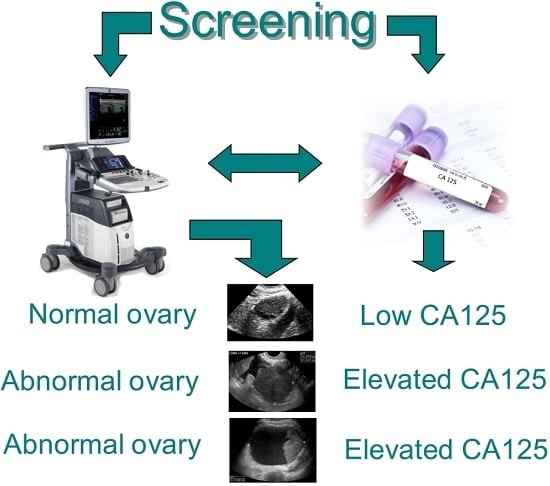
Ultrasound Monitoring of Extant Adnexal Masses in the Era of Type 1 and Type 2 Ovarian Cancers: Lessons Learned From Ovarian Cancer Screening Trials
Abstract
:1. Introduction
2. Type 1 and Type 2 Ovarian Cancers Found in Ultrasound Imaging
2.1. Summary of Information from Recent Prospective Ovarian Cancer Screening Trials
2.2. Can Type 2 Ovarian Cancers Be Detected by Ultrasound?
3. Risk of Ovarian Cancer When There Is an Adnexal Mass
3.1. The Risk Profile for Abnormal Ultrasound Findings
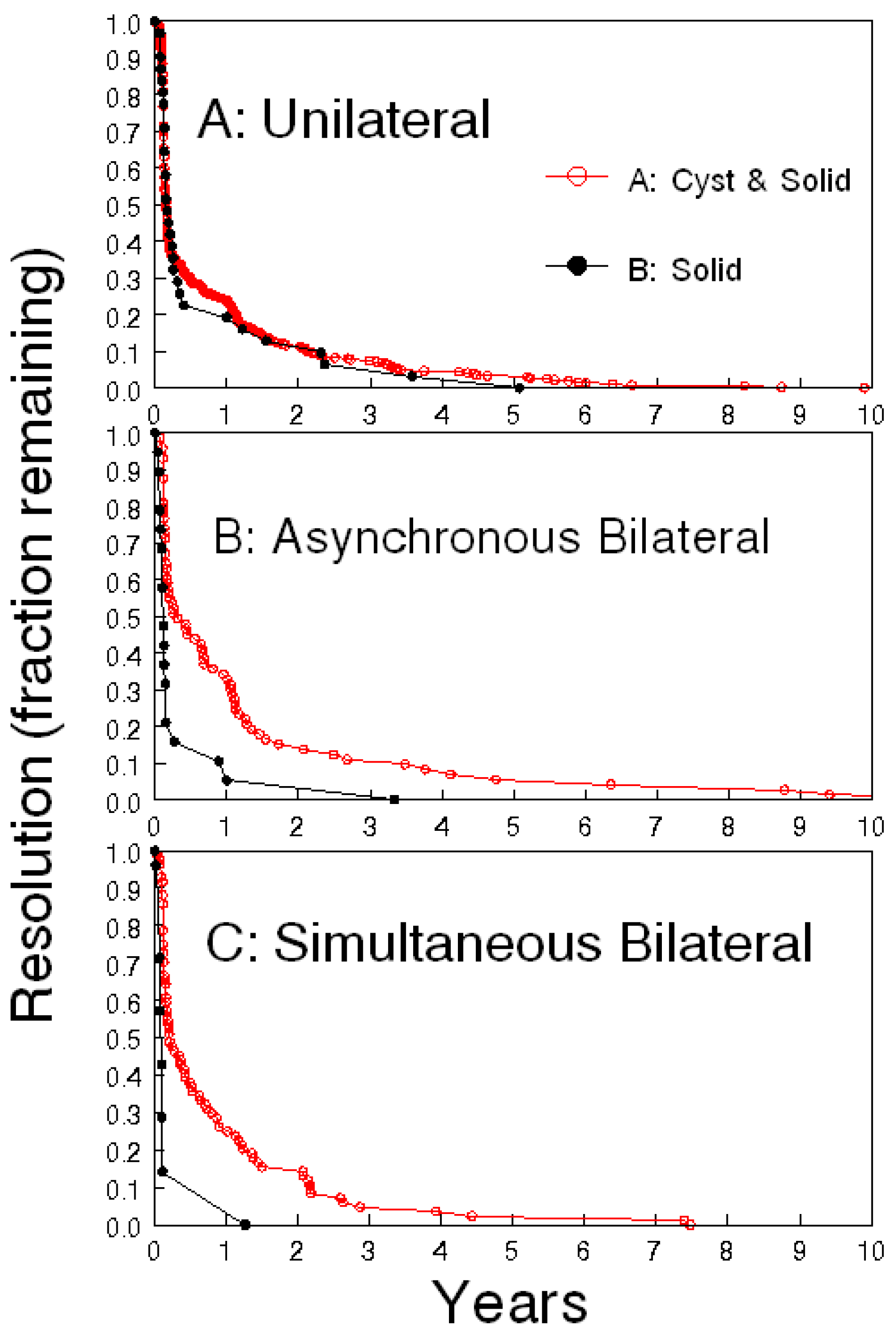
3.2. Benefit of Serial Ultrasound Follow-Up
4. Subjectivity
4.1. Does Stability Over Time Argue Against Malignancy?
4.2. The Conundrum of Ultrasound: Subjectivity and Technical Considerations
5. Ovarian Mass Ultrasound Morphology
5.1. Malignant Degeneration of Benign Masses
5.2. Psychosocial Elements in Prospective Ovarian Cancer Screening Trials
6. Executive Summary of What We Already Know
- (1)
- there are benefits in ultrasound monitoring of persisting indeterminate masses;
- (2)
- resolution of sonographic abnormality defines benign status;
- (3)
- stability over time may not equate with benign status particularly for Type 1 tumors;
- (4)
- for certain types of tumors benign lesions are precursors of malignant lesions;
- (5)
- repeated ultrasound monitoring does not negatively impact psychosocial well-being.
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute: Atlanta, 2016. Available online: www.cdc.gov/uscs (accessed on 25 April 2017).
- American Cancer Society Surveillance Research 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044512.pdf (accessed on 25 April 2017).
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer Statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Van Nagell, J.R., Jr.; Miller, R.W.; DeSimone, C.P.; Ueland, F.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef]
- Salani, R.; Bristow, R.E. Surgical management of epithelial ovarian cancer. Clin. Obstet. Gynecol. 2013, 55, 75–95. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force. Final Recommendation Statement Ovarian Cancer: Screening. September 2012. Available online: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening (accessed on 29 December 2016).
