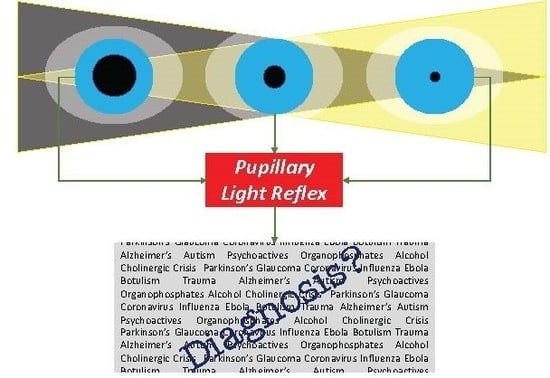
Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics
Abstract
:1. Introduction
2. Pupillary Light Reflex
3. Measuring the PLR
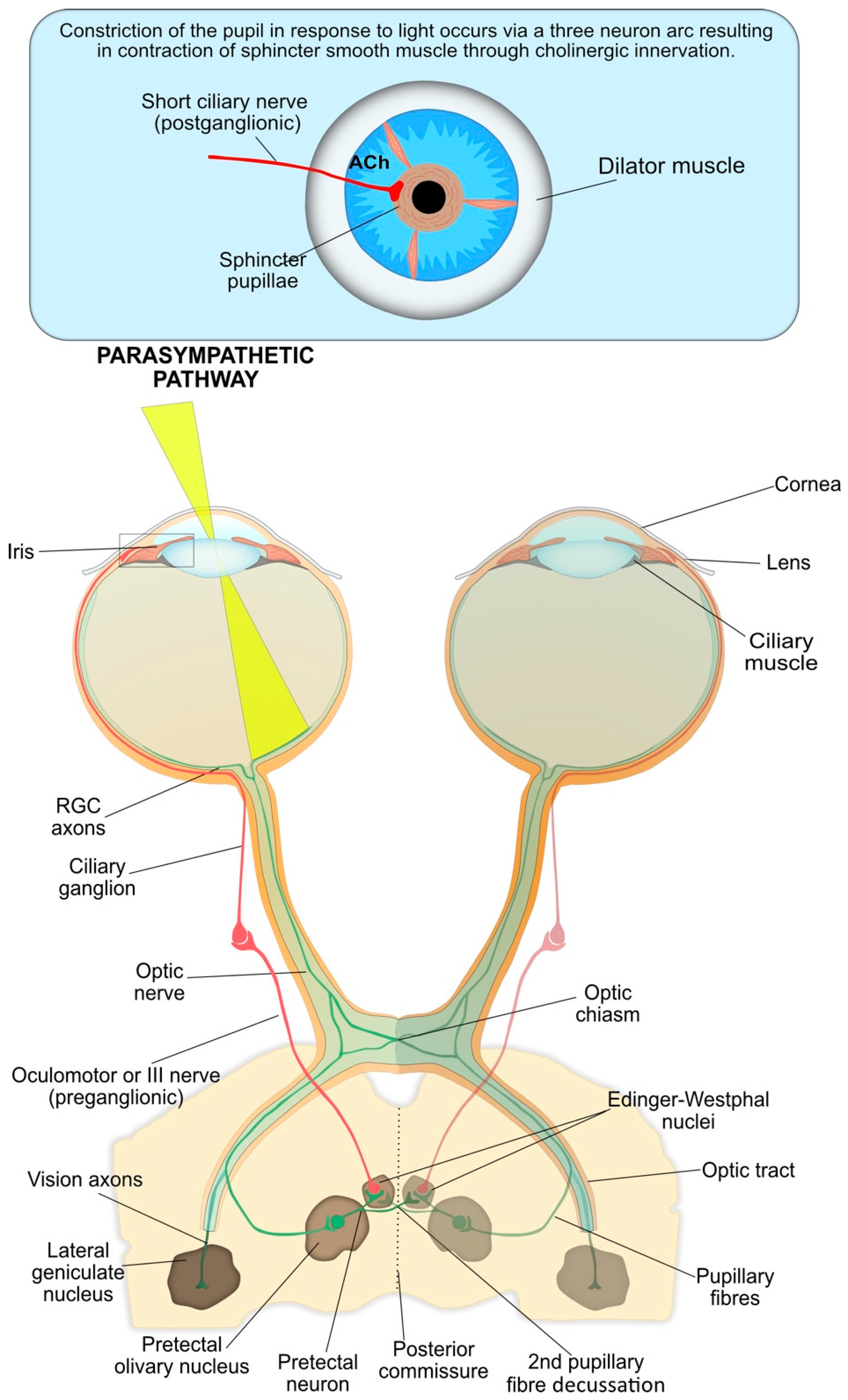
4. Neuronal Basis for the Pupillary Light Reflex
4.1. Pupil Constriction
4.2. Afferent Arm of Pupil Constriction
4.3. The Interneuron and Efferent Arms of Pupil Constriction
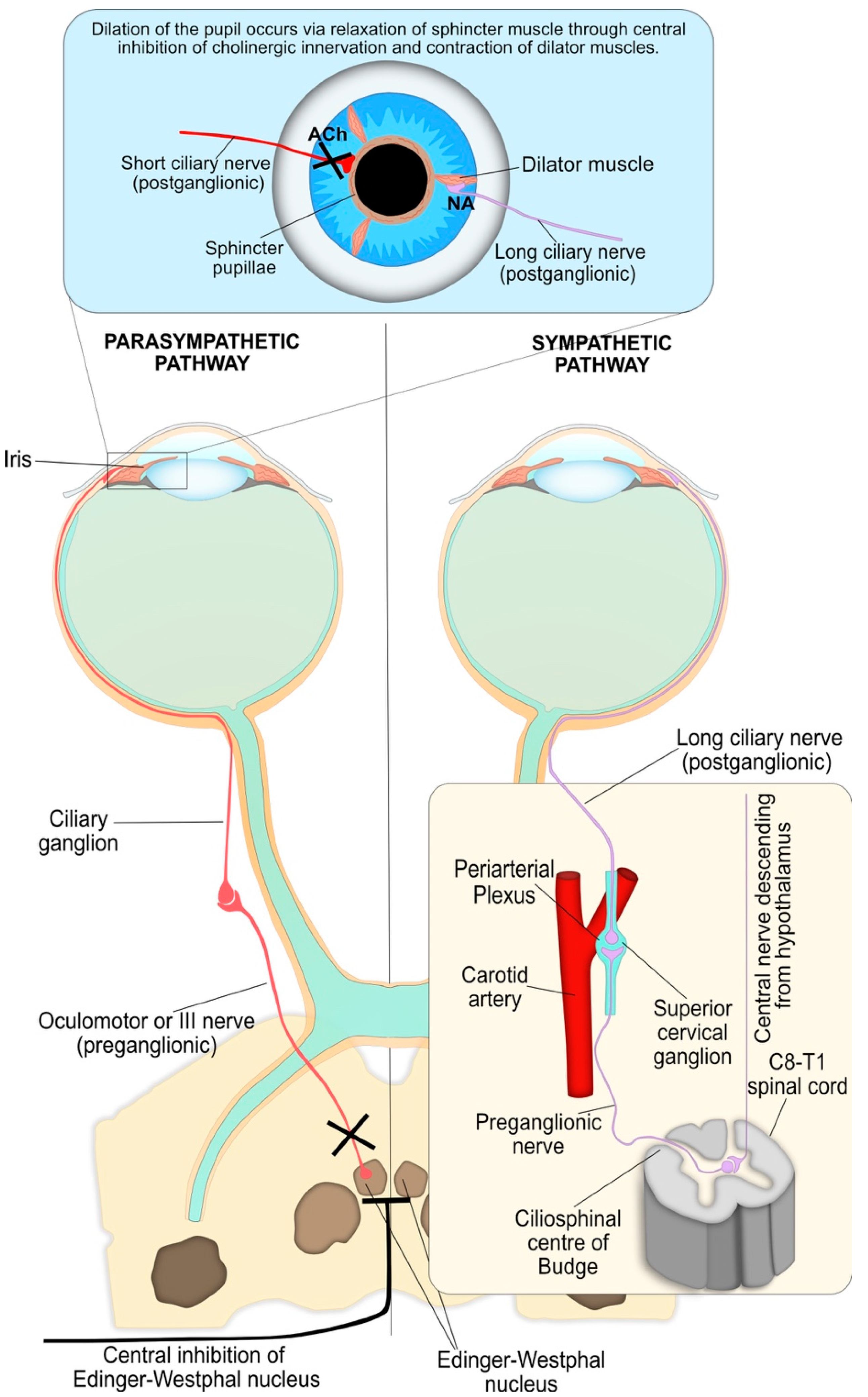
4.4. Pupil Reflex Dilation: Central and Peripheral Nervous System Integration
4.5. Other Inputs to the Iris
5. Clinical Applications of Pupillometry
5.1. Neurodegenerative Disorders
5.2. Trauma
5.3. Autism
5.4. Alcohol and Recreational Drugs
5.4.1. Alcohol
5.4.2. Recreational Drugs
5.5. Exposure to Toxins and Toxic Chemicals
5.6. Response to Infection
6. Limitations
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hirata, Y.; Yamaji, K.; Sakai, H.; Usui, S. Function of the pupil in vision and information capacity of retinal image. Syst. Comput. Jpn. 2003, 34, 48–57. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef] [PubMed]
- Girkin, C. Evaluation of the pupillary light response as an objective measure of visual function. Ophthalmol. Clin. N. Am. 2003, 16, 143–153. [Google Scholar] [CrossRef]
- Loewenfeld, I.E. The Pupil: Anatomy, Physiology, and Clinical Applications, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 1999. [Google Scholar]
- Winn, B.; Whitaker, D.; Elliott, D.B.; Phillips, N.J. Factors affecting light-adapted pupil size in normal human subjects. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1132–1137. [Google Scholar]
- Adhikari, P.; Pearson, C.A.; Anderson, A.M.; Zele, A.J.; Feigl, B. Effect of age and refractive error on the melanopsin mediated post-illumination pupil response (PIPR). Sci. Rep. 2015, 5, 17610. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.J. The pupillary light reflex in normal subjects. Br. J. Ophthalmol. 1981, 65, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, O.; Loewenfeld, I.E. The sleep-waking cycle and pupillary activity. Ann. N. Y. Acad. Sci. 1964, 117, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, O.; Kardon, R.H. Latency of the pupil light reflex: Sample rate, stimulus intensity, and variation in normal subjects. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1546–1554. [Google Scholar] [CrossRef]
- Barbur, J. Learning from the pupil: Studies of basic mechanisms and clinical applications. In The Visual Neurosciences; MIT: Cambridge, MA, USA, 2004; pp. 641–656. [Google Scholar]
- Kawasaki, A.; Kardon, R.H. Intrinsically photosensitive retinal ganglion cells. J. Neuroophthalmol. 2007, 27, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.S.; Feigl, B.; Zele, A.J. Melanopsin-mediated post-illumination pupil response in the peripheral retina. J. Vis. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Kankipati, L.; Girkin, C.A.; Gamlin, P.D. Post-illumination pupil response in subjects without ocular disease. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2764–2769. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Feigl, B.; Zele, A.J. Rhodopsin and melanopsin contributions to the early redilation phase of the post-illumination pupil response (PIPR). PLoS ONE 2016, 11, e0161175. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P.D.; McDougal, D.H.; Pokorny, J.; Smith, V.C.; Yau, K.W.; Dacey, D.M. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis. Res. 2007, 47, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.S.; Gore, C.L.; Taylor, D.; Chitkara, D.; Howes, F.; Kowalewski, E. Use of a digital infrared pupillometer to assess patient suitability for refractive surgery. J. Cataract Refract. Surg. 2002, 28, 1433–1438. [Google Scholar] [CrossRef]
- Smith, G.T. Repeatability of the procyon p3000 pupillometer. J. Refract. Surg. 2011, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Vakil-Gilani, K.; Williamson, K.L.; Cecil, S. Infrared pupillometry, the Neurological Pupil index and unilateral pupillary dilation after traumatic brain injury: Implications for treatment paradigms. Springerplus 2014, 3, 548. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.K.; Pinto, M.A.; Vieira, P.G.; Baron, J.; Tierra-Criollo, C.J. An open-source, FireWire camera-based, Labview-controlled image acquisition system for automated, dynamic pupillometry and blink detection. Comput. Methods Programs Biomed. 2013, 112, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Bremner, F.D. Pupillometric evaluation of the dynamics of the pupillary response to a brief light stimulus in healthy subjects. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7343–7347. [Google Scholar] [CrossRef] [PubMed]
