Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring
Abstract
:1. Introduction
2. Human Breath for Diagnosis of Diseases
Volatile Organic Compounds in Breath
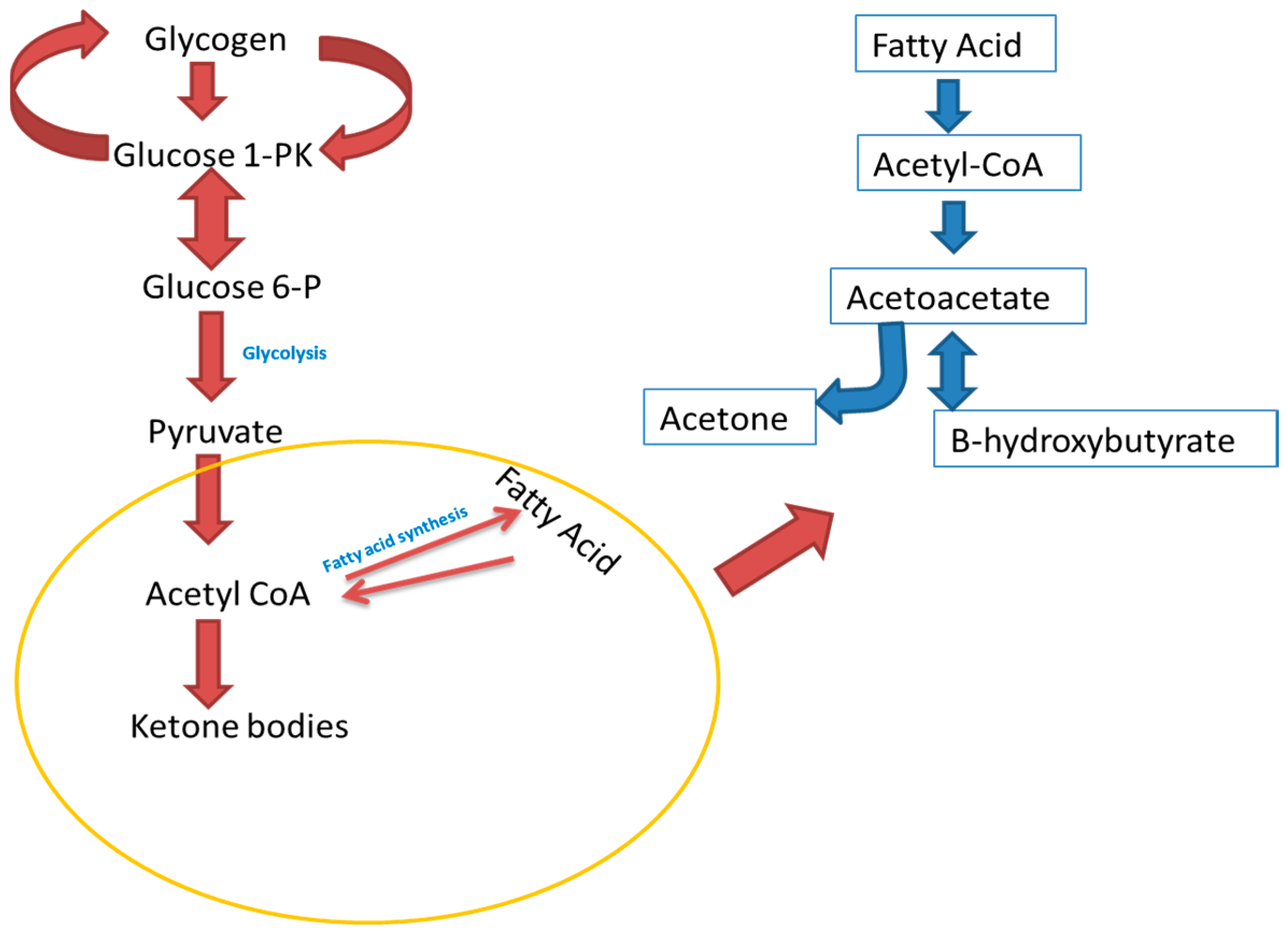
3. Acetone Metabolism
4. Various Techniques Used in Diabetes Monitoring and Diagnosis
5. Nanomaterial-Based Approaches for Detection of Acetone
6. Limitations of Semiconducting Metal Oxides (SMOs)
- sensitivity, a change of measured signal per analyte unit, that is, the slope of a calibration graph;
- selectivity, a characteristic that determines whether a sensor can respond selectively to a single analyte;
- stability, the ability of a sensor to provide reproducible results for a certain period of time. This includes retaining the sensitivity, selectivity, response and recovery time;
- durability, the ability to withstand damage due to temperature, chemical addition and so on;
- response time, the time required for the sensor to respond to a stepped concentration change from zero to a certain concentration value;
- recovery time, the time it takes for the sensor signal to return to its initial value after a stepped concentration change from a certain value to zero;
- room temperature operation and so on, the ability to detect gases at room temperature.
7. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Surya, S.; Salam, A.D.; Tomy, D.V.; Carla, B.; Kumar, R.A.; Sunil, C. Diabetes mellitus and medicinal plants—A review. Asian Pac. J. Trop. Dis. 2014, 4, 337–347. [Google Scholar] [CrossRef]
- Karalliedde, J.; Gnudi, L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol. Dial. Transplant. 2014, 31, 206–313. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Banerjee, M.; Vats, P. Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. RedoxBiol. 2014, 2, 170–177. [Google Scholar]
- Baynes, H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015, 6, 2. [Google Scholar]
- Poretsky, L. Principles of Diabetes Mellitus; Springer: Boston, MA, USA, 2010. [Google Scholar]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S16. [Google Scholar]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ngo, J.; Blake, D.; Meinardi, S.; Pontello, A.M.; Newcomb, R.; Galassetti, P.R. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J. Appl. Physiol. 2009, 107, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hu, W. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Ethn. Dis. 2005, 15, 32–34. [Google Scholar]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. J. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Parekh, B.; Walton, C.; Španěl, P.; Smith, D.; Evans, M. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.; Oliver, S.R.; Ngo, J.; Flores, R.; Midyett, J.; Meinardi, S.; Carlson, M.K.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1175. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, X.; Yin, H.; Wang, Z.; Jiang, C.; Liu, W.; Chen, Z.; Yuan, Y.; Li, Y.; Wang, C. Study of breath acetone and its correlations with blood glucose and blood beta-hydroxybutyrate using an animal model with lab-developed type 1 diabetic rats. RSC Adv. 2015, 5, 71002–71010. [Google Scholar] [CrossRef]
- Rydosz, A. A negative correlation between blood glucose and acetone measured in healthy and type 1 diabetes mellitus patient breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Semenya, S.; Potgieter, M.; Tshisikhawe, M.; Shava, S.; Maroyi, A. Medicinal utilization of exotic plants by Bapedi traditional healers to treat human ailments in Limpopo province, South Africa. J. Ethnopharmacol. 2012, 144, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si: WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Nikan, E.; Khodadadi, A.A.; Mortazavi, Y. Highly sensitive carbon nanotubes–SnO2 nanocomposite sensor for acetone detection in diabetes mellitus breath. Sens. Actuators B Chem. 2014, 205, 261–267. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, X.; Zhao, Z.; Li, L.; Zhang, L.; Yao, H.; Wang, J.S.; Li, Z.J. Highly sensitive and selective acetone sensor based on C-doped WO3 for potential diagnosis of diabetes mellitus. Sens. Actuators B Chem. 2014, 199, 210–219. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Mochalski, P.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Filipiak, W.; Teschl, S.; Hinterhuber, H.; Amann, A. Dynamic profiles of volatile organic compounds in exhaled breath as determined by a coupled PTR-MS/GC-MS study. Physiol. Meas. 2010, 31, 1169. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Kupferthaler, A.; Frauscher, B.; Hackner, H.; Unterkofler, K.; Teschl, G.; Hinterhuber, H.; Amann, A.; Högl, B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012, 33, 413. [Google Scholar] [CrossRef] [PubMed]
