Dynamic Contrast-Enhanced Ultrasound of Colorectal Liver Metastases as an Imaging Modality for Early Response Prediction to Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Protocol
2.3. Imaging Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Baccala, A., Jr.; Hedgepeth, R.; Kaouk, J.; Magi-Galluzzi, C.; Gilligan, T.; Fergany, A. Pathological evidence of necrosis in recurrent renal mass following treatment with sunitinib. Int. J. Urol. 2007, 14, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.Q.; Liu, L.Z.; Xiong, Y.H.; Zou, R.H.; Chen, M.S.; Li, A.H.; Cai, M.Y. Quantitative analysis of contrast-enhanced ultrasonography: Differentiating focal nodular hyperplasia from hepatocellular carcinoma. Br. J. Radiol. 2013, 86, 20120536. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Wilson, S.R. CEUS: Where are we in 2015? Eur. J. Radiol. 2015, 84, 1621–1622. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D. Ultrasound contrast agents: An overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsoe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013, 34, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; Garcovich, M.; Lupascu, A.; Stasio, E.D.; Roccarina, D.; Annicchiarico, B.E.; Riccardi, L.; Ainora, M.E.; Ponziani, F.; Caracciolo, G.; et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: The role of dynamic contrast enhanced ultrasound. J. Hepatol. 2013, 59, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Bonastre, J.; Kind, M.; Vilgrain, V.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A.; Labbe-Ceviliers, C.; et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: The French multicenter support for innovative and expensive techniques study. Investig. Radiol. 