- Hartge, P.; Hayes, R.; Reding, D.; Sherman, M.E.; Prorok, P.; Schiffman, M.; Buys, S. Complex ovarian cysts in postmenopausal women are not associated with ovarian cancer risk factors: Preliminary data from the prostate, lung, colon, and ovarian cancer screening trial. Am. J. Obstet. Gynecol. 2000, 183, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.L.; Ueland, F.R.; Land, G.L.; DePriest, P.D.; Gallion, H.H.; Kryscio, R.J.; van Nagell, J.R., Jr. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol. Oncol. 1998, 69, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Modesitt, S.C.; Pavlik, E.J.; Ueland, F.R.; DePriest, P.D.; Kryscio, R.J.; Nagell, J.R., Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet. Gynecol. 2003, 102, 594–599. [Google Scholar] [CrossRef]
- Saunders, B.A.; Podzielinski, I.; Ware, R.A.; Goodrich, S.; Desimone, C.P.; Ueland, F.R.; Seamon, L.; Ubellacker, J.; Pavlik, E.J.; Kryscio, R.J.; et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol. Oncol. 2010, 118, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Apostolidou, S.; Burnell, M.; Campbell, S.; Habib, M.; Gentry-Maharaj, A.; Amso, N.; Seif, M.W.; Fletcher, G.; Singh, N.; et al. Risk of epithelial ovarian cancer in asymptomatic women with ultrasound-detected ovarian masses: A prospective cohort study within the UK collaborative trial of ovarian cancer screening (UKCTOCS). Ultrasound Obstet. Gynecol. 2012, 40, 338–344. [Google Scholar] [CrossRef]
- Ormsby, E.L.; Pavlik, E.J.; Van Nagell, J.R. Ultrasound follow up of an adnexal mass has the potential to save lives. Am. J. Obstet. Gynecol. 2015, 213, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Brown, D.L.; Andreotti, R.F.; Benacerraf, B.; Benson, C.B.; Brewster, W.R.; Coleman, B.; DePriest, P.; Doubilet, P.M.; Goldstein, S.R.; et al. Society of Radiologists in Ultrasound. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010, 26, 121–131. [Google Scholar]
- Glanc, P.; Benacerraf, B.; Bourne, T.; Brown, D.; Coleman, B.; Crum, C.; Dodge, J.; Levine, D.; Pavlik, E.; Timmerman, D.; et al. First International Consensus Report on Adnexal Masses: Management Recommendations. J. Ultrasound Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Olivia, D.E. Precursors and pathogenesis of ovarian carcinoma. Pathology 2013, 45, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih, I.