- Nyström, P.; Falck-Ytter, T.; Gredebäck, G. The TimeStudio Project: An open source scientific workflow system for the behavioral and brain sciences. Behav. Res. Methods 2016, 48, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Keivanidou, A.; Fotiou, D.; Arnaoutoglou, C.; Arnaoutoglou, M.; Fotiou, F.; Karlovasitou, A. Evaluation of autonomic imbalance in patients with heart failure: A preliminary study of pupillomotor function. Cardiol. J. 2010, 17, 65–72. [Google Scholar] [PubMed]
- Wang, Y.; Zekveld, A.A.; Naylor, G.; Ohlenforst, B.; Jansma, E.P.; Lorens, A.; Lunner, T.; Kramer, S.E. Parasympathetic nervous system dysfunction, as identified by pupil light reflex, and its possible connection to hearing impairment. PLoS ONE 2016, 11, e0153566. [Google Scholar] [CrossRef] [PubMed]
- Cepko, C.L. The Determination of Rod and Cone Photoreceptor Fate. Annu. Rev. Vis. Sci. 2015, 1, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D. Why rods and cones? Eye 2016, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Tachibanaki, S. Explaining the functional differences of rods versus cones. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 675–683. [Google Scholar] [CrossRef]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M.; Liao, H.W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-W.; Ren, X.; Peterson, B.B.; Marshak, D.W.; Yau, K.-W.; Gamlin, P.D.; Dacey, D.M. Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J. Comp. Neurol. 2016, 524, 2845–2872. [Google Scholar] [CrossRef] [PubMed]
- Nasir-Ahmad, S.; Lee, S.C.S.; Martin, P.R.; Grünert, U. Melanopsin-expressing ganglion cells in human retina: Morphology, distribution, and synaptic connections. J. Comp. Neurol. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.R.; Hattar, S.; Hankins, M.W.; Lucas, R.J. Melanopsin regulates visual processing in the mouse retina. Curr. Biol. 2006, 16, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Wong, K.Y.; Shapiro, P.; Frederick, C.; Pattabiraman, K.; Berson, D.M. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 2008, 99, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Hartwick, A.T.; Bramley, J.R.; Yu, J.; Stevens, K.T.; Allen, C.N.; Baldridge, W.H.; Sollars, P.J.; Pickard, G.E. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J. Neurosci. 2007, 27, 13468–13480. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, S.; Lall, G.S.; Ralphs, K.L.; Wolstenholme, A.J.; Lucas, R.J.; Foster, R.G.; Hankins, M.W. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J. Neurosci. 2007, 27, 3981–3986. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.J.; Allen, C.N.; Brown, R.L.; Robinson, D.W. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur. J. Neurosci. 2006, 23, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Markwell, E.L.; Feigl, B.; Zele, A.J. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin. Exp. Optom. 2010, 93, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, P.; Zele, A.J.; Feigl, B. The post-illumination pupil response (PIPR). Investig. Ophthalmol. Vis. Sci. 2015, 56, 3838–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Yau, K.W. Phototransduction in mouse rods and cones. Pflugers Arch. Eur. J. Physiol. 2007, 454, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, W.F.; Luis, O. The primate chiasm. Details of visual fiber organization studied by silver impregnation techniques. Arch. Ophthalmol. 1963, 70, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Kozicz, T.; Bittencourt, J.C.; May, P.J.; Reiner, A.; Gamlin, P.D.; Palkovits, M.; Horn, A.K.; Toledo, C.A.; Ryabinin, A.E. The Edinger-Westphal nucleus: A historical, structural, and functional perspective on a dichotomous terminology. J. Comp. Neurol. 2011, 519, 1413–1434. [Google Scholar] [CrossRef] [PubMed]
- Remington, L.A. Clinical Anatomy of the Visual System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; 303p. [Google Scholar]
- Kardon, R.; Anderson, S.C.; Damarjian, T.G.; Grace, E.M.; Stone, E.; Kawasaki, A. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology 2011, 118, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Hill, R.A.; Bartholomew, M.J. Correlation of afferent pupillary defect with visual field loss on automated perimetry. Ophthalmology 1988, 95, 1649–1655. [Google Scholar] [CrossRef]