- Ligor, T.; Ligor, M.; Amann, A.; Ager, C.; Bachler, M.; Dzien, A.; Buszewski, B. The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J. Breath Res. 2008, 2, 046006. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J. Thorac. Oncol. 2008, 3, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, R.; Mon, T.R.; Robinson, A.B.; Cary, P.; Pauling, L. Gas chromatography of volatiles from breath and urine. Anal. Chem. 1972, 44, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- O’hara, M.E.; Clutton-Brock, T.H.; Green, S.; Mayhew, C.A. Endogenous volatile organic compounds in breath and blood of healthy volunteers: Examining breath analysis as a surrogate for blood measurements. J. Breath Res. 2009, 3, 027005. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Kęsy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Worrall, A.D.; Bernstein, J.A.; Angelopoulos, A.P. Portable method of measuring gaseous acetone concentrations. Talanta 2013, 112, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.S.; Khalid, T.; Ahmed, I.; Johnson, E.; Smith, S.; Ratcliffe, N.M. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointest. Liver Dis. 2009, 18, 337–343. [Google Scholar]
- Marczin, N.; Yacoub, M. Disease Markers in Exhaled Breath: Basic Mechanisms and Clinical Applications; IOS Press: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–36451. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, F.; Wang, Y.; Pan, Y.; Lu, D.; Wang, P.; Ying, K.; Chen, E.; Zhang, W. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer 2007, 110, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Van den Velde, S.; Nevens, F.; van Steenberghe, D.; Quirynen, M. GC–MS analysis of breath odor compounds in liver patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index; Merck & Co. Inc.: Rahway, NJ, USA, 1989; p. 104. [Google Scholar]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.L.; Pownraj, P.; Abdulla, S.; Pullithadathil, B. Technologies for clinical diagnosis using expired human breath analysis. Diagnostics 2015, 5, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Kheel, H.; Park, S.; Lee, S.; Park, S.E.; Lee, C. Synthesis of TiO2 nanorods decorated with NiO nanoparticles and their acetone sensing properties. Ceram. Int. 2016, 42, 1063–1069. [Google Scholar] [CrossRef]
- Yamane, N.; Tsuda, T.; Nose, K.; Yamamoto, A.; Ishiguro, H.; Kondo, T. Relationship between skin acetone and blood β-hydroxybutyrate concentrations in diabetes. Clin. Chim. Acta 2006, 365, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Yang, C.; Lin, C.; Chen, C.; Shih, C. Applications of Hadamard transform-gas chromatography/mass spectrometry to the detection of acetone in healthy human and diabetes mellitus patient breath. Talanta 2014, 120, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Choi, S.; Kim, S.; Jang, J. Exhaled breath sensors. In Smart Sensors for Health and Environment Monitoring; Springer: Dordrecht, The Netherlands, 2015; pp. 19–49. [Google Scholar]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K. From highly sophisticated analytical techniques to life-saving diagnostics: Technical developments in breath analysis. TrAC Trends Anal. Chem. 2006, 25, 665–673. [Google Scholar] [CrossRef]
- Stashenko, E.; Martinez, J.R. Gas Chromatography-Mass Spectrometry. In Advances in Gas Chromatography; InTech: London, UK, 2014. [Google Scholar]
- Ueta, I.; Saito, Y.; Hosoe, M.; Okamoto, M.; Ohkita, H.; Shirai, S.; Tamura, H.; Jinno, K. Breath acetone analysis with miniaturized sample preparation device: In-needle preconcentration and subsequent determination by gas chromatography–mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Jordan, A. Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998, 27, 347–375. [Google Scholar] [CrossRef]
- Zhan, X.; Duan, J.; Duan, Y. Recent developments of proton-transfer reaction mass spectrometry (PTR-MS) and its applications in medical research. Mass Spectrom. Rev. 2013, 32, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Gastl, G.A.; Amann, A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.W.; Bartlome, R.; Marinov, D.; Rey, J.M.; Vogler, D.E.; Wachter, H. Trace gas monitoring with infrared laser-based detection schemes. Appl. Phys. B 2008, 90, 289–300. [Google Scholar] [CrossRef]
