Advancing Point-of-Care (PoC) Testing Using Human Saliva as Liquid Biopsy
Abstract
:1. Introduction
1.1. Paradigm Shift from Central Laboratory (CL) to Point-of-Care
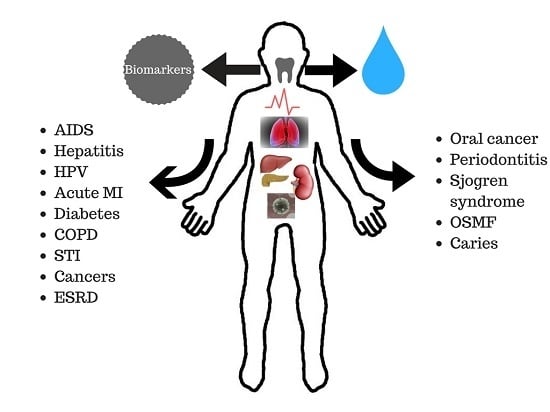
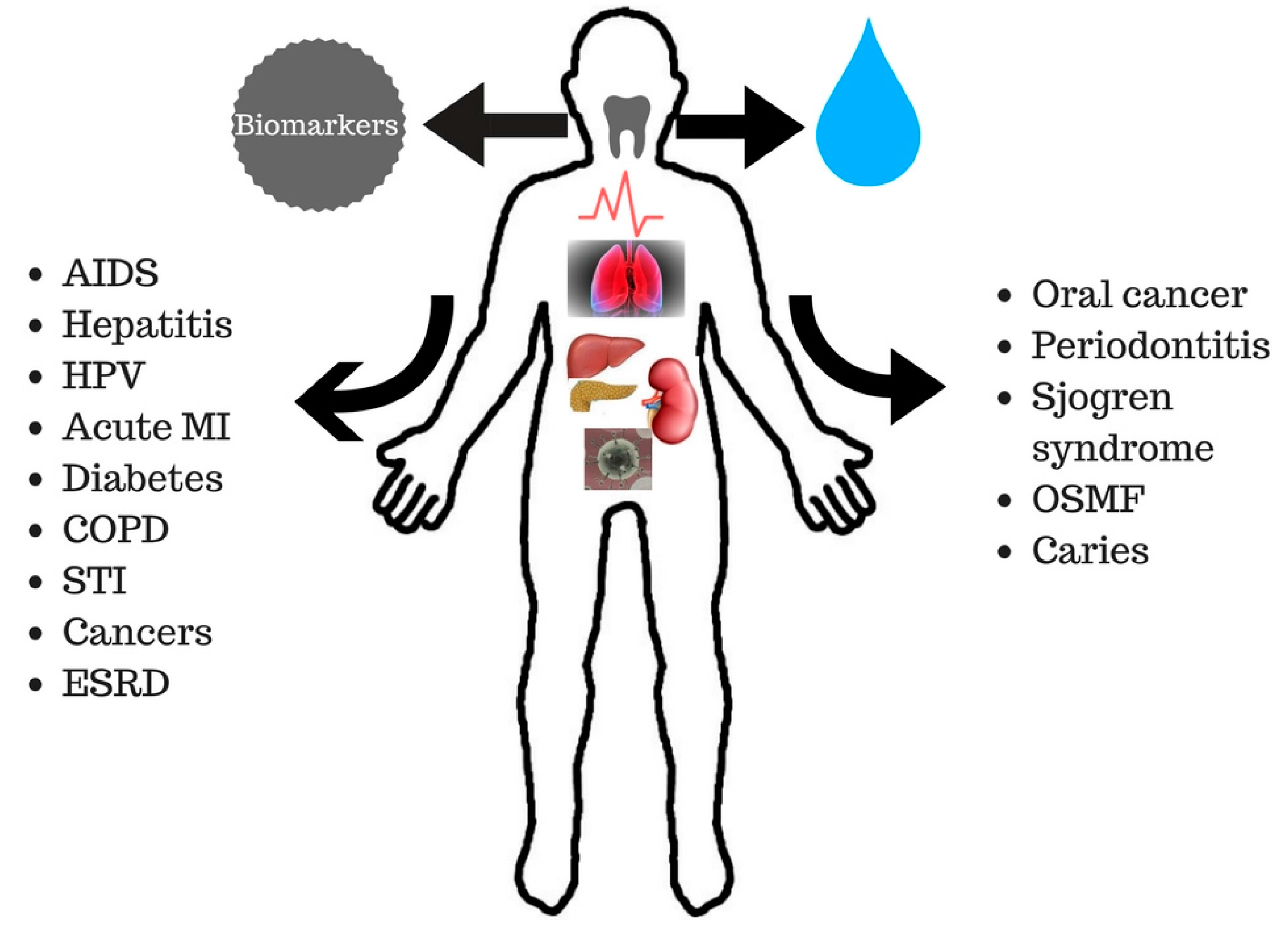
1.2. Saliva in the Diagnosis of Oral and Systemic Diseases
2. Point-of-Care Technology: An Overview
2.1. Diagnostic Targets
2.2. Diagnostic Toolboxes
2.3. Salivary Biomarker-Based PoC Platforms
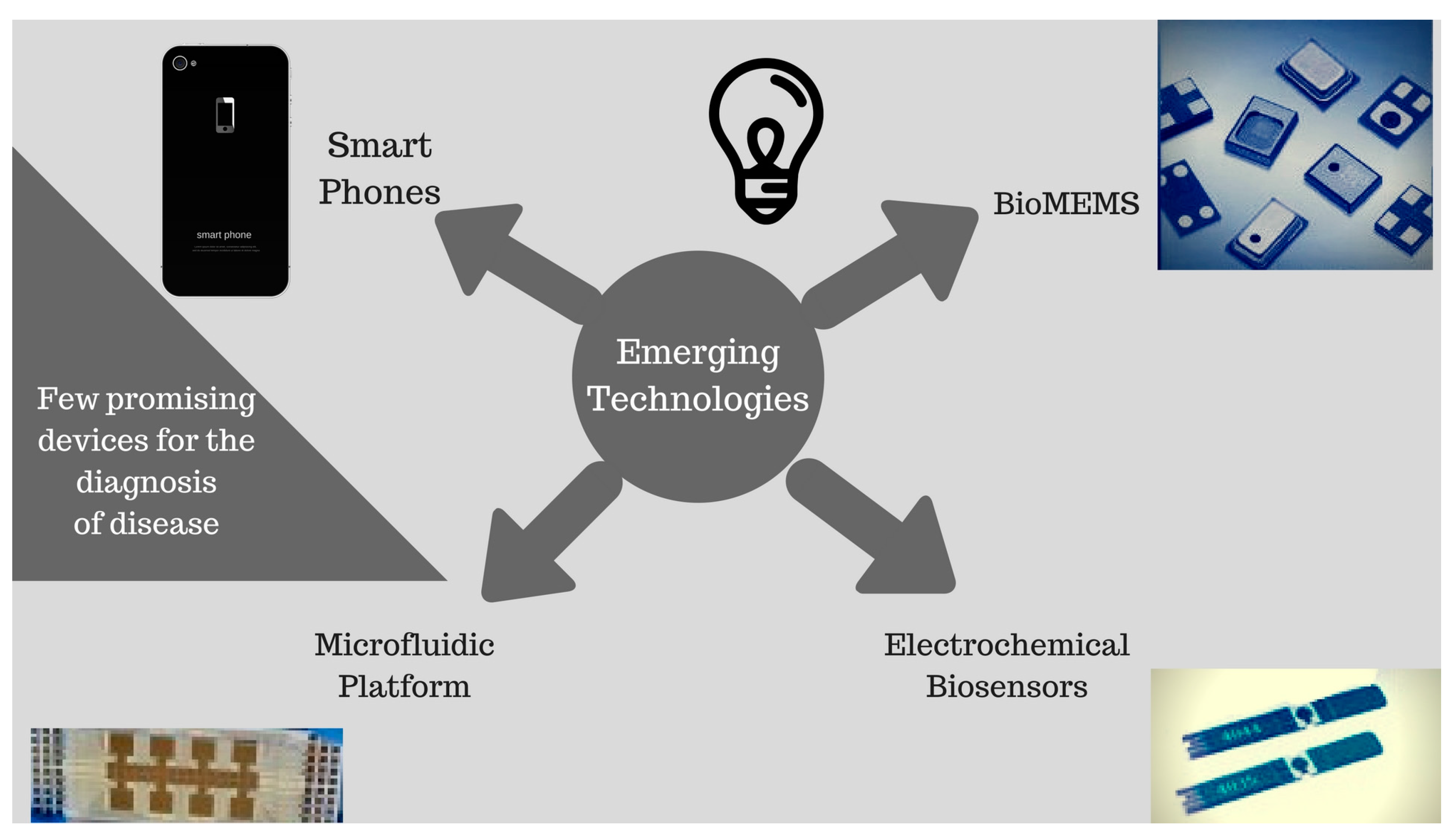
3. Emerging Novel PoC Technologies
3.1. Biosensors
3.2. Fluorescent Biosensors
3.3. Biological Micro-Electro-Mechanical Systems (BioMEMS)
3.4. Microfluidics/Paper-Based Technology
3.5. Electric Field-Induced Release and Measurement (EFIRM)
3.6. Smartphone-Based Biosensors
4. Future Direction and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. Aust. Assoc. Clin. Biochem. 2014, 35, 155–167. [Google Scholar]
- Jani, I.V.; Peter, T.F. How point-of-care testing could drive innovation in global health. New Engl. J. Med. 2013, 368, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Yamaguchi, M. Salivary biosensors for screening trauma-related psychopathology. Oral Maxillofac. Surg. Clin. North Am. 2010, 22, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Walt, D.R. Salivary diagnostics using a portable point-of-service platform: A review. Clin. Ther. 2015, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.; Rehman, R.; Rehman, I. Advances of proteomic sciences in dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: An update. Proteomes 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2015, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M. Human gingival crevicular fluids (GCF) proteomics: An overview. Dent. J. 2017, 5, 12. [Google Scholar] [CrossRef]
- Khurshid, Z.; Haq, J.A.; Khan, R.S.; Sohail Zafar, M.; Altaf, M.; Najeeb, S. Human saliva and its role in oral & systemic health. J. Pak. Dent. Assoc. 2016, 25, 170–174. [Google Scholar]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2015, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.; Slowey, P.; Almas, K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Tabak, L.A. In defense of the oral cavity: The protective role of the salivary secretions. Pediatr. Dent. 2006, 28, 110–198. [Google Scholar] [PubMed]
- Tsuchida, S.; Satoh, M.; Sogawa, K.; Kawashima, Y.; Kado, S.; Ishige, T.; Beppu, M.; Sawai, S.; Nishimura, M.; Kodera, Y.; et al. Application of proteomic technologies to discover and identify biomarkers for periodontal diseases in gingival crevicular fluid: A review. Proteom. Clin. App. 2014, 8, 232–240. [Google Scholar] [CrossRef]