- Saari, M.; Koskela, P.; Masar, S.E. Effect of vehicle on pilocarpine-induced miosis. Acta Ophthalmol. 1978, 56, 496–503. [Google Scholar] [CrossRef]
- Liu, J.H.; Dacus, A.C. Central cholinergic stimulation affects ocular functions through sympathetic pathways. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1332–1338. [Google Scholar]
- Hansen, M.S.; Sander, B.; Kawasaki, A.; Brøndsted, A.E.; Nissen, C. Prior light exposure enhances the pupil response to subsequent short wavelength (blue) light. J. Clin. Exp. Ophthalmol. 2011, 2, 1000152. [Google Scholar] [CrossRef]
- Rubin, L.S. Pupillometric studies of alcoholism. Int. J. Neurosci. 1980, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.S.; Gottheil, E.; Roberts, A.; Alterman, A.; Holstine, J. Effects of alcohol on autonomic reactivity in alcoholics. Pupillometric studies. III. J. Stud. Alcohol 1980, 41, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Roecklein, K.; Wong, P.; Ernecoff, N.; Miller, M.; Donofry, S.; Kamarck, M.; Wood-Vasey, W.M.; Franzen, P. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 2013, 210, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.J.; Boettger, M.K.; Schulz, S.; Harzendorf, C.; Agelink, M.W.; Yeragani, V.K.; Chokka, P.; Voss, A. The interaction between pupil function and cardiovascular regulation in patients with acute schizophrenia. Clin. Neurophysiol. 2008, 119, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Bakes, A.; Bradshaw, C.M.; Szabadi, E. Attenuation of the pupillary light reflex in anxious patients. Br. J. Clin. Pharmacol. 1990, 30, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.M.; Wieseler, I.; Wilhelm, H.; Riepe, M.W.; Müller, N.G. Repetitive pupil light reflex: Potential marker in Alzheimer’s disease? J. Alzheimer Dis. 2014, 42, 1469–1477. [Google Scholar]
- Fotiou, F.; Fountoulakis, K.N.; Tsolaki, M.; Goulas, A.; Palikaras, A. Changes in pupil reaction to light in Alzheimer’s disease patients: A preliminary report. Int. J. Psychophysiol. 2000, 37, 111–120. [Google Scholar] [CrossRef]
- Tales, A.; Troscianko, T.; Lush, D.; Haworth, J.; Wilcock, G.K.; Butler, S.R. The pupillary light reflex in aging and Alzheimer’s disease. Aging 2001, 13, 473–478. [Google Scholar] [PubMed]
- Fotiou, D.F.; Stergiou, V.; Tsiptsios, D.; Lithari, C.; Nakou, M.; Karlovasitou, A. Cholinergic deficiency in Alzheimer’s and Parkinson’s disease: Evaluation with pupillometry. Int. J. Psychophysiol. 2009, 73, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Karlovasitou, A. Pupil light reflex in Parkinson’s disease: Evaluation with pupillometry. Int. J. Neurosci. 2011, 121, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Micieli, G.; Tassorelli, C.; Martignoni, E.; Pacchetti, C.; Bruggi, P.; Magri, M.; Nappi, G. Disordered pupil reactivity in Parkinson’s disease. Clin. Auton. Res. 1991, 1, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, V.; Fotiou, D.; Tsiptsios, D.; Haidich, B.; Nakou, M.; Giantselidis, C.; Karlovasitou, A. Pupillometric findings in patients with Parkinson’s disease and cognitive disorder. Int. J. Psychophysiol. 2009, 72, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Miles, J.H.; Takahashi, N.; Yao, G. Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.S. Patterns of pupillary dilatation and constriction in psychotic adults and autistic children. J. Nerv. Ment. Dis. 1961, 133, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Kankipati, L.; Girkin, C.A.; Gamlin, P.D. The post-illumination pupil response is reduced in glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Mattes, D.; Thomas, R.; Zele, A.J. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4362–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, C.; Sander, B.; Milea, D.; Kolko, M.; Herbst, K.; Hamard, P.; Lund-Andersen, H. Monochromatic pupillometry in unilateral glaucoma discloses no adaptive changes subserved by the ipRGCs. Front. Neurol. 2014, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigl, B.; Zele, A.J.; Fader, S.M.; Howes, A.N.; Hughes, C.E.; Jones, K.A.; Jones, R. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 2012, 90, e230–e234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dütsch, M.; Marthol, H.; Michelson, G.; Neundörfer, B.; Hilz, M.J. Pupillography refines the diagnosis of diabetic autonomic neuropathy. J. Neurol. Sci. 2004, 222, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.L.; Marques, J.L.; Gandhi, R.A.; Heller, S.R.; Schneider, F.K.; Tesfaye, S.; Gamba, H.R. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: A pilot study. Biomed. Eng. Online 2010, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Taniguchi, H.; Baba, S.; Yamamoto, M. The pupillary light reflex in borderline diabetics. J. Int. Med. Res. 1989, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Spaeth, E.B.; Vernino, S.; Muppidi, S. Disproportionate pupillary involvement in diabetic autonomic neuropathy. Clin. Auton. Res. 2014, 24, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, L.P.; Sen, A.; Stanko, C.M.; Rawal, B.; Heckman, M.G.; Hoyne, J.B.; Dimberg, E.L.; Freeman, M.L.; Ng, L.K.; Rabinstein, A.A.; et al. Early Absent Pupillary Light Reflexes After Cardiac Arrest in Patients Treated with Therapeutic Hypothermia. Ther. Hypothermia Temp. Manag. 2016, 6, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, D.F.; Brozou, C.G.; Haidich, A.B.; Tsiptsios, D.; Nakou, M.; Kabitsi, A.; Giantselidis, C.; Fotiou, F. Pupil reaction to light in Alzheimer’s disease: Evaluation of pupil size changes and mobility. Aging Clin. Exp. Res. 2007, 19, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Granholm, E.; Morris, S.; Galasko, D.; Shults, C.; Rogers, E.; Vukov, B. Tropicamide effects on pupil size and pupillary light reflexes in Alzheimer’s and Parkinson’s disease. Int. J. Psychophysiol. 2003, 47, 95–115. [Google Scholar] [CrossRef]
- Prettyman, R.; Bitsios, P.; Szabadi, E. Altered pupillary size and darkness and light reflexes in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Gerasimou, G.; Gotzamani-Psarrakou, A.; Karlovasitou, A. Pupillometry and 123I-DaTSCAN imaging in Parkinson’s disease: A comparison study. Int. J. Neurosci. 2012, 122, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, E.; Molaschi, M.; Villa, L.; Varetto, O.; Bogetto, C.; Nuzzi, R. Is videopupillography useful in the diagnosis of Alzheimer’s disease? Neurology 1998, 50, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; McAnany, J.J. Effect of stimulus size and luminance on the rod-, cone-, and melanopsin-mediated pupillary light reflex. J. Vis. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Hirata, Y.; Usui, S. A method for monitoring autonomic nervous activity by pupillary flash response. Syst. Comput. Jpn. 2000, 31, 22–31. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Gautille, T.; Blunt, B.A.; Klauber, M.R.; Marshall, L.E. The localizing value of asymmetry in pupillary size in severe head injury: Relation to lesion type and location. Neurosurgery 1994, 34, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Moss, H.E.; McAnany, J.J. The Pupillary Light Reflex in Idiopathic Intracranial Hypertension. Investig. Ophthalmol. Vis. Sci. 2016, 57, 23–29. [Google Scholar] [CrossRef]
- Taylor, W.R.; Chen, J.W.; Meltzer, H.; Gennarelli, T.A.; Kelbch, C.; Knowlton, S.; Richardson, J.; Lutch, M.J.; Farin, A.; Hults, K.N.; et al. Quantitative pupillometry, a new technology: Normative data and preliminary observations in patients with acute head injury. J. Neurosurg. 2003, 98, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Gombart, Z.J.; Rogers, S.; Gardiner, S.K.; Cecil, S.; Bullock, R.M. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg. Neurol. Int. 2011, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.F.; Juul, N.; Marshall, S.B.; Benedict, B.; Marshall, L.F. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Neurosurgery 1998, 43, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Meeker, M.; Du, R.; Bacchetti, P.; Privitera, C.M.; Larson, M.D.; Holland, M.C.; Manley, G. Pupil examination: Validity and clinical utility of an automated pupillometer. J. Neurosci. Nurs. 2005, 37, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Gibb, T.; Jurkovich, G.J. Evaluation and significance of the pupillary light reflex in trauma patients. Ann. Emerg. Med. 1993, 22, 1052–1057. [Google Scholar] [CrossRef]
- Couret, D.; Boumaza, D.; Grisotto, C.; Triglia, T.; Pellegrini, L.; Ocquidant, P.; Bruder, N.J.; Velly, L.J. Reliability of standard pupillometry practice in neurocritical care: An observational, double-blinded study. Crit. Care 2016, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.D.; Muhiudeen, I. Pupillometric analysis of the ‘absent light reflex’. Arch. Neurol. 1995, 52, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Stutzman, S.; Saju, C.; Wilson, M.; Zhao, W.; Aiyagari, V. Interrater Reliability of Pupillary Assessments. Neurocrit. Care 2016, 24, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.S.; Boland, M.V.; Arora, K.S.; Supakontanasan, W.; Chen, B.B.; Friedman, D.S. Symmetry of the pupillary light reflex and its relationship to retinal nerve fiber layer thickness and visual field defect. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5596–5601. [Google Scholar] [CrossRef] [PubMed]
