Prospective Comparison of F-18 Choline PET/CT Scan Versus Axial MRI for Detecting Bone Metastasis in Biochemically Relapsed Prostate Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
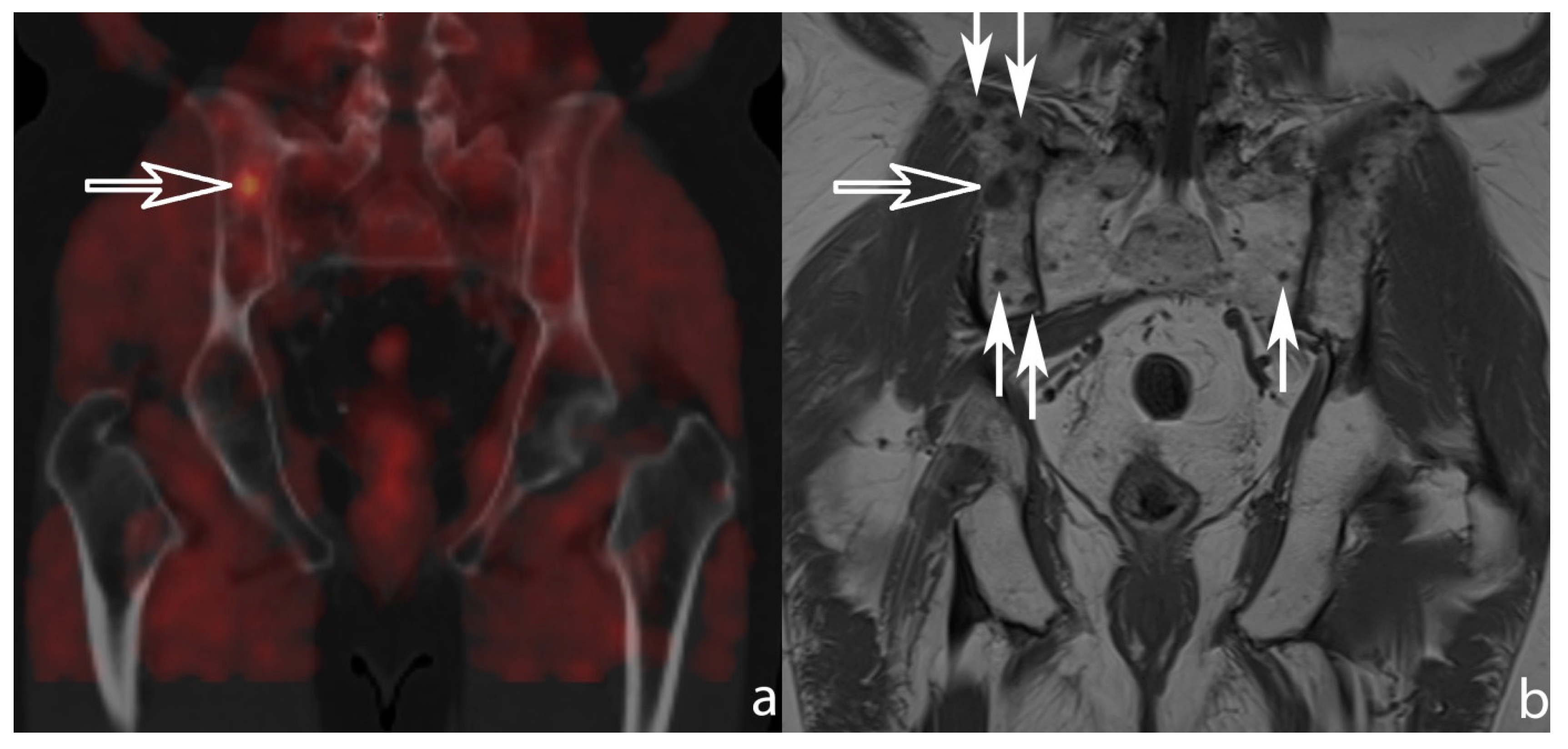
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Ost, P.; Bossi, A.; Decaestecker, K.; de Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach, M.; Briganti, A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Beresford, M.J.; Gillatt, D.; Benson, R.J.; Ajithkumar, T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. 2010, 22, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, E.; Tran, P.T.; Ost, P. Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Curr. Opin. Urol. 2017, 27, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Decaestecker, K.; de Meerleer, G.; Ameye, F.; Fonteyne, V.; Lambert, B.; Joniau, S.; Delrue, L.; Billiet, I.; Duthoy, W.; Junius, S.; et al. Surveillance or metastasis-directed therapy for OligoMetastatic prostate cancer recurrence (STOMP): Study protocol for a randomized phase II trial. BMC Cancer 2014, 14, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruycker, A.; Lambert, B.; Claeys, T.; Delrue, L.; Mbah, C.; de Meerleer, G.; Villeirs, G.; de Vos, F.; de Man, K.; Decaestecker, K.; et al. Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; El Mouedden, J.; Collette, L.; Coche, E.; Danse, E.; Jamar, F.; Machiels, J.-P.; Vande Berg, B.; Omoumi, P.; Tombal, B. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 2012, 62, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Geukens, D.; Stainier, A.; Jamar, F.; Jamart, J.; d’Othee, B.J.; Therasse, P.; Vande Berg, B.; Tombal, B. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: Diagnostic and cost-effectiveness and comparison with current detection strategies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Decaestecker, K.; Lambert, B.; Fonteyne, V.; Delrue, L.; Lumen, N.; Ameye, F.; de Meerleer, G. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate 2014, 74, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Zhou, X.C.; Wang, H.; Yang, T.; Shaukat, F.; Partin, A.W.; Eisenberger, M.A.; Antonarakis, E.S. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Wetter, A.; Lipponer, C.; Nensa, F.; Heusch, P.; Rubben, H.; Schlosser, T.W.; Poppel, T.D.; Lauenstein, T.C.; Nagarajah, J. Quantitative evaluation of bone metastases from prostate cancer with simultaneous 18F choline PET/MRI: Combined SUV and ADC analysis. Ann. Nucl. Med. 2014, 28, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. (68)Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2016. [Google Scholar] [CrossRef] [PubMed]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; La Fougère, C. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef] [PubMed]