2014, 49, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Lamuraglia, M.; Chami, L.; Leclere, J.; Bonvalot, S.; Terrier, P.; Roche, A.; Le Cesne, A. Gastrointestinal stromal tumors treated with imatinib: Monitoring response with contrast-enhanced sonography. Am. J. Roentgenol. 2006, 187, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Maddalena, M.E.; Cui, X.W.; Schreiber-Dietrich, D.; Ignee, A. Liver tumor characterization—Review of the literature. Ultraschall Med. 2012, 33, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.F.; Smolock, A.; Cantisani, V.; Bezzi, M.; D’Ambrosio, F.; Proiti, M.; Lee, F.; Aiani, L.; Calliada, F.; Ferraioli, G. Contrast enhanced ultrasound in the evaluation and percutaneous treatment of hepatic and renal tumors. Eur. J. Radiol. 2015, 84, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Cosgrove, D.; Albrecht, T.; Bolondi, L.; Bosio, M.; Calliada, F.; Correas, J.M.; Darge, K.; Dietrich, C.; D’Onofrio, M.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)-update 2008. Ultraschall Med. 2008, 29, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Rojas Llimpe, F.L.; Di Fabio, F.; Ercolani, G.; Giampalma, E.; Cappelli, A.; Serra, C.; Castellucci, P.; D’Errico, A.; Golfieri, R.; Pinna, A.D.; et al. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: Results of the PROMETEO-01 study. Br. J. Cancer 2014, 111, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Leen, E.; Averkiou, M.; Arditi, M.; Burns, P.; Bokor, D.; Gauthier, T.; Kono, Y.; Lucidarme, O. Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur. Radiol. 2012, 22, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Frampas, E.; Lassau, N.; Zappa, M.; Vullierme, M.P.; Koscielny, S.; Vilgrain, V. Advanced Hepatocellular Carcinoma: Early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur. J. Radiol. 