- Tabak, L.A. Point-of-care diagnostics enter the mouth. Ann. N. Y. Acad. Sci. 2007, 1098, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wong, D.T.W. Point-of-care platforms for salivary diagnostics. Chin. J. Dent. Res. 2012, 15, 7–15. [Google Scholar] [PubMed]
- Slomiany, B.L.; Murty, V.L.N.; Slomiany, A. Salivary lipids in health and disease. Prog. Lipid Res. 1985, 24, 311–324. [Google Scholar] [CrossRef]
- Actis, A.B.; Perovic, N.R.; Defagó, D.; Beccacece, C.; Eynard, A.R. Fatty acid profile of human saliva: A possible indicator of dietary fat intake. Arch. Oral Biol. 2005, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 2016, 6, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, O.; Krief, G.; Konttinen, Y.T.; Zaks, B.; Wong, D.T.; Aframian, D.J.; Palmon, A. Identification of Sjogren’s syndrome oral fluid biomarker candidates following high-abundance protein depletion. Rheumatology 2015, 54, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Delaleu, N.; Mydel, P.; Kwee, I.; Brun, J.G.; Jonsson, M.V.; Jonsson, R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjgren’s syndrome. Arthritis Rheumatol. 2015, 67, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Langie, S.A.S.; Szarc Vel Szic, K.; Declerck, K.; Traen, S.; Koppen, G.; Van Camp, G.; Schoeters, G.; Vanden Berghe, W.; De Boever, P. Whole-genome saliva and blood DNA methylation profiling in individuals with a respiratory allergy. PLoS ONE 2016, 11, e0151109. [Google Scholar] [CrossRef] [PubMed]
- Maria, N.I.; Brkic, Z.; Waris, M.; van Helden-Meeuwsen, C.G.; Heezen, K.; van de Merwe, J.P.; van Daele, P.L.; Dalm, V.A.S.H.; Drexhage, H.A.; Versnel, M.A. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren’s syndrome. Ann. Rheum. Dis. 2014, 73, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.M.; Klimatcheva, E.; Rothstein, T.L. CXCL13 is elevated in Sjogren’s syndrome in mice and humans and is implicated in disease pathogenesis. J. Leukoc. Biol. 2013, 94, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Shiao, Y.M.; Wu, T.H.; Chen, W.S.; Hsu, Y.H.; Tsai, S.F.; Tsai, C.Y. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J. Rheum. 2010, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Out, D.; Hall, R.J.; Granger, D.A.; Page, G.G.; Woods, S.J. Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav. Immun. 2012, 26, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Mirzaii-Dizgah, I.; Riahi, E. Salivary high-sensitivity cardiac troponin T levels in patients with acute myocardial infarction. Oral Dis. 2013, 19, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Mirzaii-Dizgah, I.; Jafari-Sabet, M. Unstimulated whole saliva creatine phosphokinase in acute myocardial infarction. Oral Dis. 2011, 17, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.Y.Y.; Wan, Y.; Kostner, K.; Arivalagan, A.; Atherton, J.