Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer
Abstract
:1. Introduction
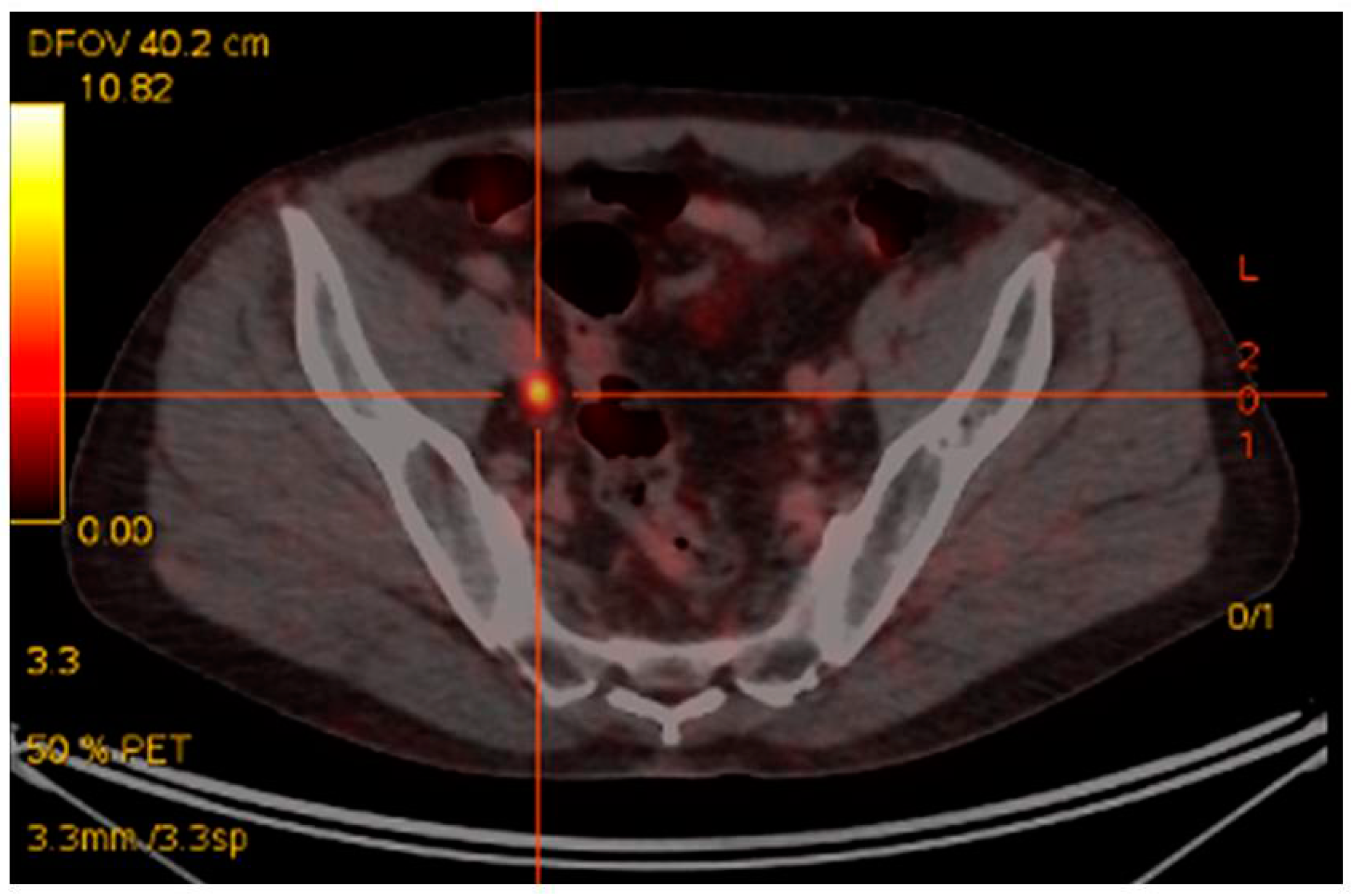
2. 68Ga PSMA PET/CT in Recurrent Disease
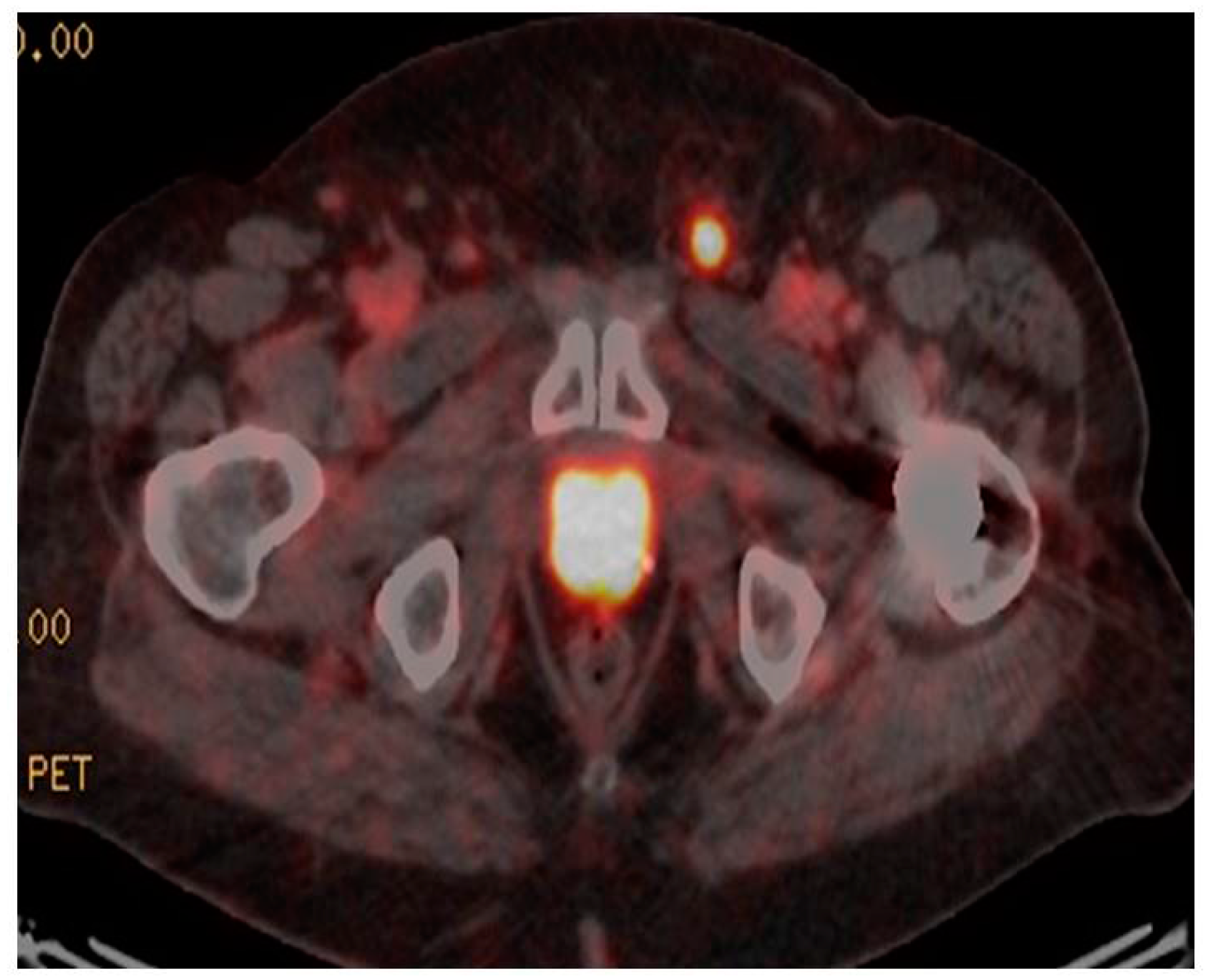
3. 68Ga-PSMA PET/CT in Primary Disease
4. New Clinical Applications
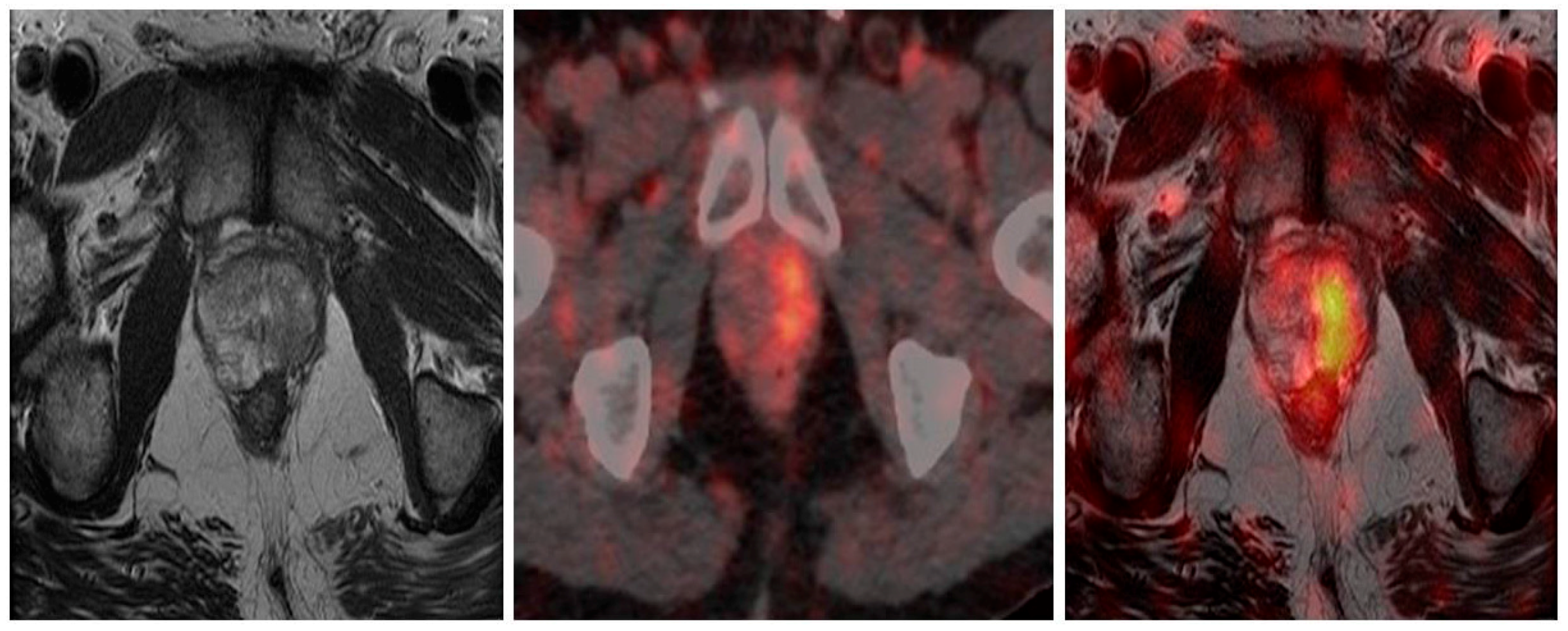
4.1. 68Ga-PSMA PET/CT and PET/MRI Guided Biopsy
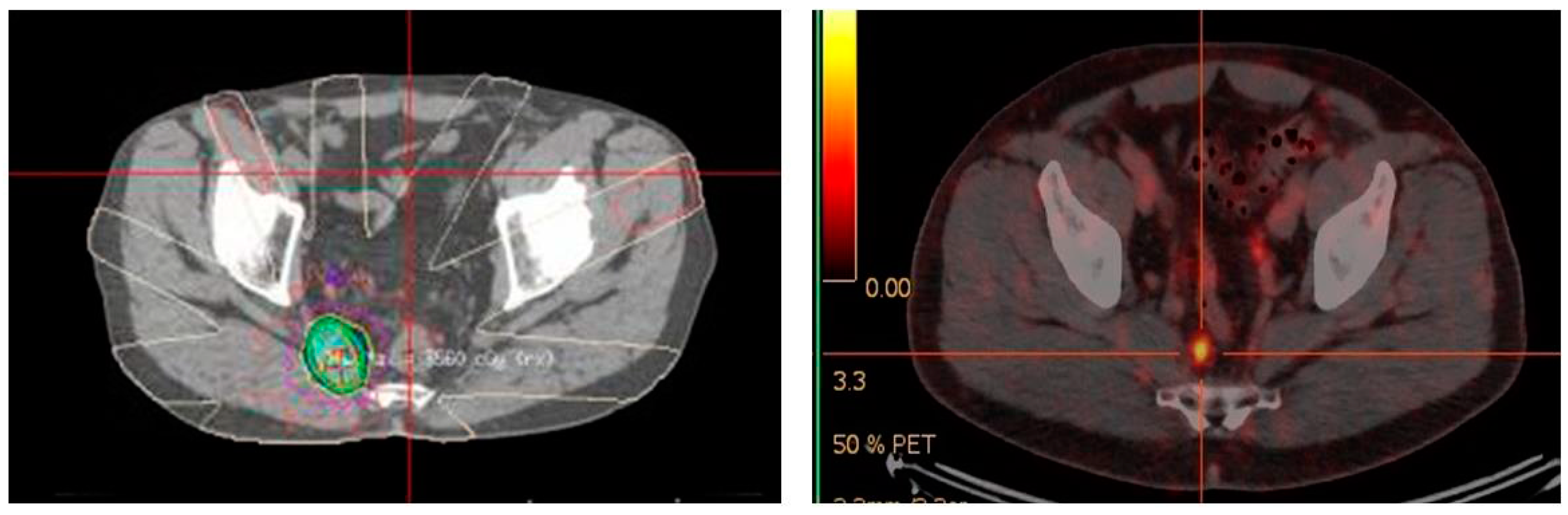
4.2. 68Ga-PSMA PET/CT-Directed Surgery and Radiotherapy
4.3. Monitoring Treatment Response
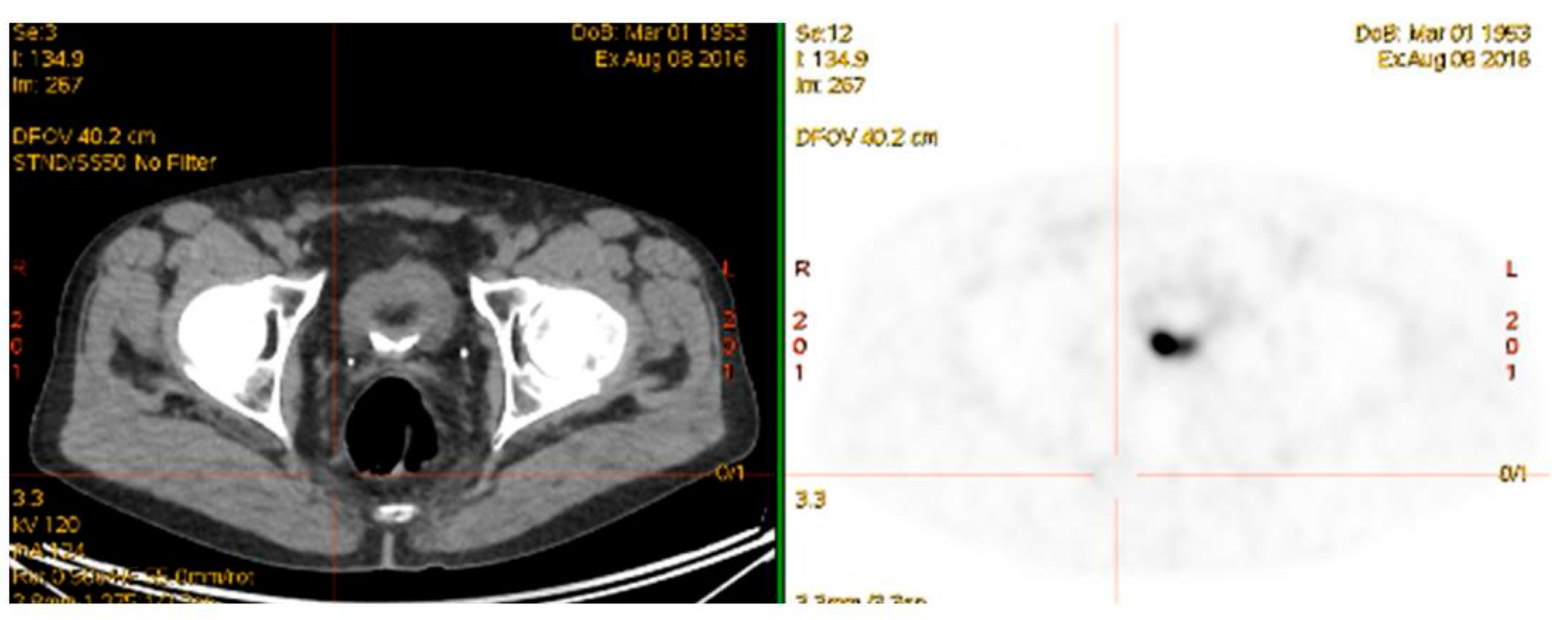
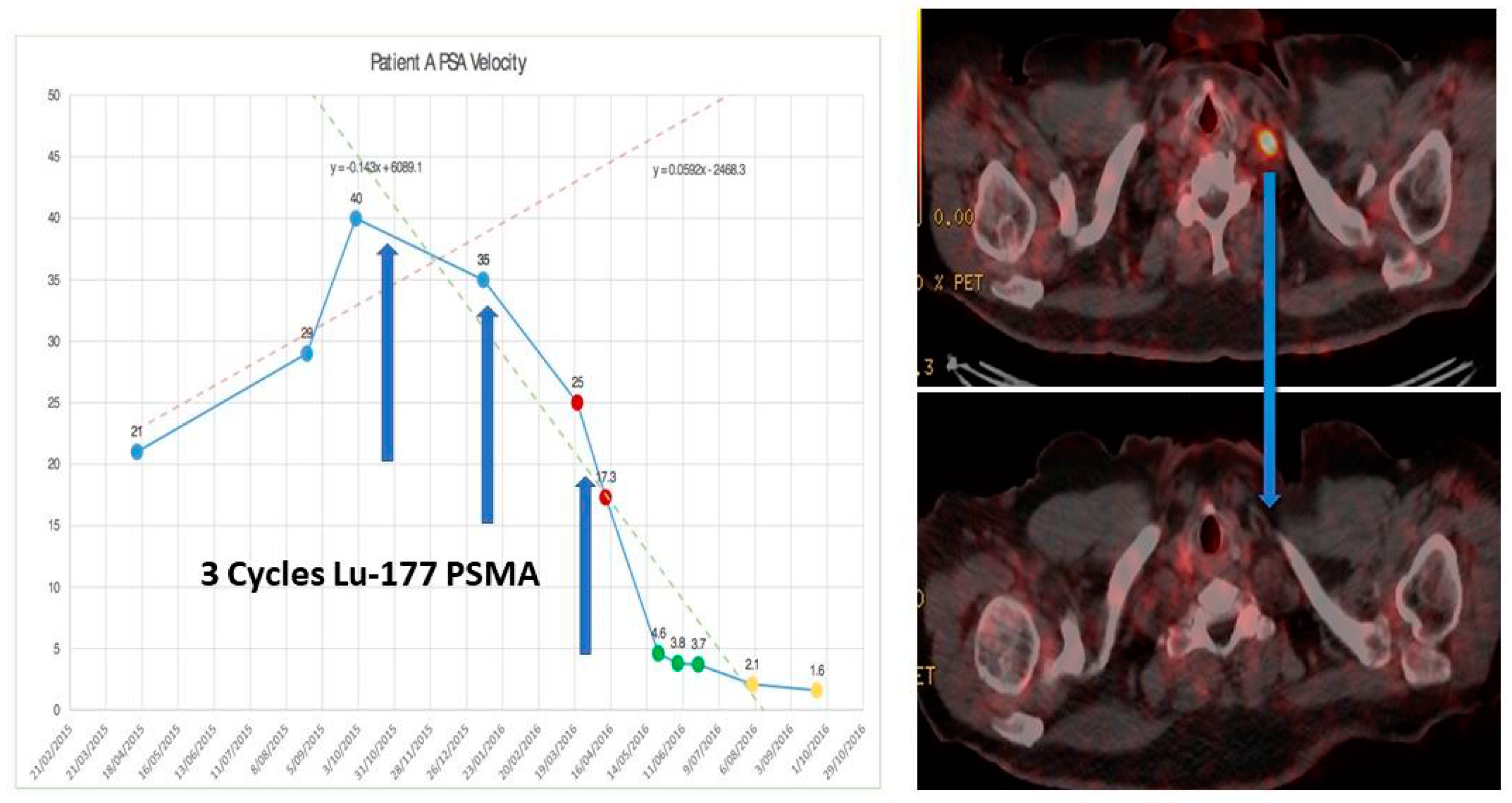
4.4. 68Ga-PSMA PET/CT Guided Theranostic Pair Therapy with 177Lu-PSMA or 225Ac-PSMA