2013, 82, e205–e211. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Chebil, M.; Chami, L.; Bidault, S.; Girard, E.; Roche, A. Dynamic contrast-enhanced ultrasonography (DCE-US): A new tool for the early evaluation of antiangiogenic treatment. Target. Oncol. 2010, 5, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Schirin-Sokhan, R.; Winograd, R.; Roderburg, C.; Bubenzer, J; Nicole Cabral Do, O.; Guggenberger, D.; Hecker, H.; Trautwein, C.; Tischendorf, J.J. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J. Gastroenterol. 2012, 18, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Koscielny, S.; Albiges, L.; Chami, L.; Benatsou, B.; Chebil, M.; Roche, A.; Escudier, B.J. Metastatic renal cell carcinoma treated with sunitinib: Early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin. Cancer Res. 2010, 16, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Koscielny, S.; Chami, L.; Chebil, M.; Benatsou, B.; Roche, A.; Ducreux, M.; Malka, D.; Boige, V. Advanced hepatocellular carcinoma: Early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—Preliminary results. Radiology 2011, 258, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Chami, L.; Koscielny, S.; Chebil, M.; Massard, C.; Benatsou, B.; Bidault, S.; Cioffi, A.; Blay, J.Y.; Le Cesne, A. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Investig. New Drugs 2012, 30, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Chapotot, L.; Benatsou, B.; Vilgrain, V.; Kind, M.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A.; et al. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: The French multicenter Support for Innovative and Expensive Techniques Study. Investig. Radiol. 