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. NT-ProBNP levels in saliva and its clinical relevance to heart failure. PLoS ONE 2012, 7, e48452. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.S.; Chang, K.M.; Loke, M.F.; Angela Loo, V.P.; Subrayan, V. Association of potential salivary biomarkers with diabetic retinopathy and its severity in type-2 diabetes mellitus: A proteomic analysis by mass spectrometry. PeerJ 2016, 4, e2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Cheng, X.Q.; Li, J.Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ladgotra, A. Estimation of salivary and serum biomarkers in diabetic and non diabetic patients—A comparative study. J. Clin. Diagn. Res. 2016, 10, ZC56–ZC61. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Zigler, C.; Robles, T.F.; Elashoff, D.; Yamaguchi, M. Developmental validation of a point-of-care, salivary? Amylase biosensor. Psychoneuroendocrinology 2011, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A.; Solis, M.; Hsieh, Y.H.; Jett-Goheen, M.; Nour, S.; Rothman, R.E. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int. J. STD AIDS 2013, 24, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Zachary, D.; Mwenge, L.; Muyoyeta, M.; Shanaube, K.; Schaap, A.; Bond, V.; Kosloff, B.; de Haas, P.; Ayles, H. Field comparison of oraquick advance rapid HIV-1/2 antibody test and two blood-based rapid HIV antibody tests in Zambia. BMC Infect. Dis. 2012, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Bien, C.H.; Peeling, R.W. Point-of-care testing for sexually transmitted infections: Recent advances and implications for disease control. Curr. Opin. Infect. Dis. 2013, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Choi, Y. Point-of-care diagnosis of periodontitis using saliva: Technically feasible but still a challenge. Front. Cell. Infect. Microbiol. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Floriano, P.N.; Christodoulides, N.; Miller, C.S.; Ebersole, J.L.; Spertus, J.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; Steinhubl, S.; Acosta, S.; et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: A feasibility study. Clin. Chem. 2009, 55, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Blicharz, T.M.; Siqueira, W.L.; Helmerhorst, E.J.; Oppenheim, F.G.; Wexler, P.J.; Little, F.F.; Walt, D.R. Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva. Anal. Chem. 2009, 81, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodrguez, R.M.; Campuzano, S.; Ruiz-Valdepeas Montiel, V.; Gamella, M.; Pingarrn, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Walt, D.R.; Blicharz, T.M.; Hayman, R.B.; Rissin, D.M.; Bowden, M.; Siqueira, W.L.; Helmerhorst, E.J.; Grand-Pierre, N.; Oppenheim, F.G.; Bhatia, J.S.; et al. Microsensor arrays for saliva diagnostics. Ann. N. Y. Acad. Sci. 2007, 1098, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Barnfather, K.D.; Cope, G.F.; Chapple, I.L. Effect of incorporating a 10 min point of care test for salivary nicotine metabolites into a general practice based smoking cessation programme: Randomised controlled trial. BMJ Clin. Res. 2005, 331, 999. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.H.; Burbelo, P.D.; Gonzalez-Begne, M.; Roberts, M.E.P.; Coca, A.; Sanz, I.; Iadarola, M.J. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjogren’s Syndrome. J. Dent. Res. 2011, 90, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, W.; Wang, M.L. Sensing of salivary glucose using nano-structured biosensors. Biosensors 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. BioSens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef]
- Christodoulides, N.; Pierre, F.N.; Sanchez, X.; Li, L.; Hocquard, K.; Patton, A.; Muldoon, R.; Miller, C.S.; Ebersole, J.L.; Redding, S.; et al. Programmable bio-nanochip technology for the diagnosis of cardiovascular disease at the point-of-care. Methodist DeBakey Cardiovasc. J. 2012, 8, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Salivary sensors in point-of-care testing. Sens. Mater. 2010, 22, 143–153. [Google Scholar]
- Cone, E.J.; Clarke, J.; Tsanaclis, L. Prevalence and disposition of drugs of abuse and opioid treatment drugs in oral fluid. J. Anal. Toxicol. 2007, 31, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Gau, V.; Wong, D. Oral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platform. Ann. N. Y. Acad. Sci. 2007, 1098, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2016, 242, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K.; Daar, A.S.; Thorsteinsdottir, H.; Martin, D.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Silberring, J.; Ciborowski, P. Biomarker discovery and clinical proteomics. Trends Anal. Chem. 2010, 29, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.E.; Belkowski, S.M.; Plata-Salamán, C.R. Biomarker discovery and validation: Technologies and integrative approaches. Trends Biotechnol. 2004, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, C.; Tu, M.; Wong, D. Oral biofluid biomarker research: Current status and emerging frontiers. Diagnostics 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- St. John, M.A.R.; Li, Y.; Zhou, X.; Denny, P.; Ho, C.M.C.M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Sinha, U.; et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.L.; Wilson, C.H.; Perri, L.P.; Hannibal, S.L.; O’Connell, R.J. Usefulness of a rapid human immunodeficiency virus-1 antibody test for the management of occupational exposure to blood and body fluid. Infect. Control Hosp. Epidemiol. 