Driving Forces Behind the Past and Future Emergence of Personalized Medicine
Abstract
:1. Introduction
2. Personalized Medicine as a Continuously Developing Approach
3. Personalized Medicine as a Business Diversification Strategy
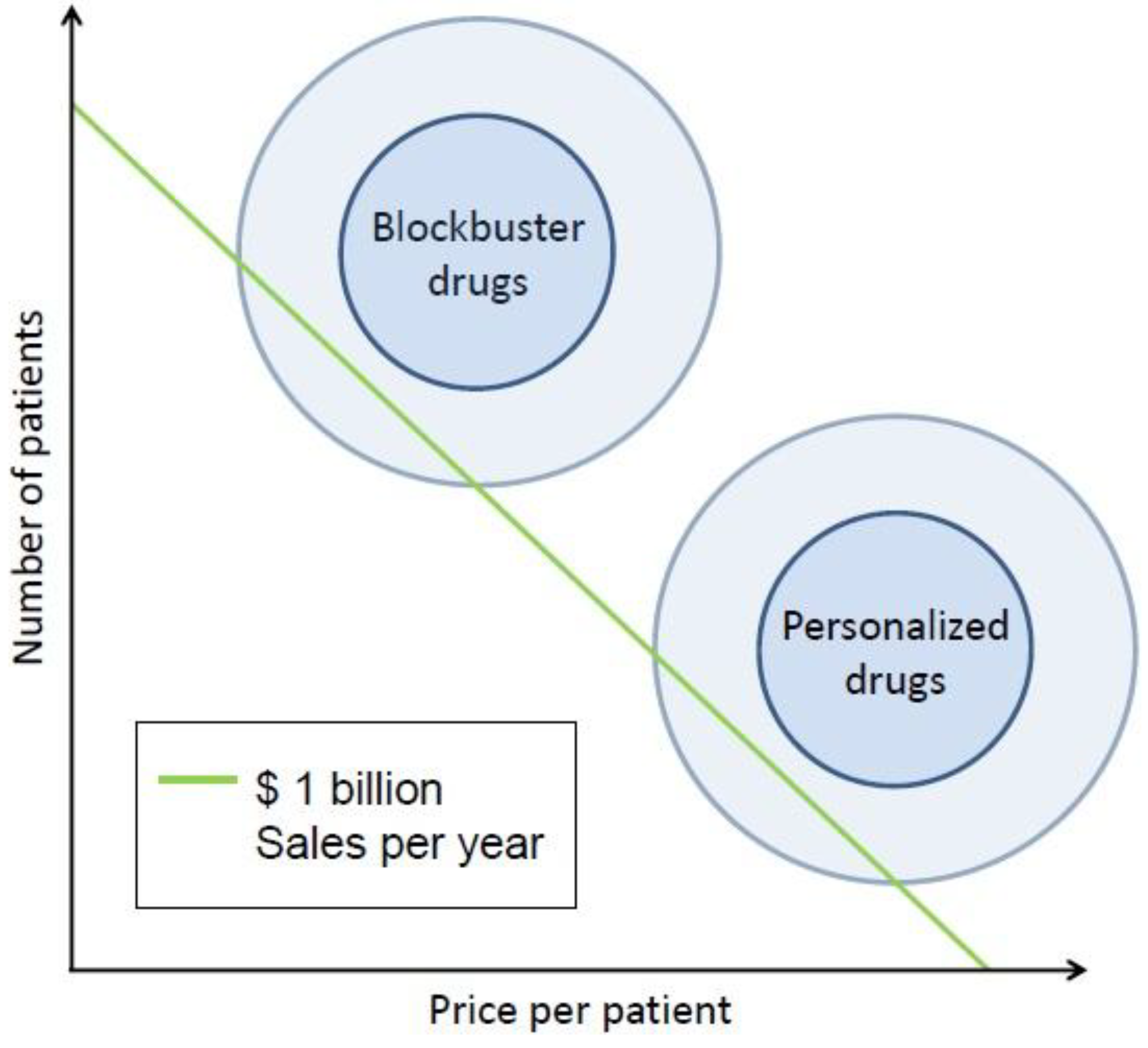
4. An Integrated Perspective
5. Conclusions
Conflict of Interest
References and Notes
- President’s Council of Advisors on Science and Technology. Priorities for Personalized Medicine. Available online: http://www.whitehouse.gov/files/documents/ostp/PCAST/pcast_report_v2.pdf (accessed on 3 June 2012).
- Shaw, A.T. The Crizotinib Story: From Target to FDA Approval and Beyond. Available online: http://www.informedicalcme.com/lucatoday/crizotinib-story-from-target-to-fda-approval/ (accessed on10 August 2012).