- Kosterev, A.; Wysocki, G.; Bakhirkin, Y.; So, S.; Lewicki, R.; Fraser, M.; Tittel, F.; Curl, R.F. Application of quantum cascade lasers to trace gas analysis. Appl. Phys. B 2008, 90, 165–176. [Google Scholar] [CrossRef]
- Faist, J.; Capasso, F.; Sivco, D.L.; Sirtori, C.; Hutchinson, A.L.; Cho, A.Y. Quantum cascade laser. Science 1994, 264, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Werle, P. A review of recent advances in semiconductor laser based gas monitors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 197–236. [Google Scholar] [CrossRef]
- Bai, Y.; Bandyopadhyay, N.; Tsao, S.; Slivken, S.; Razeghi, M. Room temperature quantum cascade lasers with 27% wall plug efficiency. Appl. Phys. Lett. 2011, 98, 181102. [Google Scholar] [CrossRef]
- Wagner, T.; Schöning, M.J. Light-addressable potentiometric sensors (LAPS): Recent trends and applications. Compr. Anal. Chem. 2007, 49, 87–128. [Google Scholar]
- Dean, G.; Hafeman, J.; Wallace, P. Light-addressable po-tentiometric sensor for biochemical systems. Science 1988, 240, 1182–1185. [Google Scholar]
- Zhang, Q.; Wang, P.; Li, J.; Gao, X. Diagnosis of diabetes by image detection of breath using gas-sensitive laps. Biosens. Bioelectron. 2000, 15, 249–256. [Google Scholar] [CrossRef]
- Kim, I.; Rothschild, A.; Tuller, H.L. Advances and new directions in gas-sensing devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Marín, P.; Periago, J.F. Application of solid-phase microextraction and gas chromatography–mass spectrometry to the determination of volatile organic compounds in end-exhaled breath samples. J. Chromatogr. A 2003, 1011, 125–134. [Google Scholar] [CrossRef]
- Massick, S. Biomedical Optics & Medical Imaging Portable Breath Acetone Measurements Combine Chemistry and Spectroscopy. Available online: http://spie.org/newsroom/0948-portable-breath-acetone-measurements-combine-chemistry-and-spectroscopy?highlight=x2416&SSO=1 (accessed on 6 December 2007).
- King, J.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Teschl, S.; Teschl, G.; Miekisch, W.; Schubert, J.; Hinterhuber, H.; Amann, A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009, 3, 027006. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Pizzini, A.; Arendacká, B.; Zerlauth, K.; Filipiak, W.; Schmid, A.; Dzien, A.; Neuner, S.; Lechleitner, M.; Scholl-Bürgi, S.; et al. Breath acetone—Aspects of normal physiology related to age and gender as determined in a PTR-MS study. J. Breath Res. 2009, 3, 027003. [Google Scholar] [CrossRef] [PubMed]
- Mihranyan, A.; Ferraz, N.; Strmme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Xu, H.; Wei, Y.; Zhu, L.; Huang, J.; Li, Y.; Liu, F.; Wang, S.; Liu, S. Bifunctional magnetic nanoparticles for analysis of aldehyde metabolites in exhaled breath of lung cancer patients. J. Chromatogr. A 2014, 1324, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xu, L.; Duan, G.; Li, T.; Zhang, H.; Li, Y.; Wang, Y.; Wang, Y.; Cai, W. Fast-response, sensitivitive and low-powered chemosensors by fusing nanostructured porous thin film and IDEs-microheater chip. Sci. Rep. 2013, 3, 1669. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Dubourg, G.; Segkos, A.; Katona, J.; Radović, M.; Savić, S.; Niarchos, G.; Tsamis, C.; Crnojević-Bengin, V. Fabrication and Characterization of Flexible and Miniaturized Humidity Sensors Using Screen-Printed TiO2 Nanoparticles as Sensitive Layer. Sensors 2017, 17, 1854. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.D.; Duy, N.V.; El-Safty, S.A.; Hieu, N.V. Meso-/nanoporous semiconducting metal oxides for gas sensor applications. J. Nanomater. 2015, 16, 186. [Google Scholar] [CrossRef]
- Hill, D.; Binions, R. Breath Analysis for Medical Diagnosis. Int. J. Smart Sens. Intell. Syst. 2012, 5. [Google Scholar] [CrossRef]
