Percutaneous, Imaging-Guided Biopsy of Bone Metastases
Abstract
:1. Introduction
2. Indications—Contraindications
3. Pre-Procedural Imaging
4. Techniques
5. Efficacy and Safety
6. Conclusions
Author Contributions
Conflicts of Interest
References
- ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25, iii124–iii137. [Google Scholar]
- ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, vii139–vii154. [Google Scholar]
- Czervionke, L.F.; Fenton, D.S. (Eds.) Percutaneous Spine biopsy. In Image Guided Spine Intervention; Saunders: New South Wales, Australia, 2003; pp. 141–187. [Google Scholar]
- Monfardini, L.; Preda, L.; Aurilio, G.; Rizzo, S.; Bagnardi, V.; Renne, G.; Maccagnoni, S.; Vigna, P.D.; Davide, D.; Bellomi, M. CT-guided bone biopsy in cancer patients with suspected bone metastases: Retrospective review of 308 procedures. Radiol. Med. 2014, 119, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wallace, M.J.; Cardella, J.F.; Kundu, S.; Miller, D.L.; Rose, S.C. Quality improvement guidelines for percutaneous needle biopsy. J. Vasc. Interv. Radiol. 2010, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.A.M.S.; Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Interv. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Tehranzadeh, J.; Tao, C.; Browning, C.A. Percutaneous needle biopsy of the spine. Acta Radiol. 2007, 48, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Le, H.B.; Lee, S.T.; Munk, P.L. Image-guided musculoskeletal biopsies. Semin. Interv. Radiol. 2010, 27, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; André, F.; Thompson, A.M.; De Laurentiis, M.; Esposito, A.; Gelao, L.; Fumagalli, L.; Locatelli, M.; Minchella, I.; Orsi, F.; et al. Biopsy confirmation of metastatic sites in breast cancer patients: Clinical impact and future perspectives. Breast Cancer Res. 2014, 16, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himes, N.C.; Chansakul, T.; Lee, T.C. Magnetic Resonance Imaging-Guided Spine Interventions. Magn. Reson. Imaging Clin. 2015, 23, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Shellikeri, S.; Setser, R.M.; Vatsky, S.; Srinivasan, A.; Krishnamurthy, G.; Zhu, X.; Keller, M.S.; Cahill, A.M. Prospective evaluation of MR overlay on real-time fluoroscopy for percutaneous extremity biopsies of bone lesions visible on MRI but not on CT in children in the interventional radiology suite. Pediatr. Radiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Garnon, J.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Chari, B.; Cazzato, R.L.; Gangi, A. Ultrasound-Guided Biopsies of Bone Lesions without Cortical Disruption Using Fusion Imaging and Needle Tracking: Proof of Concept. Cardiovasc. Interv. Radiol. 2017, 40, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Tselikas, L.; Joskin, J.; Roquet, F.; Farouil, G.; Dreuil, S.; Hakimé, A.; Teriitehau, C.; Auperin, A.; de Baere, T.; Deschamps, F. Percutaneous bone biopsies: Comparison between flat-panel cone-beam CT and CT-scan guidance. Cardiovasc. Interv. Radiol. 2015, 38, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Mavrogenis, A.F.; Mazioti, A.; Palialexis, K.; Megaloikonomos, P.D.; Papagelopoulos, P.J.; Kelekis, A. Metastatic bone disease from breast cancer: A review of minimally invasive techniques for diagnosis and treatment. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.R.; Kelekis, A.; Alvarez, L.G.; Filippiadis, D.K. Interventional procedures of the spine. Semin. Musculoskelet. Radiol. 2014, 18, 309–317. [Google Scholar] [PubMed]
- Matti, A.; Farolfi, A.; Frisoni, T.; Fanti, S.; Nanni, C. FDG-PET/CT Guided Biopsy in Angiosarcoma of Bone: Diagnosis, Staging and Beyond. Clin. Nucl. Med. 2018, 43, e48–e49. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, W.M.; Pastor, C.; Gnannt, R.; Parra, D.A.; Amaral, J.G.; Temple, M.J.; Sochett, E.B.; Connolly, B.L. Technique, Safety, and Yield of Bone Biopsies for Histomorphometry in Children. J. Vasc. Interv. Radiol. 