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET/CT imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. (177)Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. A short history of the origins of radiography in Australia. Radiography 2009, 15 (Suppl. 1), e42–e47. [Google Scholar] [CrossRef]
- Robb, W.L. Perspective on the first 10 years of the CT scanner industry. Acad. Radiol. 2003, 10, 756–760. [Google Scholar] [CrossRef]
- Imagination at Work. Available online: https://www.ge.com/au/company/history/1971–1985 (accessed on 28 November 2017).
- Australian Government Policy Regarding MRI Services. Available online: http://www.users.on.net/~spinupdownunder/misc/medicare.htm (accessed on 28 November 2017).
- Mahipal, A.; Kothari, N.; Gupta, S. Epidermal growth factor receptor inhibitors: Coming of age. Cancer Control 2014, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, V.; Berkman, C.E.; Choi, J.K.; O’Keefe, D.S.; Bacich, D.J. Expression of Prostate-Specific Membrane Antigen (PSMA) Increases Cell Folate Uptake and Proliferation and Suggests a Novel Role for PSMA in the Uptake of the Non-Polyglutamated Folate, Folic Acid. Prostate 2010, 70, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Available online: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/prostate-cancer-statistics (accessed on 28 November 2017).
- Cancer Research UK. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence (accessed on 28 November 2017).
- Cancer Australia Prostate Cancer. Available online: https://prostate-cancer.canceraustralia.gov.au/statistics (accessed on 28 November 2017).
- Sadi, M.V. PSA screening for prostate cancer. Rev. Assoc. Med. Bras. 1992 2017, 63, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Neuberger, M.M.; Djulbegovic, M.; Dahm, P. Screening for prostate cancer. Cochrane Database Syst. Rev. 2013, CD004720. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S. Guideline of guidelines: Prostate cancer screening. BJU Int. 2014, 114, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Sadetsky, N.; Konety, B.R.; Resnick, M.I.; Carroll, P.R. Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer 2008, 112, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Rajcic, B.; Foreman, D.; Moretti, K.; O’Callaghan, M.E. Men presenting with prostate-specific antigen (PSA) values of over 100 ng/mL. BJU Int. 2016, 117 (Suppl. 4), 68–75. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on Prostate Cancer. European Association of Urology. 2015. Available online: http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf (accessed on 28 November 2017).
- Diagnostics Imaging Pathways. Available online: http://www.imagingpathways.health.wa.gov.au/index.php/imaging-pathways/urological/staging-of-prostate-cancer#pathway (accessed on 28 November 2017).