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R.; Shih, I. Ovarian cancer. Ann. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Senturk, E.; Cohen, S.; Dottino, P.R.; Senturk, E.; Cohen, S.; Dottino, P.R.; Martignetti, J.A. A critical re-appraisal of BRCA1 methylation studies in ovarian cancer. Gynecol. Oncol. 2010, 119, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Cabrero, I.; Navani, S.S.; Young, R.H.; Scully, R.E. Tumors of the Fimbriated End of the Fallopian Tube: A Clinicopathologic Analysis of 20 Cases, Including Nine Carcinomas. Int. J. Gynecol. Pathol. 1997, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Colgan, T.J.; Murphy, J.; Cole, D.E.; Narod, S.; Rosen, B. Occult carcinoma in prophylactic oophorectomy specimens: Prevalence and association with BRCA germline mutation status. Am. J. Surg. Pathol. 2001, 25, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Cass, I.; Holschneider, C.; Datta, N.; Barbuto, D.; Walts, A.E.; Karlan, B.Y. BRCA-mutation-associated fallopian tube carcinoma: A distinct clinical phenotype? Obstet. Gynecol. 2005, 106, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 2006, 30, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial Carcinoma of the Fimbriae and Pelvic Serous Carcinoma: Evidence for a Causal Relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.R.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Guth, U.; Huang, D.J.; Bauer, G.; Stieger, M.; Wight, E.; Singer, G. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer 2007, 110, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Birrer, M.J.; Sood, A.K. Early Events in the Pathogenesis of Epithelial Ovarian Cancer. J. Clin. Oncol. 2008, 26, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.P.; Mckeon, F.D.; Xian, X. The oviduct and ovarian cancer: Causality, clinical implications, and “targeted prevention”. Clin. Obstet. Gynecol. 2012, 55, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.C.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading ovarian serous carcinoma using a two-tier system. Am. J. Surg. Pathol. 2004, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Pavlik, E.J.; Ueland, F.R.; Miller, R.W.; Ubellacker, J.M.; Desimone, C.P.; Elder, J.; Hoff, J.; Baldwin, L.; Kryscio, R.J.; van Nagell, J.R., Jr. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet. Gynecol. 2013, 122, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Van Nagell, J.R., Jr.; Miller, R.W. Evaluation and Management of Ultrasonographically Detected Ovarian Tumors in Asymptomatic Women. Obstet. Gynecol. 2016, 127, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Van Nagell, J.R., Jr.; Hoff, J.T. Transvaginal ultrasonography in ovarian cancer screening: Current perspectives. Int. J. Womens Health. 2014, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.W.; Pavlik, E.J.; Long, A.; Miller, R.W.; Desimone, C.P.; Hoff, J.T.; Ueland, W.R.; Kryscio, R.J.; Nagell, J.R., Jr.; Ueland, F.R. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol. Oncol. 2014, 135, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kaijser, J.; van Gorp, T.; van hoorde, k.; van Holsbeke, C.; Sayasneh, A.; Vergote, I.; Bourne, T.; Timmerman, D.; van Calster, B. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to assess the risk of malignancy in women with an adnexal mass. Gynecol. Oncol. 2013, 129, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Thorpe, J.D.; Bergan, L.A.; Forrest, R.M.; Kampani, A.V.; Scholler, N.; O’Briant, K.C.; Anderson, G.L.; Cramer, D.W.; Berg, C.D.; et al. Potential role of HE4 in multimodal screening for epithelial ovarian cancer. J. Natl. Cancer Inst. 2011, 103, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; MacLaughlan, S.; Bast, R.C. Current state of biomarker development for clinical application in epithelial ovarian cancer. Gynecol. Oncol. 2010, 116, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Skates, S.J.; Mai, P.; Horick, N.K.; Piedmonte, M.; Drescher, C.W.; Isaacs, C.; Armstrong, D.K.; Buys, S.S.; Rodriguez, G.C.; Horowitz, I.R.; et al. Large Prospective Study of Ovarian Cancer Screening in High risk Women: CA-125 Cut-point Defined by Menopausal Status. Cancer Prev. Res. (Phila) 2011, 4, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009, 10, 327–340. [Google Scholar] [CrossRef]
- Havrilesky, L.; Sanders, G.; Kulasingam, S.; Chino, J.; Berchuck, A.; Marks, J.; Evan, R.; Myers, E. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity. Cancer 2010, 117, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.O.; Palmer, C. The Preclinical Natural History of Serous Ovarian Cancer: Defining the Target for Early Detection. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.S.; Gambhir, S.S. Mathematical Model Identifies Blood Biomarker–Based Early Cancer Detection Strategies and Limitations. Sci. Transl. Med. 2011, 3, 109ra116. [Google Scholar]