2017, 28, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Tutton, S.; Kelekis, A. Percutaneous bone lesion ablation. Radiol. Med. 2014, 119, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Gkizas, C.; Kostantos, C.; Mazioti, A.; Reppas, L.; Brountzos, E.; Kelekis, N.; Kelekis, A. Percutaneous Biopsy and Radiofrequency Ablation of Osteoid Osteoma with Excess Reactive New Bone Formation and Cortical Thickening Using a Battery-Powered Drill for Access: A Technical Note. Cardiovasc. Interv. Radiol. 2016, 39, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.K.; Ng, A.W.; Griffith, J.F. CT-guided bone biopsy with a battery-powered drill system: Preliminary results. Am. J. Roentgenol. 2013, 201, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.G.; McMahon, C.J.; Kung, J.W.; Wu, J.S. Comparison of Battery-Powered and Manual Bone Biopsy Systems for Core Needle Biopsy of Sclerotic Bone Lesions. Am. J. Roentgenol. 2016, 206, W83–W86. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, J.S.; Murphey, M.D.; Welker, J.A.; Henshaw, R.M.; Kransdorf, M.J.; Shmookler, B.M.; Malawer, M.M. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: Experience with 110 tumors. Radiology 2002, 223, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, A.O.; Slavin, J.; Smith, P.J.; Schlict, S.M.; Powell, G.J.; Ngan, S.; Toner, G.; Choong, P.F. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J. Surg. 2005, 75, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hau, A.; Kim, I.; Kattapuram, S.; Hornicek, F.J.; Rosenberg, A.E.; Gebhardt, M.C.; Mankin, H.J. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skelet. Radiol. 2002, 31, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Faugere, M.C.; Malluche, H.H. Comparison of different bone-biopsy techniques for qualitative and quantitative diagnosis of metabolic bone diseases. J. Bone Jt. Surg. Am. 1983, 65, 1314–1318. [Google Scholar] [CrossRef]