- Carvalhal, G.F.; Smith, D.S.; Mager, D.E.; Ramos, C.; Catalona, W.J. Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/mL or less. J. Urol. 1999, 161, 835–839. [Google Scholar] [CrossRef]
- Cui, T.; Kovell, R.C.; Terlecki, R.P. Is it time to abandon the digital rectal examination? Lessons from the PLCO Cancer Screening Trial and peer-reviewed literature. Curr. Med. Res. Opin. 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D.; et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N. Engl. J. Med. 2004, 350, 2239–2246. [Google Scholar] [PubMed]
- Hövels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.P.; Teber, D.; Hohenfellner, M.; Hadaschik, B.A. The current and future role of magnetic resonance imaging in prostate cancer detection and management. Transl. Androl. Urol. 2015, 4, 326–341. [Google Scholar] [PubMed]
- Carlaw, K.R.; Woo, H.H. Evaluation of the changing landscape of prostate cancer diagnosis and management from 2005 to 2016. Prostate Int. 2017, 5, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Saokar, A.; Islam, T.; Jantsch, M.; Saksena, M.A.; Hahn, P.F.; Harisinghani, M.G. Detection of lymph nodes in pelvic malignancies with Computed Tomography and Magnetic Resonance Imaging. Clin. Imaging 2010, 34, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Turkbey, B.; Choyke, P.L. PSMA PET/CT and Radionuclide Therapy in Prostate Cancer. Semin. Nucl. Med. 2016, 46, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.J.; Freeman, A.; Masters, J.R.; Payne, H.A.; Harland, S.J. BAUS Section of Oncology Cancer Registry. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of <10 ng/mL. BJU Int. 2005, 96, 303–307. [Google Scholar] [PubMed]
- Rajasekaran, A.K.; Anilkumar, G.; Christiansen, J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 2005, 288, C975–C981. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Rajasekaran, S.A.; Anilkumar, G.; Oshima, E.; Bowie, J.U.; Liu, H.; Heston, W.; Bander, N.H.; Rajasekaran, A.K. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell 2003, 14, 4835–4845. [Google Scholar] [CrossRef] [PubMed]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6 (Suppl. 10), S13–S18. [Google Scholar] [PubMed]
- Fung, E.K.; Cheal, S.M.; Fareedy, S.B.; Punzalan, B.; Beylergil, V.; Amir, J.; Chalasani, S.; Weber, W.A.; Spratt, D.E.; Veach, D.R.; et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: Optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Sodee, D.B.; Malguria, N.; Faulhaber, P.; Resnick, M.I.; Albert, J.; Bakale, G. Multicenter ProstaScint imaging findings in 2154 patients with prostate cancer. The ProstaScint Imaging Centers. Urology 2000, 56, 988–993. [Google Scholar] [CrossRef]
- PET/Ctronis, J.D.; Regan, F.; Lin, K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med. 1998, 23, 672–677. [Google Scholar] [CrossRef]
- Deb, N.; Goris, M.; Trisler, K.; Fowler, S.; Saal, J.; Ning, S.; Becker, M.; Marquez, C.; Knox, S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. 1996, 2, 1289–1297. [Google Scholar] [PubMed]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Beylergil, V.; Lyashchenko, S.; Ruan, S.; Solomon, S.B.; Durack, J.C.; Carrasquillo, J.A.; Lefkowitz, R.A.; Gonen, M.; et al. ⁸⁹Zr-huJ591 immuno-PET/CT imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Hull, W.E.; Wangler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of urea-based PSMA inhibitor for PET/CT imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Lutje, S.; Heskamo, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.M.W.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA ligands for radionuclide imaging and therapy of prostate cancer: Clinical status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.; Langton, T.; Kumar, D.; Campbell, A. Comparison of PSMA-HBED and PSMA-I&T as diagnostic agents in prostate carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1455–1462. [Google Scholar] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Babich, J.W.; Osborne, J.R.; Tagawa, S.T.; Lipai, I.; Solnes, L.; Maresca, K.P.; Armor, T.; Joyal, J.L.; et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: Pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J. Nucl. Med. 2014, 55, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Gage, K.L.; Mease, R.C.; Senthamizhchelvan, S.; Holt, D.P.; Jeffrey-Kwanisai, A.; Endres, C.J.; Dannals, R.F.; Sgouros, G.; Lodge, M.; et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 2012, 53, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y.; et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET/CT Imaging of Prostate Cancer. Mol. Imaging Biol. 2015, 17, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Jethwa, K.R.; Ost, P.; Williams, S.; Kwon, E.D.; Lowe, V.J.; Davis, B.J. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract. Radiat. Oncol. 2018, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J. Nucl. Med. 2017, 58, 67S–76S. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Siew, T.; Campbell, A.; Lenzo, N.; Spry, N.; Vivian, J.; Morandeau, L. ¹⁸F-Fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: Prospective comparison with standard imaging. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Patena, E.; Mazurek, A.J.; Dziuk, M. 68Ga-PSMA PET/CT imaging in recurrent prostate cancer: Where are we now? Cent. Eur. J. Urol. 2017, 70, 37–43. [Google Scholar]
- Velikyan, I. Prospective of 68Ga-radiopharmaceutical development. Theranostics 2014, 4, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Breeman, W.A.; Klette, I.; Gottschaldt, M.; Odparlik, A.; Baehre, M.; Tworowska, I.; Schultz, M.K. Radiolabelling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat. Protoc. 2016, 11, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, D.; Lenzo, N.P.; Crouch, J.; Trifunovic, M.; Henderson, A.; Turner, J.H. Gallium-68-PSMA-11 kit formulation in routine clinical management of prostate cancer. Cancer Biother. Radiopharm. 2017. in review. [Google Scholar]
