzuto, I.; Benjamin, E.; Singh, N.; et al. Evidence of Stage Shift in Women Diagnosed with Ovarian Cancer During Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J. Clinl. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, Å.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Shenton, A.; Woodward, E.; Lalloo, F.; Howell, A.; Maher, E.R. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a clinical cancer genetics service setting: Risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J. Natl. Cancer Inst. 2015, 107, djv214. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dicks, E.; Ramus, S.J.; Tyrer, J.P.; Intermaggio, M.P.; Hayward, J.; Edlund, C.K.; Conti, D.; Harrington, P.; Fraser, L.; et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J. Clin. Oncol. 2015, 33, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Daniels, M. Endometrial and ovarian cancer in women with Lynch syndrome: Update in screening and prevention. Fam. Cancer 2013, 12, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.P. Hereditary breast and ovarian cancer (HBOC): Clinical features and counseling for BRCA1 and BRCA2, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome. Obstet. Gynecol. Clin. N. Am. 2010, 37, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press (US): Washington, DC, USA, 2016. [Google Scholar]
- King, M.C.; Levy-Lahad, E.; Lahad, A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA 2014, 312, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
| PLCO | PLCO | PLCO |
|---|---|---|
| Death due to ovarian cancer | The disease process and/or associated treatments initiated or sustained a chain of events causally responsible for death | Identify other underlying cause of death |
| Annual update questionnaire | Periodic | |
| Population-based cancer registries | Whenever possible | |
| Linkage to National Death Index | Periodic | |
| Obtained diagnostic medical records: Abstracted by registrars: stage, histology , grade, and treatment | Reviewers blinded to participation in screened vs. unscreened arm | Identify next of kin and personal physician |
| Underlying cause of death: first 2 years | Death certificate & relevant determinations underlying cause of death | Potential, ovarian cancer deaths, deaths of unknown or uncertain deaths were reviewed by at least 1 member of a panel of expertise (2 reviewers with discrepancies decided by a third) |
| Underlying cause of death: after year 2 | Primary reviewer considered records without access to death certificate | If primary review disagreed with death certificate, a second expert reviewed record & death certificate. Disagreement triggered another independent review which led to a resolution by meeting or teleconference |
| Attempt to collect identical death information from both screen-detected and non-screen detected cancers | Screen-detected cancers will have more extensive information collected | Less information for both unscreened group participants & screened false positives |
| UKCTOCS | UKCTOCS | UKCTOCS |
|---|---|---|
| Direct communication with participants | ||
| Postal follow-up questionnaires | 3–5 years after randomization | |
| Diagnosis: England & Wales | Linked by NHS number to the Health & Social Care Information Center, the National Cancer Intelligence Network, Hospital Episodes Statistics | Cancer & death registrations |
| Diagnosis: Northern Ireland | Central Services Agency and the Northern Ireland Cancer Registry | Cancer & death registrations |
| Surgery outside the trial | Hospital Episodes Statistical records | |
| Underlying cause of death | Outcomes review committee (2 pathologists & 2 gynecological oncologists) | Final diagnosis based on algorithm: disease progression, (new lesions or increase in size of original lesions by imaging, clinical worsening, or rising biomarkers) |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlik, E.J. Ten Important Considerations for Ovarian Cancer Screening. Diagnostics 2017, 7, 22. https://doi.org/10.3390/diagnostics7020022
Pavlik EJ. Ten Important Considerations for Ovarian Cancer Screening. Diagnostics. 2017; 7(2):22. https://doi.org/10.3390/diagnostics7020022
Chicago/Turabian StylePavlik, Edward J. 2017. "Ten Important Considerations for Ovarian Cancer Screening" Diagnostics 7, no. 2: 22. https://doi.org/10.3390/diagnostics7020022