- Suys, T.; Bouzat, P.; Marques-Vidal, P.; Sala, N.; Payen, J.F.; Rossetti, A.O.; Oddo, M. Automated quantitative pupillometry for the prognostication of coma after cardiac arrest. Neurocrit. Care 2014, 21, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Heimburger, D.; Durand, M.; Gaide-Chevronnay, L.; Dessertaine, G.; Moury, P.H.; Bouzat, P.; Albaladejo, P.; Payen, J.F. Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation 2016, 103, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Dunn, D.W.; Edwards-Brown, M.; Feinberg, J. Hydrogen proton magnetic resonance spectroscopy in autism: Preliminary evidence of elevated choline/creatine ratio. J. Child Neurol. 2002, 17, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Martin-Ruiz, C.; Graham, A.; Court, J.; Jaros, E.; Perry, R.; Iversen, P.; Bauman, M.; Perry, E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 2002, 125, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Lee, M.L.; Martin-Ruiz, C.M.; Court, J.A.; Volsen, S.G.; Merrit, J.; Folly, E.; Iversen, P.E.; Bauman, M.L.; Perry, R.H.; et al. Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain. Am. J. Psychiatry 2001, 158, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Karvat, G.; Kimchi, T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 2014, 39, 831–840. [Google Scholar] [CrossRef] [PubMed]
- McTighe, S.M.; Neal, S.J.; Lin, Q.; Hughes, Z.A.; Smith, D.G. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS ONE 2013, 8, e62189. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Heinrich, J.; Delorme, R.; Proepper, C.; Betancur, C.; Huguet, G.; Konyukh, M.; Chaste, P.; Ey, E.; Rastam, M.; et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012, 8, e1002521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daluwatte, C.; Miles, J.H.; Sun, J.; Yao, G. Association between pupillary light reflex and sensory behaviors in children with autism spectrum disorders. Res. Dev. Disabil. 2015, 37, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Daluwatte, C.; Miles, J.H.; Christ, S.E.; Beversdorf, D.Q.; Takahashi, T.N.; Yao, G. Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. J. Autism Dev. Disord. 2013, 43, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Nyström, P.; Gredebäck, G.; Bölte, S.; Falck-Ytter, T.; EASE Team. Hypersensitive pupillary light reflex in infants at risk for autism. Mol. Autism 2015, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Dockstader, C.; Gaetz, W.; Rockel, C.; Mabbott, D.J. White matter maturation in visual and motor areas predicts the latency of visual activation in children. Hum. Brain Mapp. 2012, 33, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Ben Bashat, D.; Kronfeld-Duenias, V.; Zachor, D.A.; Ekstein, P.M.; Hendler, T.; Tarrasch, R.; Even, A.; Levy, Y.; Ben Sira, L. Accelerated maturation of white matter in young children with autism: A high b value DWI study. Neuroimage 2007, 37, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E. Abnormal early brain development in autism. Mol. Psychiatry 2002, 7 (Suppl. 2), S21–S23. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Ben-Sira, L.; Levy, Y.; Zachor, D.A.; Ben Itzhak, E.; Artzi, M.; Tarrasch, R.; Eksteine, P.M.; Hendler, T.; Ben Bashat, D. Abnormal white matter integrity in young children with autism. Hum. Brain Mapp. 2011, 32, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J.; Gu, H.; Gerig, G.; Elison, J.T.; Styner, M.; Gouttard, S.; Botteron, K.N.; Dager, S.R.; Dawson, G.; Estes, A.M.; et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 2012, 169, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.E.; Cohen, M.X.; Geurts, H.M. Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci. Biobehav. Rev. 2012, 36, 604–625. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Campbell, K.; Solso, S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011, 1380, 138–145. [Google Scholar] [CrossRef] [PubMed]
- DeVito, T.J.; Drost, D.J.; Neufeld, R.W.; Rajakumar, N.; Pavlosky, W.; Williamson, P.; Nicolson, R. Evidence for cortical dysfunction in autism: A proton magnetic resonance spectroscopic imaging study. Biol. Psychiatry 2007, 61, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.; Priemer, F.; Hitzl, W.; Keller, T. Pupil function as an indicator for being under the influence of central nervous system-acting substances from a traffic-medicine perspective. Med. Sci. Law 2010, 50, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.C.; Hitzl, W.; Priemer, F.; Preiss, U.; Kunz, S.N.; Keller, T. The potential of infrared pupillography in routine police traffic checks. Rechtsmedizin 2015, 25, 466–473. [Google Scholar] [CrossRef]