- Mochalski, P.; Rudnicka, J.; Agapiou, A.; Statheropoulos, M.; Amann, A.; Buszewski, B. Near real-time VOCs analysis using an aspiration ion mobility spectrometer. J. Breath Res. 2013, 7, 026002. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tang, Z.; Duan, Y.; Liu, Y. GC-based techniques for breath analysis: Current status, challenges, and prospects. Crit. Rev. Anal. Chem. 2016, 46, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Banerjee, D.; Ray, I.; Sen, A. Detection of biomarker in breath: A step towards noninvasive diabetes monitoring. Curr. Sci. 2008, 94, 237–242. [Google Scholar]
- Choi, S.; Lee, I.; Jang, B.; Youn, D.; Ryu, W.; Park, C.O.; Kim, I.D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Xiao, Y.; Lu, L.; Zhang, A.; Zhang, Y.; Sun, L.; Huo, L.; Li, F. Highly enhanced acetone sensing performances of porous and single crystalline ZnO nanosheets: High percentage of exposed (100) facets working together with surface modification with Pd nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.; Youn, D.; Kim, I. Exhaled VOCs sensing properties of WO3 nanofibers functionalized by Pt and IrO2 nanoparticles for diagnosis of diabetes and halitosis. J. Electroceram. 2012, 29, 106–116. [Google Scholar] [CrossRef]
- Gunawan, P.; Mei, L.; Teo, J.; Ma, J.; Highfield, J.; Li, Q.; Zhong, Z. Ultrahigh sensitivity of Au/1D α-Fe2O3 to acetone and the sensing mechanism. Langmuir 2012, 28, 14090–14099. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ryu, W.; Kim, S.; Cho, H.; Kim, I. Bi-functional co-sensitization of graphene oxide sheets and Ir nanoparticles on p-type Co3O4 nanofibers for selective acetone detection. J. Mater. Chem. B 2014, 2, 7160–7167. [Google Scholar] [CrossRef]
- Choi, S.; Jang, B.; Lee, S.; Min, B.K.; Rothschild, A.; Kim, I. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43 (Suppl. S1), S566. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 hollow nanofibers as a highly efficient gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Yang, X.; Liang, X.; Sun, P.; Chuai, X.; Gao, Y.; Liu, F.; Lu, G. Sub-ppm YSZ-based mixed potential type acetone sensor utilizing columbite type composite oxide sensing electrode. Sens. Actuators B Chem. 2017, 238 (Suppl. C), 928–937. [Google Scholar] [CrossRef]
- Tomer, V.K.; Singh, K.; Kaur, H.; Shorie, M.; Sabherwal, P. Rapid acetone detection using indium loaded WO3/SnO2 nanohybrid sensor. Sens. Actuators B Chem. 2017, 253 (Suppl. C), 703–713. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Yang, Z.; Ge, M.; Zhang, Y. One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapor detection. Sens. Actuators B Chem. 2017, 253 (Suppl. C), 641–651. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced acetone sensing properties of titanium dioxide nanoparticles with a sub-ppm detection limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Zhu, M.; Ji, G.; Zhao, L.; Yin, J.; Bie, Y.-J. Acetone sensing of ZnO nanosheets synthesized using room-temperature precipitation. Sens. Actuators B Chem. 2017, 249 (Suppl. C), 611–623. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Park, S.; Lee, C. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B Chem. 2015, 209, 180–185. [Google Scholar] [CrossRef]
- Liu, H.; Qu, F.; Gong, H.; Jiang, H.; Yang, M. Template-free synthesis of In2O3 nanoparticles and their acetone sensing properties. Mater. Lett. 2016, 182 (Suppl. C), 340–343. [Google Scholar] [CrossRef]
- Horita, T.; Kawada, T.; Sakai, N.; Yokokawa, H.; Dokiya, M. Low temperature fabrication of (Y, Gd, Sm)-doped ceria electrolyte. Solid State Ionics 1996, 86, 1255–1258. [Google Scholar]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuators B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Choi, S.; Choi, C.; Kim, S.; Cho, H.; Jeon, S.; Kim, I. Facile synthesis of hierarchical porous WO3 nanofibers having 1D nanoneedles and their functionalization with non-oxidized graphene flakes for selective detection of acetone molecules. RSC Adv. 2015, 5, 7584–7588. [Google Scholar] [CrossRef]