- Young, J.D.; Abbate, V.; Imberti, C.; Meszaros, L.K.; Ma, M.T.; Terry, S.Y.A.; Hider, R.C.; Mullen, G.E.; Blower, P.J. 68Ga-THP-PSMA: A PET imaging agent for prostate cancer offering rapid, room temperature, one-step kit-based radiolabeling. J. Nucl. Med. 2017, 58, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Eu, P.; Jackson, P.; Hong, E.; Binns, D.; Iravani, A.; Murphy, D.; Mitchell, C.; Siva, S.; Hicks, R.J.; et al. Cold Kit PSMA PET Imaging: Phase I study of (68)Ga-THP-PSMA PET/CT in patients with prostate cancer. J. Nucl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET/CT tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic performance of (68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Asokendaran, M.; Meyrick, D.; Henderson, A.; Turner, J.H.; Lenzo, N.P. A comparison of (68)Ga-PSMA-PET/CT with diagnostic CT alone in relapsed prostate cancer. J. Mol. Imaging Dyn. 2018. in review. [Google Scholar]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.; Vach, W.; Schaal, K.; Wetterauer, U.; Schultze-Seemann, W.; Meyer, P.T. Diagnostic Accuracy of 68Ga-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics 2017, 7, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Maurer, T.; Beer, A.J.; Graner, F.P.; Haller, B.; Weirich, G.; Doherty, A.; Gschwend, J.E.; Schwaiger, M.; Eiber, M. Value of 68Ga-PSMA HBED-CC PET/CT for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J. Nucl. Med. 2016, 57, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.; Thompson, B.; Cusick, T.; et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: Results of an Australian prospective multicenter study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Albisinni, S.; Artigas, C.; Aoun, F.; Biaou, I.; Grosman, J.; Gil, T.; Hawaux, E.; Limani, K.; Otte, F.X.; Peltier, A.; et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: Preliminary analysis of a multidisciplinary approach. BJU Int. 2017, 120, 197–203. [Google Scholar] [PubMed]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; la Fougère, C. Comparison of 68Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [(18)F]DCFPyL and 68Ga-PSMA-HBED-CC for PSMA-PET/CT Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Macura, K.J.; Ciarallo, A.; Mena, E.; Blackford, A.; Nadal, R.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A.; Ross, A.E.; et al. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naïve and Castration-Resistant Metastatic Prostate Cancer. J. Nucl. Med. 2016, 57, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, A.; Wenter, V.; Kretschmer, A.; Thierfelder, K.M.; Bartenstein, P.; Faber, C.; Gildehaus, F.J.; Stief, C.G.; Gratzke, C.; Fendler, W.P. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur. Urol. 2016, 70, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Piert, M. Performance of 68Ga-PSMA PET/CT for Prostate Cancer Management at Initial Staging and Time of Biochemical Recurrence. Curr. Urol. Rep. 2017, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, D.P.; Asokendaran, M.; Skelly, L.A.; Lenzo, N.P.; Henderson, A. The role of 68Ga-PSMA-I&T PET/CT in the pretreatment staging of primary prostate cancer. Nucl. Med. Commun. 2017, 38, 956–963. [Google Scholar] [PubMed]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET/CT for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.C.; Meißner, S.; Woythal, N.; Prasad, V.; Brenner, W.; Diederichs, G.; Hamm, B.; Makowski, M.R. Comparison of hybrid 68Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. Eur. Radiol. 2018, 28, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Sterzing, F.; Schlemmer, H.P.; Holland-Letz, T.; Mier, W.; Rius, M.; Afshar-Oromieh, A.; Kopka, K.; Debus, J.; Haberkorn, U.; et al. Intra-individual comparison of 68Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/CT/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Drendel, V.; Jilg, C.A.; Rischke, H.C.; Beck, T.I.; Schultze-Seemann, W.; Krauss, T.; Mix, M.; Schiller, F.; Wetterauer, U.; et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017, 7, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M.; et al. 68Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics 2016, 6, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, M.; Costantino, L.; Salvo, V.; Grompone, M.D.; Pecoraro, M.; Stanzione, A.; Campa, R.; Vullo, F.; Sciarra, A.; Catalano, C.; et al. MRI/US fusion-guided biopsy: Performing exclusively targeted biopsies for the early detection of prostate cancer. Radiol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bastian-Jordan, M. Magnetic resonance imaging of the prostate and targeted biopsy, Comparison of PIRADS and Gleason grading. J. Med. Imaging Radiat. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Elkjær, M.C.; Andersen, M.H.; Høyer, S.; Pedersen, B.G.; Borre, M. Prostate cancer: In-bore magnetic resonance guided biopsies at active surveillance inclusion improve selection of patients for active treatment. Acta Radiol. 2017, 284185117723372. [Google Scholar] [CrossRef] [PubMed]
- Monni, F.; Fontanella, P.; Grasso, A.; Wiklund, P.; Ou, Y.C.; Randazzo, M.; Rocco, B.; Montanari, E.; Bianchi, G. Magnetic resonance imaging in prostate cancer detection and management: A systematic review. Minerva Urol. Nefrol. 2017, 69, 567–578. [Google Scholar] [PubMed]