2005, 26, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005, 76, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M. Comparative proteomic analysis of human whole saliva. Arch. Oral Biol. 2004, 49, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; St. John, M.A.R.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.K.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012. [Google Scholar] [CrossRef]
- Kageyama, G.; Saegusa, J.; Irino, Y.; Tanaka, S.; Tsuda, K.; Takahashi, S.; Sendo, S.; Morinobu, A. Metabolomics analysis of saliva from patients with primary Sjgren’s syndrome. Clin. Exp. Immunol. 2015, 182, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J. Pathogenic microorganisms and pancreatic cancer. Gastrointest. Tumors 2015, 2, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Vissink, A.; Arellano, M.; Roozendaal, C.; Zhou, H.; Kallenberg, C.G.; Wong, D.T. Identification of autoantibody biomarkers for primary Sjogren’s syndrome using protein microarrays. Proteomics 2011, 11, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Umeda, M.; Benno, Y. Molecular analysis of human oral microbiota. J. Periodontal Res. 2005, 40, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zimmermann, B.G.; Zhou, H.; Wang, J.; Henson, B.S.; Yu, W.; Elashoff, D.; Krupp, G.; Wong, D.T. Exon-level expression profiling: A comprehensive transcriptome analysis of oral fluids. Clin. Chem. 2008, 54, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Idle, J.R.; Gonzalez, F.J. Metabolomics. Cell Metab. 2007, 6, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, F.; Fan, X.; Gao, J.; Chen, N.; Wong, T.; Wu, J.; Wen, S.W. Detection of hepatitis B surface antigen, hepatitis B core antigen, and hepatitis B virus DNA in parotid tissues. Int. J. Infect. Dis. 2009, 13, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Nasidze, I.; Quinque, D.; Li, J.; Li, M.; Tang, K.; Stoneking, M. Comparative analysis of human saliva microbiome diversity by barcoded pyrosequencing and cloning approaches. Anal. Biochem. 2009, 391, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Soper, S.A.; Brown, K.; Ellington, A.; Frazier, B.; Garcia-Manero, G.; Gau, V.; Gutman, S.I.; Hayes, D.F.; Korte, B.; Landers, J.L.; et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens. Bioelectron. 2006, 21, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.F.; Caruso, F. Biosensors: Recent advances. Rep. Prog. Phys. 1997, 60, 1397–1445. [Google Scholar] [CrossRef]