- Cross, R.; De Souza, M.M.; Narayanan, E.S. A low temperature combination method for the production of ZnO nanowires. Nanotechnology 2005, 16, 2188. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.G.; Whitfield, G.C.; Yang, D.J.; Kim, H.; Tuller, H.L.; Kim, I. Facile synthesis of Pt-functionalized SnO2 hollow hemispheres and their gas sensing properties. J. Electrochem. Soc. 2010, 157, J439. [Google Scholar] [CrossRef]
- Ai, Z.; Deng, K.; Wan, Q.; Zhang, L.; Lee, S. Facile microwave-assisted synthesis and magnetic and gas sensing properties of Fe3O4 nanoroses. J. Phys. Chem. C 2010, 114, 6237–6242. [Google Scholar] [CrossRef]
- Kao, K.; Hsu, M.; Chang, Y.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Batzill, M. Surface science studies of metal oxide gas sensing materials. In Metal Oxide Nanomaterials for Chemical Sensors; Springer: New York, NY, USA, 2013; pp. 35–67. [Google Scholar]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases-A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Sun, Y.; Liu, S.; Meng, F.; Liu, J.; Jin, Z.; Kong, L.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Phanichphant, S. Semiconductor metal oxides as hydrogen gas sensors. Procedia Eng. 2014, 87, 795–802. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Dong, M.; Wang, X. Nanosized tin oxide as the novel material with simultaneous detection towards CO, H2 and CH4. Sens. Actuators B Chem. 2000, 66, 225–227. [Google Scholar] [CrossRef]
- Ansari, S.G.; Boroojerdian, P.; Sainkar, S.R.; Karekar, R.N.; Aiyer, R.C.; Kulkarni, S.K. Grain size effects on H2 gas sensitivity of thick film resistor using SnO2 nanoparticles. Thin Solid Films 1997, 295, 271–276. [Google Scholar] [CrossRef]
- Tan, O.K.; Zhu, W.; Yan, Q.; Kong, L.B. Size effect and gas sensing characteristics of nanocrystallinex SnO2-(1 − x) α-Fe2O3 ethanol sensors. Sens. Actuators B Chem. 2000, 65, 361–365. [Google Scholar] [CrossRef]
- Michel, C.R.; Mena, E.L.; Martinez, A.H. Grain-size effects on gas response in nanostructured Gd0.9 Ba0.1 CoO3. Talanta 2007, 74, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, selectivity, and stability of gas-sensitive metal-oxide nanostructures. Metal Oxide Nanostruct. Their Appl. 2010, 3, 31–52. [Google Scholar]
- Shimizu, Y.; Hyodo, T.; Egashira, M. Mesoporous semiconducting oxides for gas sensor application. J. Eur. Ceram. Soc. 2004, 24, 1389–1398. [Google Scholar] [CrossRef]
- Choi, U.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Sensing properties of Au-loaded SnO2–Co3O4 composites to CO and H2. Sens. Actuators B Chem. 2005, 107, 397–401. [Google Scholar] [CrossRef]
- Gong, J.; Chen, Q.; Lian, M.; Liu, N.; Stevenson, R.G.; Adami, F. Micromachinednanocrystalline silver doped SnO2 H2S sensor. Sens. Actuators B Chem. 2006, 114, 32–39. [Google Scholar] [CrossRef]
- Galdikas, A.; Mironas, A.; Šetkus, A. Copper-doping level effect on sensitivity and selectivity of tin oxide thin-film gas sensor. Sens. Actuators B Chem. 1995, 26, 29–32. [Google Scholar] [CrossRef]
- Fryberger, T.B.; Semancik, S. Conductance response of Pd/SnO2 (110) model gas sensors to H2 and O2. Sens. Actuators B Chem. 1990, 2, 305–309. [Google Scholar] [CrossRef]
- Semancik, S.; Fryberger, T.B. Model studies of SnO2-based gas sensors: Vacancy defects and Pd additive effects. Sens. Actuators B Chem. 1990, 1, 97–102. [Google Scholar] [CrossRef]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimarb, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Nakata, S.; Okunishi, H.; Nakashima, Y. Distinction of gases with a semiconductor sensor under a cyclic temperature modulation with second-harmonic heating. Sens. Actuators B Chem. 2006, 119, 556–561. [Google Scholar] [CrossRef]
- Baschirotto, A.; Capone, S.; D’Amico, A.; Di Natale, C.; Ferragina, V.; Ferri, G.; Francioso, L.; Grassi, M.; GuerrinI, N.; Malcovati, P.; et al. A portable integrated wide-range gas sensing system with smart A/D front-end. Sens. Actuators B Chem. 2008, 130, 164–174. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Mwakikunga, B.W. A Field Effect Transistor and a Gas Detector Including a Plurality of Field Effect Transistors. U.S. Patent CN105474006A, 26 May 2014. [Google Scholar]