| Patient-Based Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| BVC for bone metastases | ||||||||
| Image modality | Scan result | Negative (n = 49) No. | Positive (n = 15) No. | Total (n = 64) No. | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
| Ax MRI | Negative | 47 | 0 | 47 | 100 (75–100) | 96 (84–99) | 88 (62–98) | 100 (91–100) |
| Positive | 2 | 15 | 17 | |||||
| F-18 Choline PET | Negative | 49 | 2 | 51 | 87 (58–98) | 100 (91–100) | 100 (72–100) | 96 (85–99) |
| Positive | 0 | 13 | 13 | p = 0.5 | p = 0.5 | |||
| Lesion-based analysis | ||||||||
| Image modality | Scan result | Negative (n = 55) No. | Positive (n = 24) No. | Total (n = 79) No. | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
| Ax MRI | Negative | 51 | 0 | 51 | 100 (83–100) | 93 (82–98) | 86 (66–95) | 100 (91–100) |
| Positive | 4 | 24 | 28 | |||||
| F-18 Choline PET | Negative | 55 | 6 | 61 | 75 (53–89) | 100 (91–100) | 100 (78–100) | 90 (79–96) |
| Positive | 0 | 18 | 18 | p = 0.031 | p = 0.125 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huysse, W.; Lecouvet, F.; Castellucci, P.; Ost, P.; Lambrecht, V.; Artigas, C.; Denis, M.-L.; Man, K.D.; Delrue, L.; Jans, L.; et al. Prospective Comparison of F-18 Choline PET/CT Scan Versus Axial MRI for Detecting Bone Metastasis in Biochemically Relapsed Prostate Cancer Patients. Diagnostics 2017, 7, 56. https://doi.org/10.3390/diagnostics7040056
Huysse W, Lecouvet F, Castellucci P, Ost P, Lambrecht V, Artigas C, Denis M-L, Man KD, Delrue L, Jans L, et al. Prospective Comparison of F-18 Choline PET/CT Scan Versus Axial MRI for Detecting Bone Metastasis in Biochemically Relapsed Prostate Cancer Patients. Diagnostics. 2017; 7(4):56. https://doi.org/10.3390/diagnostics7040056
Chicago/Turabian StyleHuysse, Wouter, Frédéric Lecouvet, Paolo Castellucci, Piet Ost, Valerie Lambrecht, Carlos Artigas, Marie-Laurence Denis, Kathia De Man, Louke Delrue, Lennart Jans, and et al. 2017. "Prospective Comparison of F-18 Choline PET/CT Scan Versus Axial MRI for Detecting Bone Metastasis in Biochemically Relapsed Prostate Cancer Patients" Diagnostics 7, no. 4: 56. https://doi.org/10.3390/diagnostics7040056