2012, 47, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, H.M.; Fallentin, E.; Lauridsen, C.; Law, I; Federspiel, B.; Baeksgaard, L.; Svendsen, L.B.; Nielsen, M.B. Computed tomography (CT) perfusion as an early predictive marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer—A prospective study. PLoS ONE 2014, 9, e97605. [Google Scholar]
- Wang, H.; Hristov, D.; Qin, J.; Tian, L.; Willmann, J.K. Three-dimensional Dynamic Contrast-enhanced US Imaging for Early Antiangiogenic Treatment Assessment in a Mouse Colon Cancer Model. Radiology 2015, 277, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Williams, R.; Tremblay-Darveau, C.; Sheeran, P.S.; Milot, L.; Bjarnason, G.A.; Burns, P.N. Dynamic contrast enhanced ultrasound for therapy monitoring. Eur. J. Radiol. 2015, 84, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-E.; Joo, B.; Park, M.-S.; Shin, S.J.; Ahn, J.B.; Kim, M.J. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Surrogate Biomarker for Bevacizumab in Colorectal Cancer Liver Metastasis: A Single-Arm, Exploratory Trial. Cancer Res. Treat. 2016, 48, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
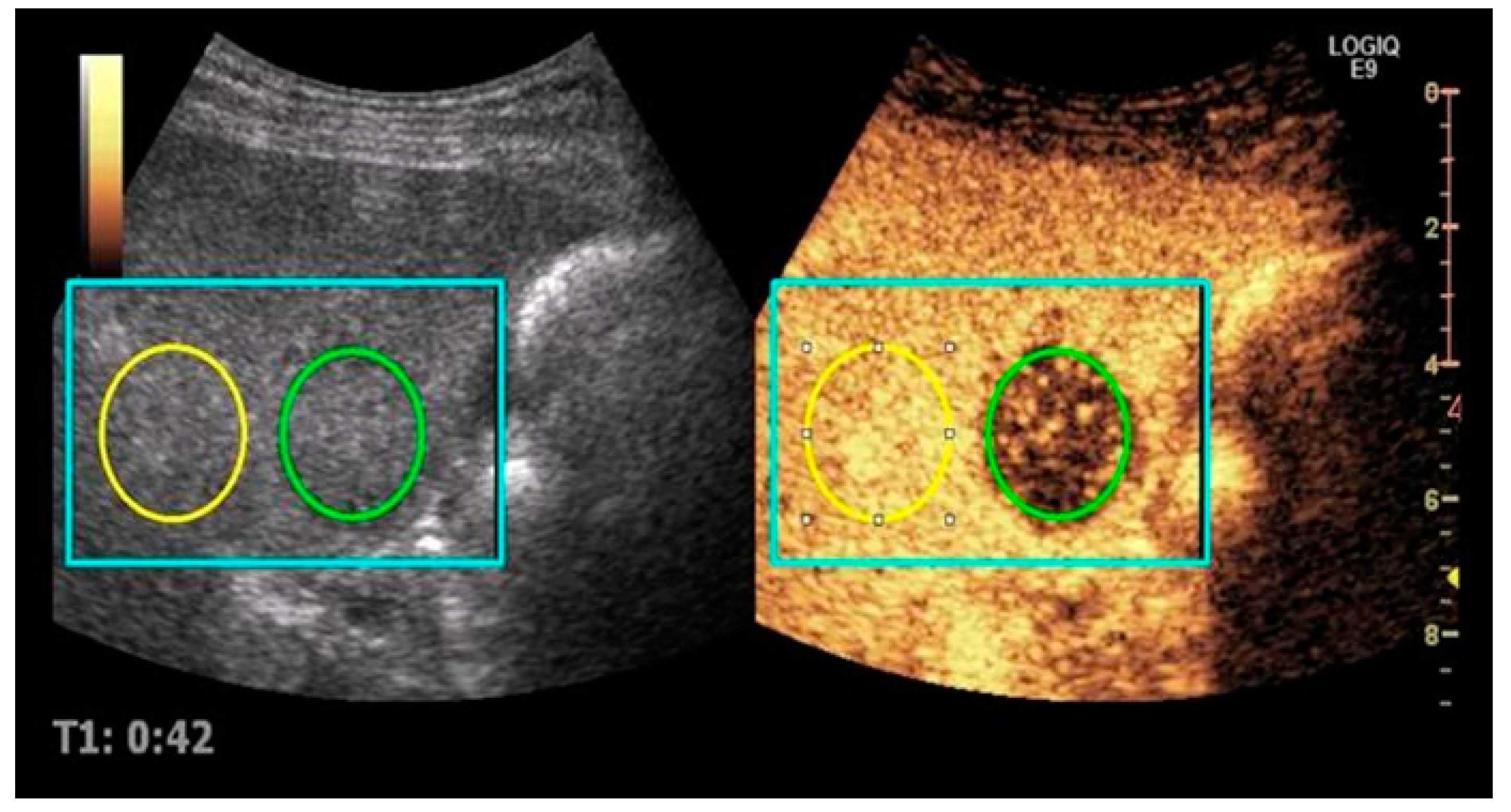
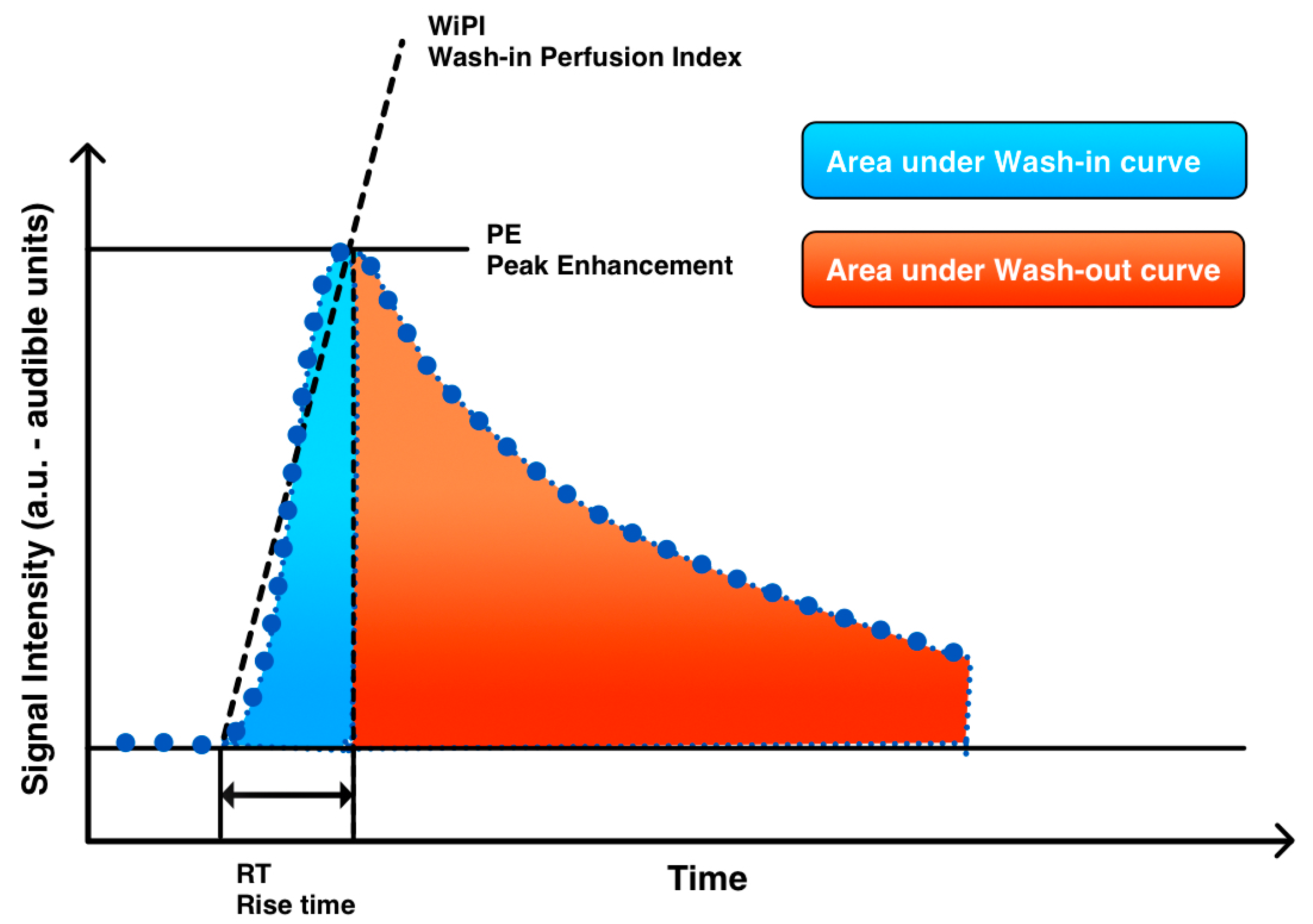
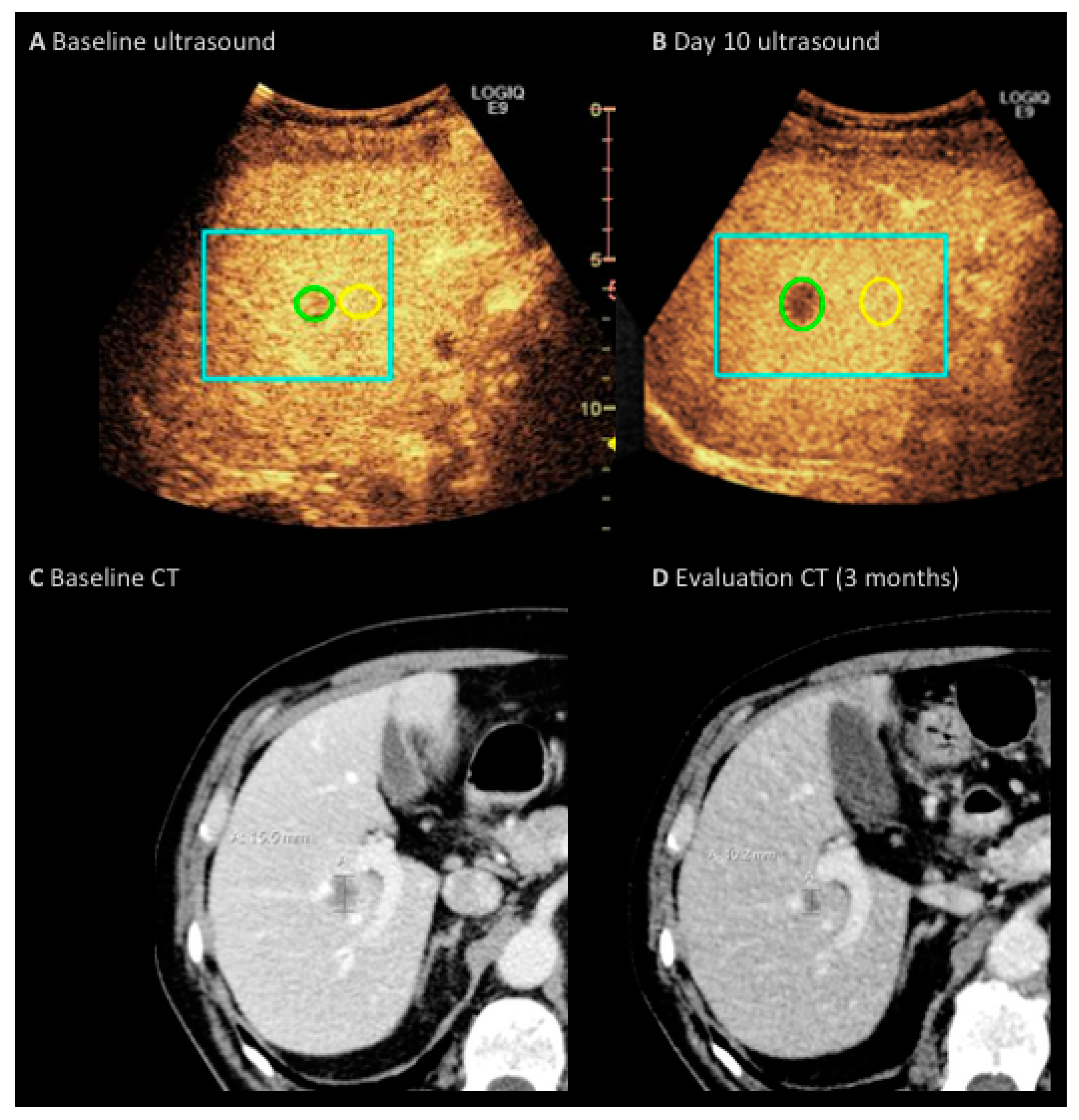
| Variable | n = 12 | Percentage |
|---|---|---|
| Gender | ||
| Female | 4 | 33% |
| Male | 8 | 67% |
| Age (years) | Median 66.5 (range 42–77) | |
| Primary tumor location | ||
| Rectum | 4 | 33% |
| Left colon | 5 | 42% |
| Right colon | 3 | 25% |
| Tumor burden | ||
| Primary tumor resected | 5 | 42% |
| Primary tumor in situ | 7 | 58% |
| Liver metastases ≥ 3 | 11 | 92% |
| Liver metastases < 3 | 1 | 8% |
| Lymph node metastases | 7 | 58% |
| Lung metastases | 4 | 33% |
| Peritoneal carcinosis | 2 | 16% |
| Treatment | ||
| CAPOX + bevacizumab | 8 | 67% |
| CAPOX | 3 | 25% |