- Monticelli, F.C.; Tutsch-Bauer, E.; Hitzl, W.; Keller, T. Pupil function as a parameter for assessing impairment of the central nervous system from a traffic-medicine perspective. Leg. Med. 2009, 11 (Suppl. 1), S331–S332. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.L.; Richman, J.E.; Hayes, C.E.; Huestis, M.A. Drug Recognition Expert (DRE) examination characteristics of cannabis impairment. Accid. Anal. Prev. 2016, 92, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kosnoski, E.M.; Yolton, R.L.; Citek, K.; Hayes, C.E.; Evans, R.B. The Drug Evaluation Classification Program: Using ocular and other signs to detect drug intoxication. J. Am. Optom. Assoc. 1998, 69, 211–227. [Google Scholar] [PubMed]
- Lobato-Rincón, L.L.; Cabanillas Campos, M.C.; Navarro-Valls, J.J.; Bonnin-Arias, C.; Chamorro, E.; Sánchez-Ramos Roda, C. Utility of dynamic pupillometry in alcohol testing on drivers. Adicciones 2013, 25, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pickworth, W.B.; Rohrer, M.S.; Fant, R.V. Effects of abused drugs on psychomotor performance. Exp. Clin. Psychopharmacol. 1997, 5, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tennant, F. The rapid eye test to detect drug abuse. Postgrad. Med. 1988, 84, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, Y.; Kondo, H.; Matsubuchi, N.; Takemura, T.; Kanayama, H.; Kaneko, Y.; Kanbayashi, T.; Hishikawa, Y.; Shimizu, T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol. Clin. Exp. Res. 2011, 35, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Jochum, T.; Hoyme, J.; Schulz, S.; Weißenfels, M.; Voss, A.; Bär, K.J. Diverse autonomic regulation of pupillary function and the cardiovascular system during alcohol withdrawal. Drug Alcohol Depend. 2016, 159, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Engberg, G.; Hajós, M. Alcohol withdrawal reaction as a result of adaptive changes of excitatory amino acid receptors. Naunyn Schmiedeberg Arch. Pharmacol. 1992, 346, 437–441. [Google Scholar] [CrossRef]
- Knapp, D.J.; Duncan, G.E.; Crews, F.T.; Breese, G.R. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: Further evidence for withdrawal-induced anxiety. Alcohol. Clin. Exp. Res. 1998, 22, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Fant, R.V.; Heishman, S.J.; Bunker, E.B.; Pickworth, W.B. Acute and residual effects of marijuana in humans. Pharmacol. Biochem. Behav. 1998, 60, 777–784. [Google Scholar] [CrossRef]
- Hysek, C.M.; Liechti, M.E. Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology 2012, 224, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Hepler, R.S.; Frank, I.M.; Ungerleider, J.T. Pupillary constriction after marijuana smoking. Am. J. Ophthalmol. 1972, 74, 1185–1190. [Google Scholar] [CrossRef]
- Burgen, A.S.; Dickens, F.; Zatman, L.J. The action of botulinum toxin on the neuro-muscular junction. J. Physiol. 1949, 109, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Sellin, L.C. The pharmacological mechanism of botulism. Trends Pharmacol. Sci. 1985, 6, 80–82. [Google Scholar] [CrossRef]
- Hemmerdinger, C.; Srinivasan, S.; Marsh, I.B. Reversible pupillary dilation following botulinum toxin injection to the lateral rectus. Eye 2006, 20, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Penas, S.C.; Faria, O.M.; Serrão, R.; Capão-Filipe, J.A.; Mota-Miranda, A.; Falcão-Reis, F. Ophthalmic manifestations in 18 patients with botulism diagnosed in Porto, Portugal between 1998 and 2003. J. Neuroophthalmol. 2005, 25, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, S.; Kökcen, H.K.; Atakan, T. Unilateral transient mydriasis and ptosis after botulinum toxin injection for a cosmetic procedure. Clin. Ophthalmol. 