- McRae, M.P.; Simmons, G.W.; Wong, J.; Shadfan, B.; Gopalkrishnan, S.; Christodoulides, N.; McDevitt, J.T. Programmable bio-nano-chip system: A flexible point-of-care platform for bioscience and clinical measurements. Lab Chip 2015, 15, 4020–4031. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A novel biosensor based on hafnium oxide: Application for early stage detection of human interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Vasan, A.S.S. Point-of-care biosensor system. Front. Biosci. 2013, S5, S357. [Google Scholar] [CrossRef]
- Mastrangelo, C.H.; Burns, M.A.; Burke, D.T. Microfabricated devices for genetic diagnostics. Proc. IEEE 1998, 86, 1769–1786. [Google Scholar] [CrossRef]
- Gilleo, K. MEMS in medicine. Circuits Assem. 2005, 16, 32–33. [Google Scholar]
- Naher, S.; Orpen, D.; Brabazon, D.; Morshed, M.M. An overview of microfluidic mixing application. Adv. Mater. Res. 2009, 83–86, 931–939. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.Q.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Yang, J.; Wong, D.T.W. Detection of exosomal biomarker by electric field-induced release and measurement (EFIRM). Biosens. Bioelectron. 2013, 44, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Wang, D.S.; Fan, S.K. Microfluidic surface plasmon resonance sensors: From principles to point-of-care applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Yang, C.A. Smartphone-based chip-scale microscope using ambient illumination. Lab Chip 2014, 14, 3056. [Google Scholar] [CrossRef] [PubMed]
| Salivary Biomarkers | Diseases/Conditions | Developed PoC | References |
|---|---|---|---|
| A-amylase | Clinical judgment for stress-induced disease | Salivary α-Amylase (sAA) biosensor system | [33] |
| HIV | AIDS | Oraquick, tablet-based kiosks | [34,35] |
| Hep C | Hepatitis | OraQuick | [36] |
| HPV | HPV-associated cancers, sexually transmitted diseases | simple fluorescent and colorimetric assay that enables DNA and RNA detection | [37] |
| Cortisol | Stress levels | Label-free chemiresistor immuno-sensor | [38] |
| Proteins (Dipeptidyl peptidase etc.), metabolites, DNA | Periodontitis | Integrated Microfluidic Platform for Oral Diagnostics (IMPOD), lab-on-a-chip (LOC) | [39] |
| C-reactive protein, myoglobin, and myeloperoxidase | Acute Myocardial Infarction | Luminex, lab-on-a-chip methods | [40] |
| Cytokines | Asthma and chronic obstructive pulmonary disease (COPD) | Fiber-optic microsphere-based antibody array | [41] |
| IL-8, IL-8mRNA | Oral Cancer | Electrochemical magneto biosensors | [42] |
| (NO2− and uric acid), and pulmonary inflammation biomarkers | End-stage renal disease (ESRD), asthma and chronic obstructive pulmonary disease (COPD) patients | Optical fibre microarrays | [43] |
| Salivary nicotine metabolites | Smoking/tobacco use | Point of care test for salivary nicotine metabolites | [44] |
| Porphyromonas gingivalis | chronic periodontitis | P. gingivalis saliva kit | |