- Suh-Burgmann, E.; Hung, Y.Y.; Kinney, W. Outcomes from ultrasound follow-up of small complex adnexal masses in women over 50. Am. J. Obstet. Gynecol. 2014, 211, 623.e1–623.e7. [Google Scholar] [CrossRef] [PubMed]
- Yazbek, J.; Raju, K.S.; Ben-Nagi, J.; Holland, T.; Hillaby, K.; Jurkovic, D. Accuracy of ultrasound subjective “pattern recognition” for the diagnosis of borderline ovarian tumors. Ultrasound Obstet. Gynecol. 2007, 29, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Ameye, L.; Franchi, D.; Guerriero, S.; Jurkovic, D.; Savell, L.; Fischerova, D.; Lissoni, A.; van Holsbeke, C.; Fruscio, R.; et al. Risk of malignancy in unilocular cysts: A study of 1148 adnexal masses classified as unilocular cysts at transvaginal ultrasound and review of the literature. Ultrasound Obstet. Gynecol. 2013, 41, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Yancik, R.; Ries, L.G.; Yates, J.W. Ovarian cancer in the elderly: An analysis of surveillance. Am. J. Obstet. Gynecol. 1986, 154, 639–647. [Google Scholar] [CrossRef]
- Pavlik, E.J.; van Nagell, J.R. Early Detection of Ovarian Tumors Using Ultrasound. Womens Health 2013, 9, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Basso, O.; Sampalis, J.; Karp, I.; Martins, C.; Feng, J.; Piedimonte, S.; Quintal, L.; Ramanakumar, A.V.; Takefman, J.; et al. Assessment of symptomatic women for early diagnosis of ovarian cancer: Results from the prospective DOvE pilot project. Lancet Oncol. 2012, 285–291. [Google Scholar] [CrossRef]
- Rossing, M.A.; Wicklund, K.G.; Cushing-Haugen, K.L.; Weiss, N.S. Predictive value of symptoms for early detection of ovarian cancer. J. Natl. Cancer Inst. 2010, 102, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.L.; Nelson, D.F.; Doran, S.; Ueland, F.R.; DeSimone, C.P.; DePriest, P.D.; McDonald, J.M.; Saunders, B.A.; Ware, R.A.; Pavlik, E.J.; et al. Long-Term Survival and Cost of Treatment in Patients with Stage IIIC Epithelial Ovarian Cancer. Curr. Women’s Health Rev. 2009, 5, 44–50. [Google Scholar]