2015, 9, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.P.; Chandler, D.L.; Lee, K.A.; Superstein, R.; de Alba Campomanes, A.; Bothun, E.D.; Morin, J.; Wallace, D.K.; Kraker, R.T. Pediatric Eye Disease Investigator Group. Tonic pupil after botulinum toxin-A injection for treatment of esotropia in children. J. AAPOS 2016, 20, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Burnett, D.C.; Miller, D.B.; Jakubowski, E.M.; Muse, W.T.; Forster, J.S.; Scotto, J.A.; Jarvis, J.R.; Davis, E.A.; Hulet, S.W.; et al. Tolerance to the miotic effect of sarin vapor in rats after multiple low-level exposures. J. Ocul. Pharmacol. Ther. 2005, 21, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Miller, D.B.; Reutter, S.A.; Mioduszewski, R.J.; Thomson, S.A. Miotic tolerance to sarin vapor exposure: Role of the sympathetic and parasympathetic nervous systems. Toxicol. Sci. 2005, 85, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Morita, H.; Nakajima, T. Sarin experiences in Japan: Acute toxicity and long-term effects. J. Neurol. Sci. 2006, 249, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Rengstorff, R.H. Vision and ocular changes following accidental exposure to organophosphates. J. Appl. Toxicol. 1994, 14, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Smith, S.E. Factors determining the potency of cholinomimetic miotic drugs and their effect upon the light reflex in man. Br. J. Clin. Pharmacol. 1978, 6, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.T.; Davis, E.; Dabisch, P.; Horsmon, M.; Li, M.; Mioduszewski, R. Alterations in autonomic function in the guinea pig eye following exposure to dichlorvos vapor. J. Ocul. Pharmacol. Ther. 2008, 24, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Genovese, R.F.; Benton, B.J.; Oubre, J.L.; Fleming, P.J.; Jakubowski, E.M.; Mioduszewski, R.J. Evaluation of miosis, behavior and cholinesterase inhibition from low-level, whole-body vapor exposure to soman in African green monkeys (Chlorocebus sabeus). J. Med. Primatol. 2010, 39, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, H.; Hori, S.; Shinozawa, Y.; Fujishima, S.; Takuma, K.; Kimura, H.; Suzuki, M.; Aikawa, N. Relationship between pupil size and acetylcholinesterase activity in patients exposed to sarin vapor. Intensive Care Med. 1997, 23, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Hulet, S.W.; Sommerville, D.R.; Crosier, R.B.; Dabisch, P.A.; Miller, D.B.; Benton, B.J.; Forster, J.S.; Scotto, J.A.; Jarvis, J.R.; Krauthauser, C.; et al. Comparison of low-level sarin and cyclosarin vapor exposure on pupil size of the Gottingen minipig: Effects of exposure concentration and duration. Inhal. Toxicol. 2006, 18, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Horsmon, M.S.; Taylor, J.T.; Muse, W.T.; Miller, D.B.; Sommerville, D.R.; Mioduszewski, R.J.; Thomson, S. Gender difference in the miotic potency of soman vapor in rats. Cutan. Ocul. Toxicol. 2008, 27, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Padilla, S.; Barone, S., Jr.; Pope, C.N.; Tilson, H.A. Fenthion produces a persistent decrease in muscarinic receptor function in the adult rat retina. Toxicol. Appl. Pharmacol. 1994, 125, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Horsmon, M.S.; Muse, W.T.; Mioduszewski, R.J.; Thomson, S. Muscarinic receptor dysfunction induced by exposure to low levels of soman vapor. Toxicol. Sci. 2007, 100, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.; Bloch-Shilderman, E.; Egoz, I.; Turetz, J.; Brandeis, R. Efficacy assessment of a combined anticholinergic and oxime treatment against topical sarin-induced miosis and visual impairment in rats. Br. J. Pharmacol. 2014, 171, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Lotti, M. Clinical Toxicology of Anticholinesterase Agents in Humans. In Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1043–1085. [Google Scholar]
- Shirakawa, S.; Ishikawa, S.; Miyata, M.; Rea, W.J.; Johnson, A.R. A pupillographical study on the presence of organochlorine pesticides in autonomic nerve disturbance. Nippon Ganka Gakkai Zasshi 1990, 94, 418–423. [Google Scholar] [PubMed]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.; Hansen, M.K.; Anderson, K.; Maier, S.F.; Watkins, L.R. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton. Neurosci. Basic Clin. 2000, 85, 49–59. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yiu, H.H.; Graham, A.L.; Stengel, R.F. Dynamics of a cytokine storm. PLoS ONE 2012, 7, e45027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Chen, L.; Schmaljohn, A.L. How Ebola and Marburg viruses battle the immune system. Nat. Rev. Immunol. 2007, 7, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Kessler, B.; Rinchai, D.; Kewcharoenwong, C.; Nithichanon, A.; Biggart, R.; Hawrylowicz, C.M.; Bancroft, G.J.; Lertmemongkolchai, G. Interleukin 10 inhibits pro-inflammatory cytokine responses and killing of Burkholderia pseudomallei. Sci. Rep. 2017, 7, 42791. [Google Scholar] [CrossRef] [PubMed]