- Wu, J.S.; Goldsmith, J.D.; Horwich, P.J.; Shetty, S.K.; Hochman, M.G. Bone and soft-tissue lesions: What factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008, 248, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Tacher, V.; Le Deley, M.C.; Hollebecque, A.; Deschamps, F.; Vielh, P.; Hakime, A.; Ileana, E.; Abedi-Ardekani, B.; Charpy, C.; Massard, C.; et al. Factors associated with success of image-guided tumour biopsies: Results from a prospective molecular triage study (MOSCATO-01). Eur. J. Cancer 2016, 59, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Halpern, E.F.; Rosenthal, D.I. Incidence of delayed complications following percutaneous CT-guided biopsy of bone and soft tissue lesions of the spine and extremities: A 2-year prospective study and analysis of risk factors. Skelet. Radiol. 2013, 42, 61–68. [Google Scholar] [CrossRef] [PubMed]
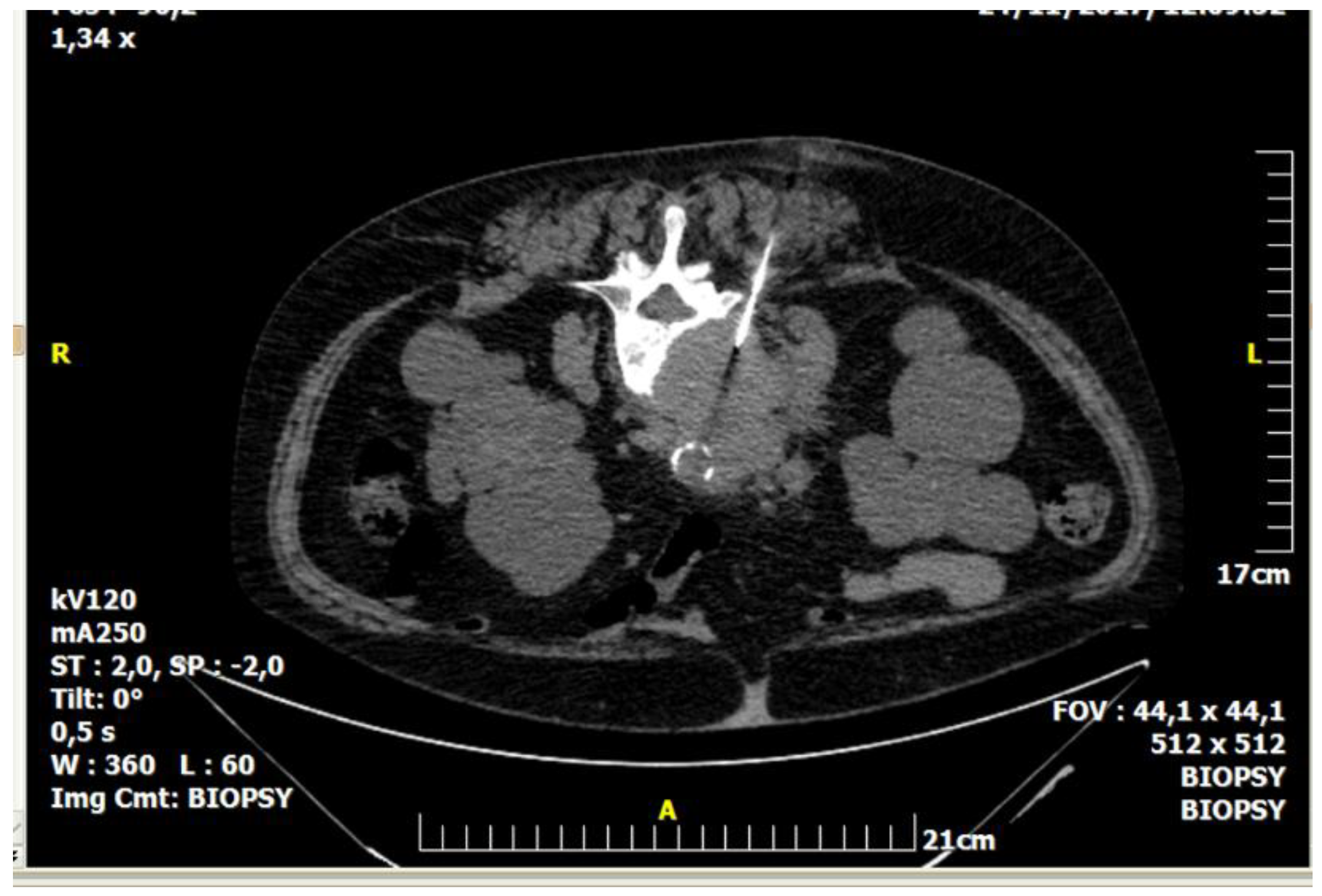
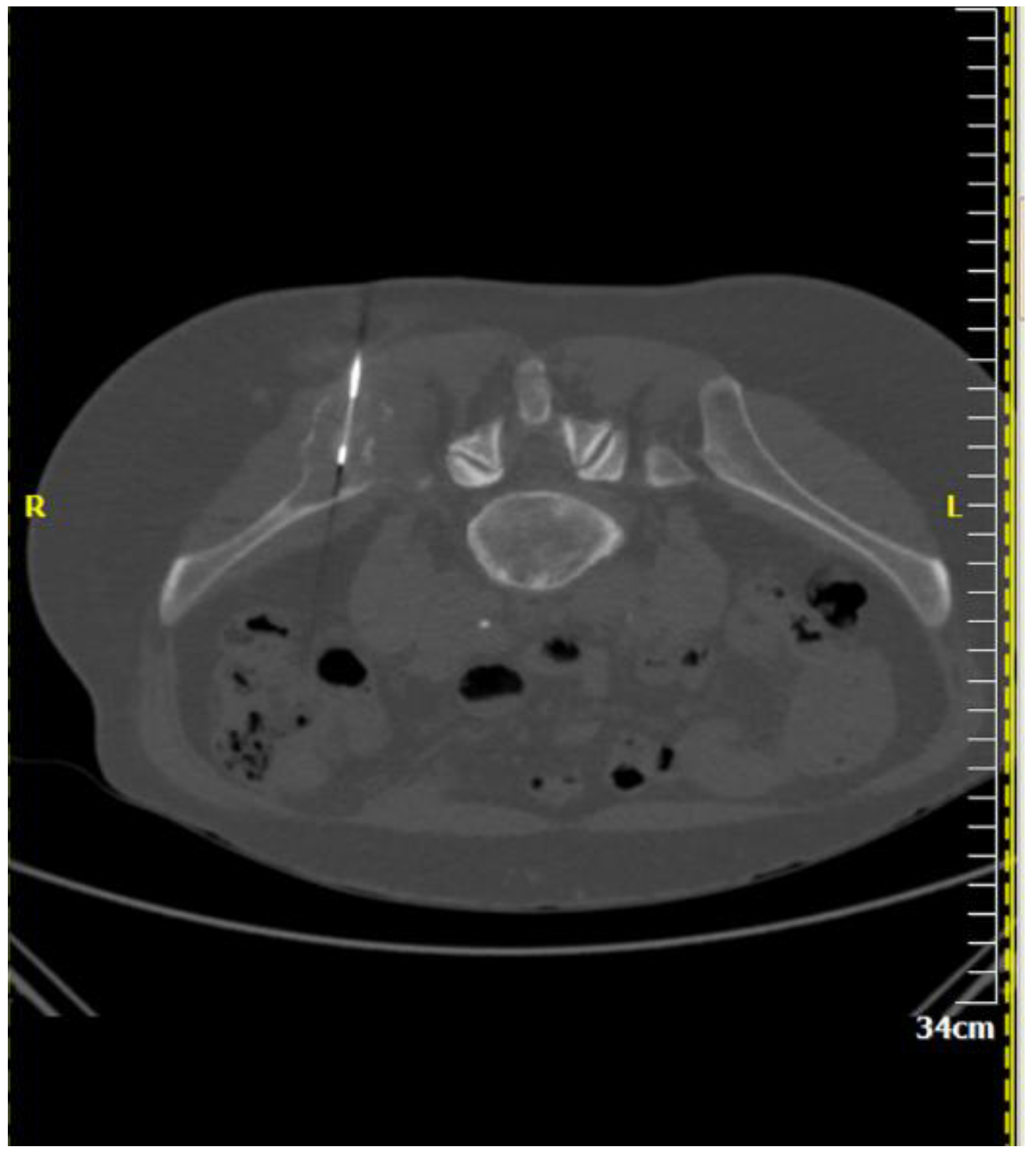
| Imaging Guidance | Fluoroscopy (incl. Cone beam CT) | Ultrasound (incl. Fusion imaging) | Computed Tomography (incl. CT fluoroscopy) | Magnetic Resonance Imaging |
| Biopsy Techniques | Co-axial technique | Tandem technique | Fine needle aspiration biopsy | Core needle biopsy |
| Diagnostic Accuracy | 70–96% depending upon target’s size and location, benign or malignant character, number of samples, on-site presence of cytopathologist | |||
| Complications | Procedure related mortality rate <0.05%—quality improvement threshold for overall incidence of complication of 2% | |||
| References | Veltri et al. CVIR 2017: CIRSE guidelines on percutaneous needle biopsy [6] | Gupta et al. JVIR 2010: Quality improvement guidelines for percutaneous needle biopsy [5] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippiadis, D.; Mazioti, A.; Kelekis, A. Percutaneous, Imaging-Guided Biopsy of Bone Metastases. Diagnostics 2018, 8, 25. https://doi.org/10.3390/diagnostics8020025
Filippiadis D, Mazioti A, Kelekis A. Percutaneous, Imaging-Guided Biopsy of Bone Metastases. Diagnostics. 2018; 8(2):25. https://doi.org/10.3390/diagnostics8020025
Chicago/Turabian StyleFilippiadis, Dimitrios, Argyro Mazioti, and Alexios Kelekis. 2018. "Percutaneous, Imaging-Guided Biopsy of Bone Metastases" Diagnostics 8, no. 2: 25. https://doi.org/10.3390/diagnostics8020025