| Gonorrhoea and chlamydia | Sexually transmitted infections (STIs) | Oral STI point-of-care (PoC) | [36] |
| Salivary anti-Ro60 and anti-Ro52 Antibody Profiles | Sjögren’s Syndrome | Luciferase Immunoprecipitation Systems (LIPS) | [45] |
| Salivary glucose | Diabetes | Glucose monitoring using saliva nanostructured biosensor | [46,47] |
| cRP, MPo, ctnl, Myo, cK-MB, d-dimer, apoa1, apoB, BnP, nt-proBnP, scd40l, McP-1, adiponectin | Cardiovascular disease (CVD) | Programmable bio-nanochip (P-BNC) system | [48] |
| cea, ca125, Her2-neu, Psa (free and complexed) | Cancer | Programmable bio-nanochip (P-BNC) system, 2D nanomaterials | [48,49] |
| Diagnostic Toolbox | Methods of Evaluation | Molecules to Be Analysed | References |
|---|---|---|---|
| Proteomics | Mass spectroscopy and 2D gel electrophoresis, ELISA, protein immunoblot techniques | Post-translational modifications and protein-enzyme complexes | [69] |
| Genomics (Transcriptomics and Epigenomics) | Gene chip arrays, DNA hybridization, qPCR, and gel electrophoresis | DNA, RNA and mRNA | [70] |
| Metabolomics | Nuclear magnetic resonance spectroscopy (NMR), gas chromatography-mass spectrometry, direct flow injection/liquid chromatography-mass spectrometry, inductively coupled plasma mass spectrometry, and high-performance liquid chromatography (HPLC), capillary electrophoresis time of flight mass spectroscopy | Small molecules end products of metabolic processes in the body such as organic species, together with non-protein hormones (epinephrine, peptide hormones and cortisol). | [71] |
| Microbiome | Bacterial microarrays, DNA hybridization, PCR, next-generation sequencing, and quantitative 16S rRNA gene sequencing, oligonucleotide microarray based on 16S rRNA, aptly named human-microbe identification microarrays (HOMIM) | Bacterial species (Streptococcus, Staphylococcus) | [68,72,73] |
| Immunomics | Immunologic analysis | Immunological markers (IgM, IgA, and IgG tests, and hepatitis B virus and hepatitis C virus, IgG) | [67] |
| PoC Platforms | System Used | Biomarker | Test Duration | Region of Origin |
|---|---|---|---|---|
| Single | Microelectromechanical technology (MEMS), optical fluorescent system followed by electrophoresis | Matrix metalloproteinase-8 MMP | 10 min | Sandia National Lab (USA) |
| Oral risk indicator ORI | MMP-8 | Less than 10 min | Dentognostics (Germany) | |
| Chromatography test strips | HIV1&2, HCV, influenza | 20 min | Orasure Technologies (USA) | |
| Handheld device | Cortisol, a-amylase | 1 min | Nipro (Japan) | |
| Multiplexed | Salivary diagnostics | Salivary proteins and nucleic acids | Less than 15 min | SDx (USA) |
| Salivary diagnostics | IL-8 mRNA, IL-8 protein | Less than 15 min | SDx (USA) |