- McColl, S.; Hicks, J.; Craig, L.; Shortreed, J. Environmental Health Risk Management: A Primer for Canadians; Graphic Services University of Waterloo: Waterloo, ON, Canada, 2000. [Google Scholar]
- Zannoni, L.; Savelli, L.; Jokubkiene, L.; Di Legge, A.; Condous, G.; Testa, A.C.; Sladkevicius, P.; Valentin, L. Intra-and interobserver agreement with regard to describing adnexal masses using International Ovarian Tumor Analysis terminology: Reproducibility study involving seven observers. Ultrasound Obstet. Gynecol. 2014, 44, 100–108. [Google Scholar] [CrossRef] [PubMed]
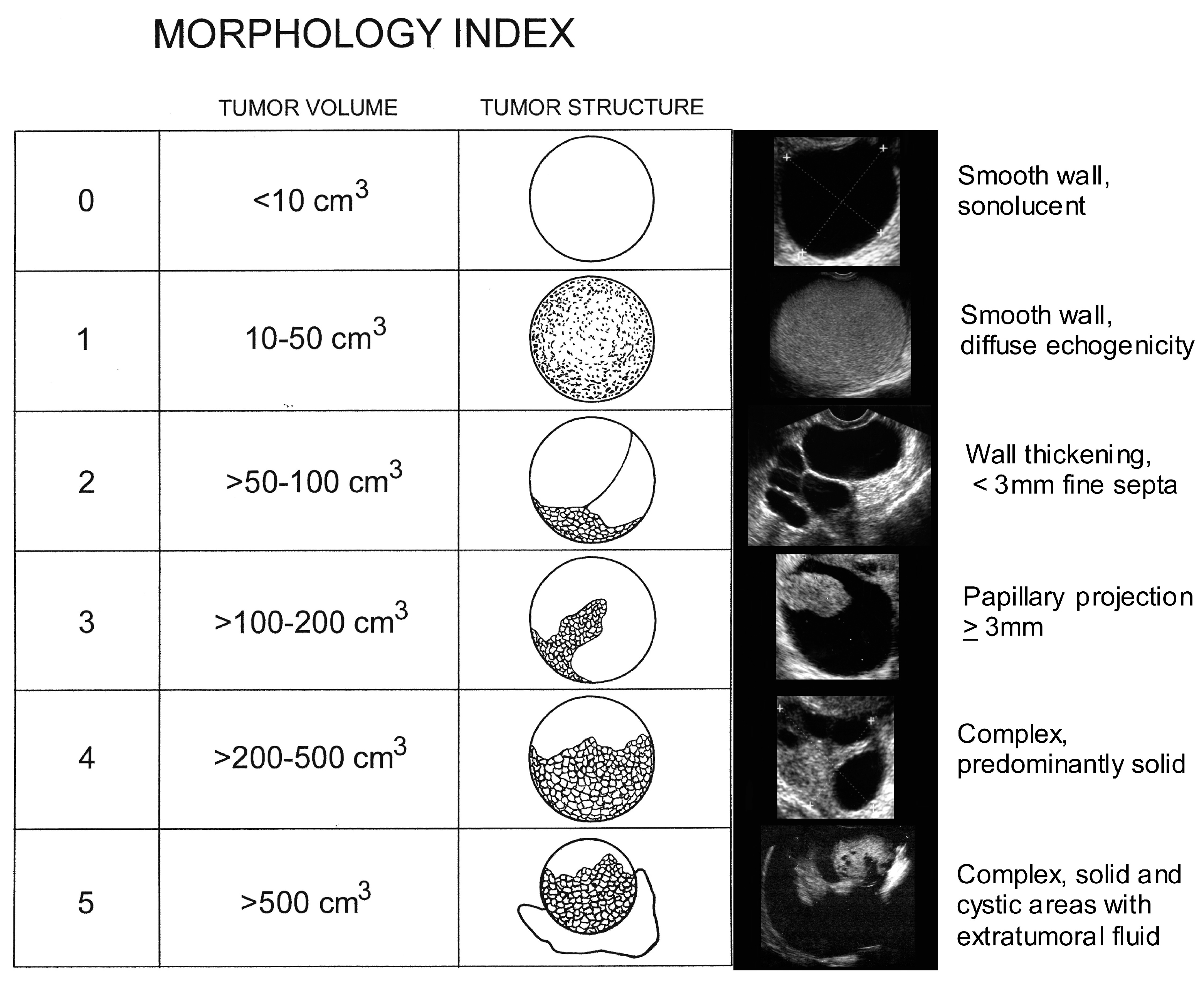
- Ueland, F.R.; DePriest, P.D.; Pavlik, E.J.; Kryscio, R.J.; Nagell, J.R., Jr. Preoperative differentiation of malignant from benign ovarian tumors: The efficacy of morphology indexing and Doppler flow sonography. Gynecol. Oncol. 2003, 91, 46–50. [Google Scholar] [CrossRef]
- Testa, A.C.; Timmerman, D.; van Hosbeke, C.; Zannoni, G.F.; Fransis, S.; Moerman, P.; Vellone, V.; Mascilini, F.; Licameli, A.; Ludovisi, M.; et al. Ovarian cancer arising in endometrioid cysts: Ultrasound findings. Ultrasound Obstet. Gynecol. 2011, 38, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Nomura, K.; Ishikawa, E.; Ushigome, S. Ovarian atypical endometriosis: Its close association with malignant epithelial tumours. Histopathology 1997, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Heaps, J.M.; Nieberg, R.K.; Berek, J.S. Malignant neoplasms arising in endometriosis. Obstet. Gynecol. 1990, 75, 1023–1028. [Google Scholar] [CrossRef]
- Moll, U.M.; Chumas, J.C.; Chalas, E.; Mann, W.J. Ovarian carcinoma arising in atypical endometriosis. Obstet. Gynecol. 1990, 75, 537–539. [Google Scholar] [PubMed]
- Sainz de la Cuesta, R.; Eichhorn, J.H.; Rice, L.W.; Fuller, A.F., Jr.; Nikrui, N.; Goff, B.A. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol. Oncol. 1996, 60, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.C.; Dash, R.; Bentley, R.C.; Snyder, M.J.; Haney, A.F.; Robboy, S.J. Malignancy in endometriosis: Frequency and comparison of ovarian and extraovarian types. Int. J. Gynecol. Pathol. 2001, 20, 133–139. [Google Scholar] [PubMed]