- Pechous, R.D.; Sivaraman, V.; Price, P.A.; Stasulli, N.M.; Goldman, W.E. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog. 2013, 9, e1003679. [Google Scholar] [CrossRef] [PubMed]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef] [PubMed]
- Szabadi, E. Modulation of physiological reflexes by pain: Role of the locus coeruleus. Front. Integr. Neurosci. 2012, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Song, X.M.; Li, J.G.; Wang, Y.L.; Hu, Z.F.; Zhou, Q.; Du, Z.H.; Jia, B.H. The protective effect of the cholinergic anti-inflammatory pathway against septic shock in rats. Shock 2008, 30, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Van Westerloo, D.J.; Giebelen, I.A.; Florquin, S.; Daalhuisen, J.; Bruno, M.J.; de Vos, A.F.; Tracey, K.J.; van der Poll, T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 2005, 191, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liao, H.; Ochani, M.; Justiniani, M.; Lin, X.; Yang, L.; Al-Abed, Y.; Wang, H.; Metz, C.; Miller, E.J.; et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004, 10, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Giebelen, I.A.; Leendertse, M.; Florquin, S.; van der Poll, T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur. Respir. J. 2009, 33, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 2006, 34, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Valdés-Ferrer, S.I.; Dancho, M.E.; Ochani, M.; Katz, D.; Cheng, K.F.; Olofsson, P.S.; Chavan, S.S.; Al-Abed, Y.; Tracey, K.J.; et al. Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav. Immun. 2015, 44, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.B.; Wolkmer, P.; Da Silva, A.S.; Paim, F.C.; Tonin, A.A.; Castro, V.S.; Felin, D.V.; Schmatz, R.; Gonçalves, J.F.; Badke, M.R.; et al. Cholinesterases as markers of the inflammatory process in rats infected with Leptospira interrogans serovar Icterohaemorrhagiae. J. Med. Microbiol. 2012, 61, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Goldie, R.G.; Henry, P.J. Influence of respiratory tract viral infection on endothelin-1-induced potentiation of cholinergic nerve-mediated contraction in mouse trachea. Br. J. Pharmacol. 1996, 119, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.P.; Lee, S.M.; Cheung, T.K.; Nicholls, J.M.; Peiris, J.S.; Ip, N.Y. Avian influenza H5N1 virus induces cytopathy and proinflammatory cytokine responses in human astrocytic and neuronal cell lines. Neuroscience 2010, 168, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Barbur, J.L.; Harlow, A.J.; Sahraie, A. Pupillary responses to stimulus structure, colour and movement. Ophthalmic Physiol. Opt. 1992, 12, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hearne, L.; Lei, B.; Miles, J.H.; Takahashi, N.; Yao, G. Weak gender effects on transient pupillary light reflex. Auton. Neurosci. Basic Clin. 2009, 147, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, D.F.; Brozou, C.G.; Tsiptsios, D.J.; Fotiou, A.; Kabitsi, A.; Nakou, M.; Giantselidis, C.; Goula, A. Effect of age on pupillary light reflex: Evaluation of pupil mobility for clinical practice and research. Electromyogr. Clin. Neurophysiol. 2007, 47, 11–22. [Google Scholar] [PubMed]
- Straub, R.H.; Thies, U.; Kerp, L. The pupillary light reflex. 1. Age-dependent and age-independent parameters in normal subjects. Ophthalmologica 1992, 204, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, O.; Schoetzau, A.; Sugimoto, K.; Zulauf, M. The influence of iris color on the pupillary light reflex. Graefe Arch. Clin. Exp. Ophthalmol. 1998, 236, 567–570. [Google Scholar] [CrossRef]
- Slooter, J.; van Norren, D. Visual acuity measured with pupil responses to checkerboard stimuli. Investig. Ophthalmol. Vis. Sci. 1980, 19, 105–108. [Google Scholar]
- Ukai, K. Spatial pattern as a stimulus to the pupillary system. J. Opt. Soc. Am. A 1985, 2, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol. Bull. 1982, 91, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Newsome, D.A.; Loewenfeld, I.E. Iris mechanics. II. Influence of pupil size on details of iris structure. Am. J. Ophthalmol. 1971, 71, 553–573. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. https://doi.org/10.3390/diagnostics8010019
Hall CA, Chilcott RP. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics. 2018; 8(1):19. https://doi.org/10.3390/diagnostics8010019
Chicago/Turabian StyleHall, Charlotte A., and Robert P. Chilcott. 2018. "Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics" Diagnostics 8, no. 1: 19. https://doi.org/10.3390/diagnostics8010019