| Types of Emerging Technologies | Biomarkers/Clinical Utility | Technique Used | Function | References |
|---|---|---|---|---|
| Biosensors | Diabetes mellitus, (glucose biosensors), UTI, cardiac markers, acute leukaemias | Electrochemical | Drug delivery, cardio MEMS to monitor heart patients, hearing aids, insulin micropumps, endoscopic pills, retinal prosthesis | [79] |
| Fluorescent biosensors/FRET biosensor | Drug discovery, arthritis, cancers, cardiovascular and neurodegenerative diseases, viral infections, chronic myeloid leukaemia | Fluorescent probes are mounted through a receptor | They are able to probe gene expression, localisation of protein, signal transduction, transcription and cell cycle apoptosis | [77] |
| Biological Microelectromechanical Systems (BioMEMS) | Drug delivery, cardio MEMS, insulin micro pumps, endoscopic pills, retinal prosthesis | lab-on-a-chip systems/micro/nano-scale fabrication | Detection of, proteins, viruses, DNA and microorganisms | [80,81] |
| Microfluidics/paper based technology | Stomach cancer biomarkers (H. pylori), detection of urine metabolites, blood glucose, pH value, liver function, infectious agents | Optoelectronic and microfluidic system | DNA extraction, polymerase chain reaction (PCR) amplification | [83] |
| Electric field induced release and measurement EFIRM | IL-8 protein and IL-8 mRNA markers for oral cancer, non-squamous cell lung cancer (NSCLC) oncogenic mutation, EGFR mutation in no small cell lung cancer | Electrochemical | Liquid biopsy technique, selective hybridization | [57,84] |
| Smartphone based biosensors | Blood samples of falciparum malaria infected and fluorescent images M. tuberculosis-positive sputum smears, self-monitoring of blood glucose, cancer | Metal-oxide semiconductor (CMOS)-based photo cameras, optical-based methods including absorbance, chemiluminescence, fluorescence, reflectance, surface plasmon resonance (SPR), bio- and electrochemilumines-cence | Detector system for reflectance, colorimetry and luminescence | [85] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.S.; Khurshid, Z.; Yahya Ibrahim Asiri, F. Advancing Point-of-Care (PoC) Testing Using Human Saliva as Liquid Biopsy. Diagnostics 2017, 7, 39. https://doi.org/10.3390/diagnostics7030039
Khan RS, Khurshid Z, Yahya Ibrahim Asiri F. Advancing Point-of-Care (PoC) Testing Using Human Saliva as Liquid Biopsy. Diagnostics. 2017; 7(3):39. https://doi.org/10.3390/diagnostics7030039
Chicago/Turabian StyleKhan, Rabia Sannam, Zohaib Khurshid, and Faris Yahya Ibrahim Asiri. 2017. "Advancing Point-of-Care (PoC) Testing Using Human Saliva as Liquid Biopsy" Diagnostics 7, no. 3: 39. https://doi.org/10.3390/diagnostics7030039