- Ogawa, S.; Kaku, T.; Amada, S.; Kobayashi, H.; Hirakawa, T.; Ariyoshi, K.; Kamura, T.; Nakano, H. Ovarian endometriosis associated with ovarian carcinoma: A clinicopathological and immunohistochemical study. Gynecol. Oncol. 2000, 77, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mostoufizadeh, M.; Scully, R.E. Malignant tumors arising in endometriosis. Clin. Obstet. Gynecol. 1980, 23, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Russell, P. The pathological assessment of ovarian neoplasms. I: Introduction to the common “epithelial” tumours and analysis of benign “epithelial” tumours. Pathology 1979, 11, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.A.; Scully, R.E. Atypical and borderline endometrioid adenofibromas of the ovary: A report of 27 cases. Am. J. Surg. Pathol. 1985, 9, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.R.; Norris, H.J.; Tavassoli, F. Endometrioid proliferative and low malignant potential tumors of the ovary: A clinicopathologic study of 46 cases. Am. J. Surg. Pathol. 1988, 12, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; van Nagell, J.R., Jr. Ovarian cancer screening—What women want. Int. J. Gynecol. Cancer 2012, 22, S21–S23. [Google Scholar] [CrossRef] [PubMed]
- Salsman, J.M.; Pavlik, E.; Boerner, L.M.; Andrykowski, M.A. Clinical, demographic, and psychological characteristics of new, asymptomatic partipants in a transvaginal ultrasound screening program for ovarian cancer. Prev. Med. 2004, 39, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lykins, E.L.; Pavlik, E.; Andrykowski, M.A. Validity of self-reports of return for routine repeat screening in an ovarian screening program. Cancer Epidemiol. Biomark. Prev. 2007, 16, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.E.; Pavlik, E.; Salsman, J.M.; Andrykowski, M.A. Pyschological and behavioral impact of receipt of a “normal” ovarian cancer screening test. Prev. Med. 2006, 42, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Andrykowski, M.A.; Boerner, L.M.; Salsman, J.M.; Pavlik, E. Psychological response to test results in an ovarian cancer screening program: A prospective, longitudinal study. Health Psychol. 2004, 23, 622–666. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.Y.; Graves, K.D.; Pavlik, E.J.; Andrykowski, M.A. Abnormal ovarian cancer screening test result: Women’s informational, psychological and practical needs. J. Psychosoc. Oncol. 