| FOLFIRI + cetuximab | 1 | 8% |
| Carcinoembryonic Antigen Baseline | Median 52 (range 5–3050) | |
| Carcinoembryonic Antigen Evaluation | Median 13 (range 5–899) |
| Perfusion Parameters | Baseline (Median) | Day 10 (Median) | ρ between Shrinkage and DCE-US Measures | Bevacizumab Subgroup: ρ between Shrinkage and DCE-US Measures |
|---|---|---|---|---|
| nPE | 0.50 (0.15–1.24) | 0.27 (0.13–1.26) | 0.455 | 0.719 (p = 0.045) |
| nWiWo | 0.21 (0.01–1.53) | 5.46 (0.12–31.70) | 0.291 | 0.108 (p = 0.799) |
| nRT | 0.50 (0.30–1.22) | 0.58 (0.25–1.50) | 0.223 | −0.108 (p = 0.799) |
| nWiPI | 0.48 (0.06–1.14) | 0.27 (0.11–1.70) | 0.140 | 0.313 (p = 0.450) |
| nWiAUC | 0.25 (0.03–0.71) | 0.17 (0.06–0.86) | 0.075 | 0.319 (p = 0.441) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogensen, M.B.; Hansen, M.L.; Henriksen, B.M.; Axelsen, T.; Vainer, B.; Osterlind, K.; Nielsen, M.B. Dynamic Contrast-Enhanced Ultrasound of Colorectal Liver Metastases as an Imaging Modality for Early Response Prediction to Chemotherapy. Diagnostics 2017, 7, 35. https://doi.org/10.3390/diagnostics7020035
Mogensen MB, Hansen ML, Henriksen BM, Axelsen T, Vainer B, Osterlind K, Nielsen MB. Dynamic Contrast-Enhanced Ultrasound of Colorectal Liver Metastases as an Imaging Modality for Early Response Prediction to Chemotherapy. Diagnostics. 2017; 7(2):35. https://doi.org/10.3390/diagnostics7020035
Chicago/Turabian StyleMogensen, Marie Benzon, Martin Lundsgaard Hansen, Birthe Merete Henriksen, Thomas Axelsen, Ben Vainer, Kell Osterlind, and Michael Bachmann Nielsen. 2017. "Dynamic Contrast-Enhanced Ultrasound of Colorectal Liver Metastases as an Imaging Modality for Early Response Prediction to Chemotherapy" Diagnostics 7, no. 2: 35. https://doi.org/10.3390/diagnostics7020035