- Herlemann, A.; Kretschmer, A.; Buchner, A.; Karl, A.; Tritschler, S.; El-Malazi, L.; Fendler, W.P.; Wenter, V.; Ilhan, H.; Bartenstein, P.; et al. Salvage lymph node dissection after (68)Ga-PSMA or (18)F-FEC PET/CT for nodal recurrence in prostate cancer patients. Oncotarget 2017, 8, 84180–84192. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, P.J.; Emmett, L.; Ho, B.; Delprado, W.; Ting, F.; Nguyen, Q.; Stricker, P.D. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017, 119, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, A.; Thompson, J.; van Leeuwen, P.J.; Doig, S.; Kalsbeek, A.; Emmett, L.; Delprado, W.; Wong, D.; Samaratunga, H.; Haynes, A.M.; et al. Initial multicentre experience of (68) gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: Acceptable safety profile but oncological benefit appears limited. BJU Int. 2017, 120, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kübler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Wirtz, M.; Eiber, M.; Maurer, T.; Wester, H.J. [(111)In]PSMA-I&T: Expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Düwel, C.; Wirtz, M.; Schottelius, M.; Wester, H.J.; Schwamborn, K.; Haller, B.; Schwaiger, M.; Gschwend, J.E.; Eiber, M.; et al. Value of (111)In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: Correlation with histopathology and clinical follow-up. BJU Int. 2017, 120, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Linke, F.; Herrmann, K.; Simunovic, I.; Eiber, M.; Kestler, C.; Buck, A.K.; Schirbel, A.; Bley, T.A.; Wester, H.J.; et al. Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Czernin, J.; Cao, M.; Kishan, A.U.; Hegde, J.V.; Shaverdian, N.; Sandler, K.A.; Chu, F.I.; King, C.R.; Steinberg, M.L.; et al. (68)Ga-PSMA PET/CT mapping of prostate cancer biochemical recurrence following radical prostatectomy in 270 patients with PSA < 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J. Nucl. Med. 2017. [Google Scholar] [CrossRef]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. (68)Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hruby, G.; Eade, T.; Kneebone, A.; Emmett, L.; Guo, L.; Ho, B.; Hsiao, E.; Schembri, G.; Hunter, J.; Kwong, C. Delineating biochemical failure with (68)Ga-PSMA-PET following definitive external beam radiation treatment for prostate cancer. Radiother. Oncol. 2017, 122, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; van Leeuwen, P.J.; Nandurkar, R.; Scheltema, M.J.; Cusick, T.; Hruby, G.; Kneebone, A.; Eade, T.; Fogarty, G.; Jagavkar, R.; et al. Treatment Outcomes from (68)Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J. Nucl. Med. 2017, 58, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Zschaeck, S.; Wust, P.; Beck, M.; Wlodarczyk, W.; Kaul, D.; Rogasch, J.; Budach, V.; Furth, C.; Ghadjar, P. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat. Oncol. 2017, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Koncz, M.; Luetzen, U.; Krause, F.; Dunst, J. Oligometastases in prostate cancer: Metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther. Onkol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.K.; Rauscher, I.; Haller, B.; Krönke, M.; Luther, S.; Heck, M.M.; Horn, T.; Gschwend, J.E.; Schwaiger, M.; Eiber, M.; et al. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, B.J.; Jang, H.J.; Kim, H.S. Comparison of the RECIST and EORTC PET criteria in the tumor response assessment: A pooled analysis and review. Cancer Chemother. Pharmacol. 2017, 80, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criterai in Solid Tumours. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschihk, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.A.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. PROstate cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J. Nucl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Brayshaw, P.A.; Price, R.A.; Turner, J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Price, R.A.; Turner, J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Turner, J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016, 103, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Turner, J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015, 30, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; van Echteld, C.J. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics 2016, 6, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.I.; van Eijck, C.H.; de Krijger, R.R.; Nieveen van Dijkum, E.J.; Teunissen, J.J.; Kam, B.L.; de Herder, W.W.; Feelders, R.A.; Bonsing, B.A.; Brabander, T.; et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J. Nucl. Med. 2015, 56, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. Recent advances in Theranostics and challenges for the future. BJR 2017. in review. [Google Scholar]
- Duffy, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Boegemann, M.; Schrader, A.J.; Rahbar, K. (177)Lu-PSMA therapy: Current evidence for use in the treatment of patients with metastatic prostate cancer. Urologe A 2017, 56, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Calopedos, R.J.S.; Chalasani, V.; Asher, R.; Emmett, L.; Woo, H.H. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lützen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [PubMed]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [PubMed]
- Kesavan, M.; Turner, J.H.; Meyrick, D.; Yeo, S.; Cardaci, G.; Lenzo, N.P. Salvage Radiopeptide Therapy of Advanced Castrate Resistant Prostate Cancer with Lutetium-177 labelled Prostate Specific Membrane Antigen (177Lu-PSMA): Efficacy and safety in Routine Practice. Cancer Biother. Radiopharm. 2017. in review. [Google Scholar]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer. Diagnostics 2018, 8, 16. https://doi.org/10.3390/diagnostics8010016
Lenzo NP, Meyrick D, Turner JH. Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer. Diagnostics. 2018; 8(1):16. https://doi.org/10.3390/diagnostics8010016
Chicago/Turabian StyleLenzo, Nat P., Danielle Meyrick, and J. Harvey Turner. 2018. "Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer" Diagnostics 8, no. 1: 16. https://doi.org/10.3390/diagnostics8010016