2007, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Andrykowski, M.A.; Pavlik, E. Response to an abnormal ovarian cancer-screening test result: Test of the social cognitive processing and cognitive social health information processing models. Psychol. Health 2011, 26, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Jenkins, V.; Farewell, V. BJOG: Psychological morbidity associated with ovarian cancer screening: Results from more than 23,000 women in the randomised trial of ovarian cancer screening (UKCTOCS). BJOG 2014, 121, 1071–1079. [Google Scholar] [CrossRef] [PubMed]

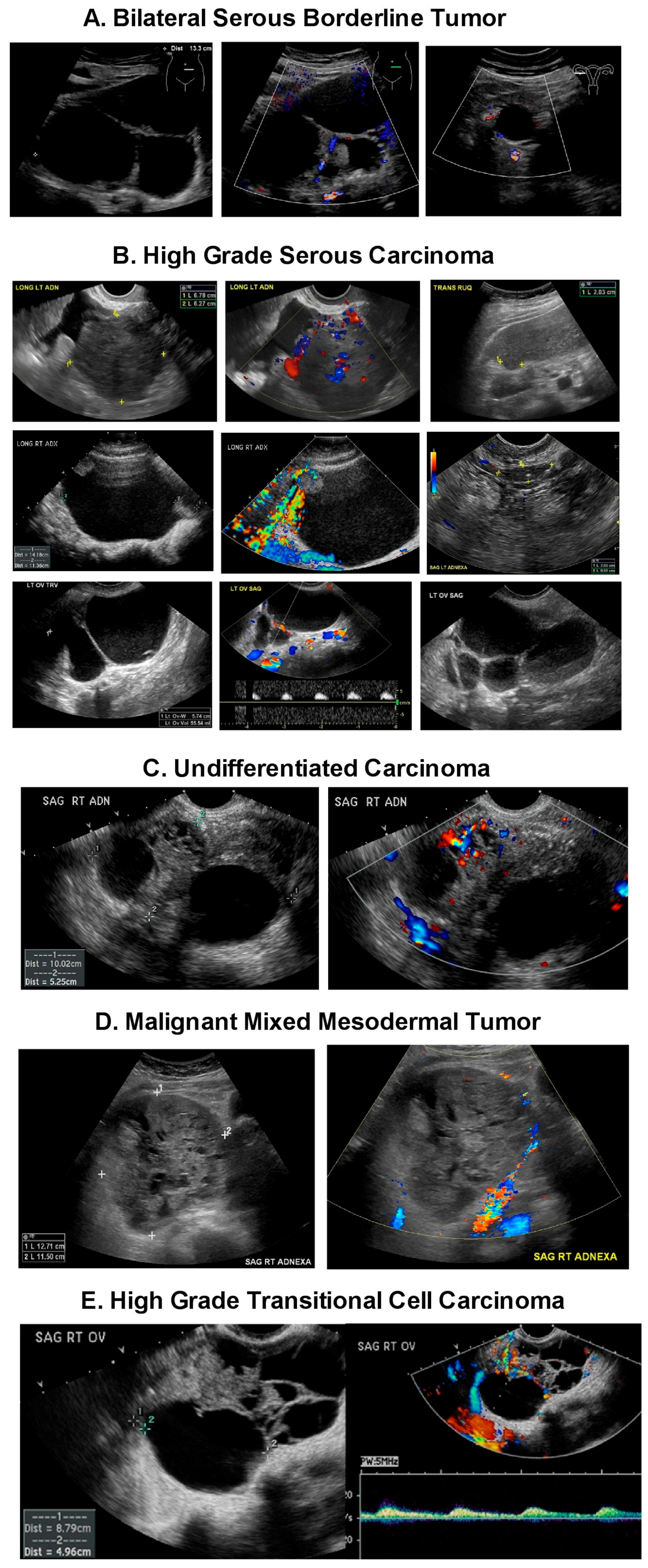
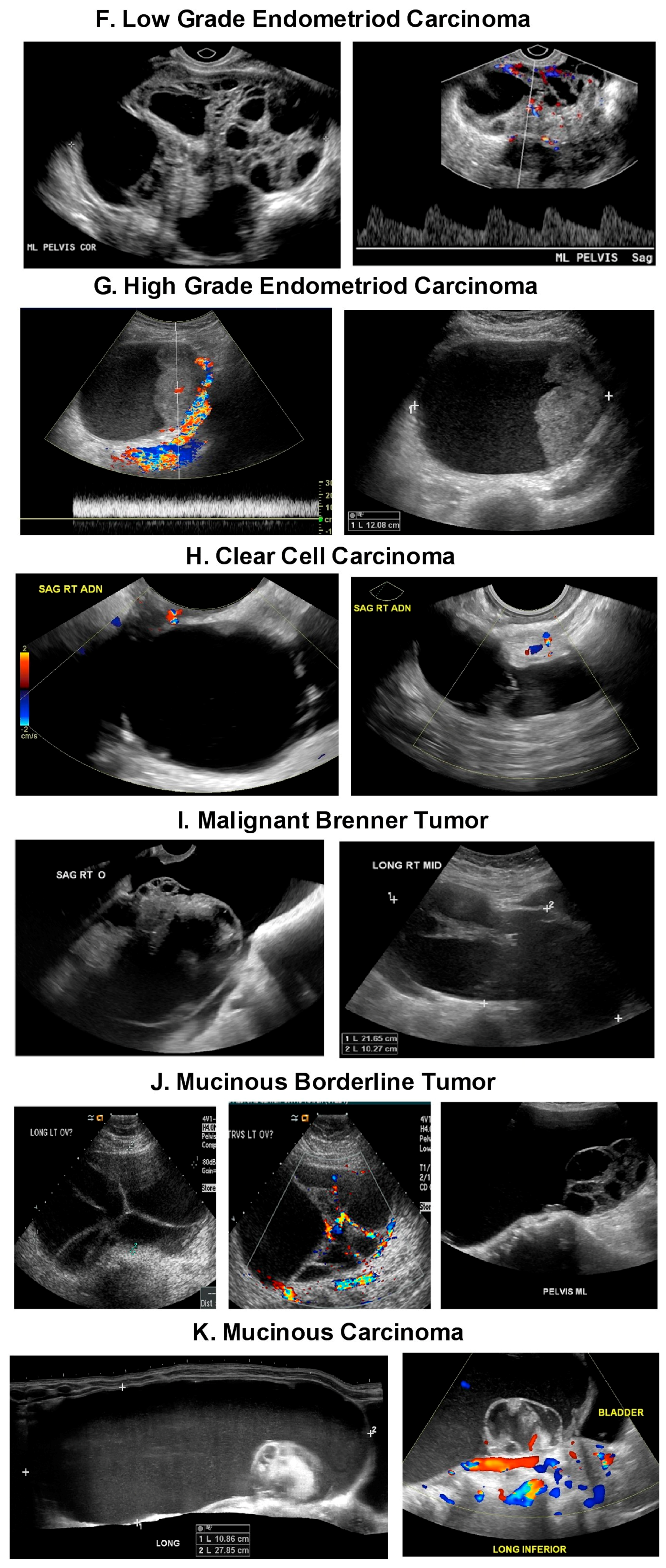
| Tumor Type | Type 1 Tumors | Type 2 Tumors |
|---|---|---|
| Behavior | Indolent | Aggressive |
| Diagnosis at | Early Stage | Advanced Stage |
| Survival Rate at 5 years | About 55% | About 30% |
| Type/Precursor | -Endometrioid carcinoma/Endometriosis -Clear cell carcinoma/Endometriosis Mucinous carcinoma/Mucinous Cystadenoma, Endometriosis, Teratoma, -Brenner Tumor, and Mucinous borderline tumor -Low grade serous carcinoma/Serous cystadenoma, Adenofibroma, Atypical proliferative serous tumor, Mullerian epithelial cyst -Transitional cell carcinoma or Malignant Brenner tumor/Brenner tumor | -High grade serous carcinoma/Probably de novo starting at the tubo, ovarian surface epithelium, serous tubal intraepithelial carcinomas (STIC) or ovarian hilum stem cell -Undifferentiated carcinoma? -Malignant mixed carcinoma? |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormsby, E.L.; Pavlik, E.J.; McGahan, J.P. Ultrasound Monitoring of Extant Adnexal Masses in the Era of Type 1 and Type 2 Ovarian Cancers: Lessons Learned From Ovarian Cancer Screening Trials. Diagnostics 2017, 7, 25. https://doi.org/10.3390/diagnostics7020025
Ormsby EL, Pavlik EJ, McGahan JP. Ultrasound Monitoring of Extant Adnexal Masses in the Era of Type 1 and Type 2 Ovarian Cancers: Lessons Learned From Ovarian Cancer Screening Trials. Diagnostics. 2017; 7(2):25. https://doi.org/10.3390/diagnostics7020025
Chicago/Turabian StyleOrmsby, Eleanor L., Edward J. Pavlik, and John P. McGahan. 2017. "Ultrasound Monitoring of Extant Adnexal Masses in the Era of Type 1 and Type 2 Ovarian Cancers: Lessons Learned From Ovarian Cancer Screening Trials" Diagnostics 7, no. 2: 25. https://doi.org/10.3390/diagnostics7020025