- Ali, H.; Bensaali, F.; Jaber, F. Novel Approach to Non-Invasive Blood Glucose Monitoring Based on Transmittance and Refraction of Visible Laser Light. IEEE Access 2017, 5, 9163–9174. [Google Scholar] [CrossRef]
- Oliver, N.S.; Toumazou, C.; Cass, A.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A. Non-invasive glucose monitoring. Curr. Opin. Biotechnol. 1996, 7, 46–49. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
| Technique | Principle | Detection Limit | Advantages | Disadvantages |
|---|---|---|---|---|
| GC–MS | Separate and analyse compounds by MS using chromatographic column (polar or non-polar) | Ppb and ppt levels | Highly selective and sensitive | Preconcentration steps, bulky, long sampling time, need for standards and requires trained operator |
| PTR–MS | Analysis of ionized molecules of target analytes by reaction with H3O+ MS | Low ppb levels | Real-time analysis | Lack of specificity, Narrow range of detectable compounds, bulky and requires trained operator |
| SIFT–MS | Analysis of ions produced by the reaction analytes and precursor ions (H3O+, NO+ or O2+) by quadrupole MS | Low ppb and ppt levels | Real-time, capability of ppt detection, broad range of detection | Cannot identify compounds, bulky and requires trained operator |
| QCL | Electrons are recycled from period to period, containing each time to the gain and the photon emission | Low ppb levels | Real-time analysis, potential for portability and miniaturization | Selectivity required for practical use and currently limited by available technology to reach sufficient specificity |
| LPAS | Analysis of trace gases. It uses the photoacoustic effect, the conversion of light to sound in all materials (solid, liquids and gases) | Ppt–ppb levels | Real-time analysis | Bulky, requires trained operator |
| SMOS-based chemoresistive sensors | Measures resistivity changes based on thinning or thickening the depletion layer of n-type SMOSs and hole accumulation layer of p-type SMOSs around the surface when exposed to oxidizing or reducing ambient gas | Ppm, ppb and ppt levels | Real-time analysis, portable, inexpensive and miniaturization | Relatively low sensitivity and less selectivity |
| Technique | Acetone Concentration | Reference |
|---|---|---|
| GC–MS | 0.049 ppb | [67] |
| 0.22–3.73 ppb | [3] | |
| 06.95–145.99 ppb | [24] | |
| 0.195–0.659 ppm | [54] | |
| PTR–MS | 0.19–1.3 ppm | [67] |
| 50 ppb | [68] | |
| 200–2000 ppb | [69] | |
| SIFT–MS | 1–20 ppm | [8] |
| 293–870 ppb | [69] |
| Material | Sensitivity (Response) (ppm) | Detection Limit (ppm) | Response/Recovery Time (s) | Operating Temperature (°C) | Reference |
|---|---|---|---|---|---|
| ZnO:Pt | 188 | 1000 | 45 | 400 | [89] |
| ZnO:Nb | 224.0 | 1000 | 56 | 400 | [89] |
| PrFeO3 | 234.4 | 500 | 6.1 | 180 | [90] |
| CdNb2O6 | 2 | 10 | 9 | 600 | [91] |
| In/WO3-SnO2 | 66.5 | 50 | 2.12 | 200 | [92] |
| 2D C3N4-SnO2 | 11 | 67 | 7 | 380 | [93] |
| TiO2 | 15.24 | 500 | 9.19 | 270 | [94] |
| 2D ZnOnanosheets | 106.1 | 500 | - | 300 | [95] |
| WO3 decorated with Au and Pd | - | 1000 | 6 | 300 | [96] |
| In2O3 nanoparticle | 21.5 | 1000 | 2 | 250 | [97] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. https://doi.org/10.3390/diagnostics8010012
Saasa V, Malwela T, Beukes M, Mokgotho M, Liu C-P, Mwakikunga B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics. 2018; 8(1):12. https://doi.org/10.3390/diagnostics8010012
Chicago/Turabian StyleSaasa, Valentine, Thomas Malwela, Mervyn Beukes, Matlou Mokgotho, Chaun-Pu Liu, and Bonex Mwakikunga. 2018. "Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring" Diagnostics 8, no. 1: 12. https://doi.org/10.3390/diagnostics8010012