- Merla, A.; Goel, S. Novel drugs targeting the epidermal growth factor receptor and its downstream pathways in the treatment of colorectal cancer: A systematic review. Chemother. Res. Pract. 2012. [Google Scholar] [CrossRef]
- Sharma, A.; Shah, S.R.; Illum, H.; Dowell, J. Vemurafenib: Targeted Inhibition of Mutated BRAF for Treatment of Advanced Melanoma and Its Potential in Other Malignancies. Drugs 2012. [Google Scholar] [CrossRef]
- Canestaro, W.J.; Martell, L.A.; Wassman, E.R.; Schatzberg, R. Healthcare payers: A gate or a translational bridge to personalized medicine. Pers. Med. 2010, 9, 73–84. [Google Scholar]
- The Age of Personalized Medicine: What is Personalized Medicine? Available online: http://www.ageofpersonalizedmedicine.org/what_is_personalized_medicine/ (accessed on 11 June 2012).
- Collins, F.S. The Language of Life, DNA and the Revolution in Personalized Medicine; HarperCollins: New York, NY, USA, 2010; p. 58. [Google Scholar]
- Salari, K.; Watkins, H.; Ashley, E.A. Personalized medicine: Hope or hype? Eur. Heart J. 2012. [Google Scholar] [CrossRef]
- Madhur, M.S.; Riaz, K.; Dreisbach, A.W.; Harrison, D.G. Hypertension. Available online: http://emedicine.medscape.com/article/241381-overview (accessed on 20 November 2012).
- Self, T.H.; Wallace, J.L.; Soberman, J.E. Cardioselective Beta-blocker treatment of hypertension in patients with asthma: When do benefits outweigh risks? J. Asthma. 2012, 49, 947–951. [Google Scholar] [CrossRef]
- Toh, S.; Reichman, M.E.; Houstoun, M.; Ross Southworth, M.; Ding, X.; Hernandez, A.F.; Levenson, M.; Li, L.; McCloskey, C.; Shoaibi, A.; et al. Comparative Risk for Angioedema Associated With the Use of Drugs That Target the Renin-Angiotensin-Aldosterone System. Arch. Intern. Med. 2012, 15, 1–8. [Google Scholar]
- Jain, K.K. Textbook of Personalized Medicine; Springer: New York, NY, USA, 2009. [Google Scholar]
- Personalized Medicine Coalition (PMC). The Case of Personalized Medicine. Available online: http://www.personalizedmedicinecoalition.org/sites/default/files/files/Case_for_PM_3rd_edition.pdf (accessed on 27 May 2012).
- What is Personalized Medicine? Available online: http://www.dukepersonalizedmedicine.org/what_is_personalized_medicine (accessed on 11 June 2012).
- The Boston Consulting Group. Medizinische Biotechnologie in Deutschland 2011. Available online: http://www.vfa-bio.de/download/bcg-report-2011-broschuere.pdf (accessed on 30 May 2012).
- Simmons, L.A.; Dinan, M.A.; Robinson, T.J.; Snyderman, R. Personalized medicine is more than genomic medicine: Confusion over terminology impedes progress towards personalized healthcare. Pers. Med. 2012, 9, 85–91. [Google Scholar] [CrossRef]
- Tavares, P.; Dias, L.; Palmeiro, A.; Rendeiro, P.; Tolias, P. Single-test parallel assessment of multiple genetic disorders. Pers. Med. 2012, 8, 375–379. [Google Scholar]
- Tran, B.; Dancey, J.E.; Kamel-Reid, S.; McPherson, J.D.; Bedard, P.L.; Brown, A.M.; Zhang, T.; Shaw, P.; Onetto, N.; Stein, L.; et al. Cancer Genomics: Technology, Discovery, and Translation. J. Clin. Oncol. 2012, 30, 647–660. [Google Scholar]
- Kampen, K.R. The discovery and early understanding of leukemia. Leuk. Res. 2012, 36, 6–13. [Google Scholar] [CrossRef]
- Ferdinand, R.; Mitchell, S.A.; Batson, S.; Tumur, I. Treatments for chronic myeloid leukemia: A qualitative systematic review. J. Blood Med. 2012, 3, 51–76. [Google Scholar]
- Breccia, M.; Alimena, G. How to treat CML patients in the tyrosine kinase inhibitors era? From imatinib standard dose to second generation drugs front-line: Unmet needs, pitfalls and advantages. Cancer Lett. 2012, 322, 127–132. [Google Scholar] [CrossRef]
- Blay, J.Y.; von Mehren, M. Nilotinib: A novel, selective tyrosine kinase inhibitor. Semin. Oncol. 2011, 38, S3–S9. [Google Scholar]
- Gora-Tybor, J. Emerging therapies in chronic myeloid leukemia. Curr. Cancer Drug Targets 2012, 12, 458–470. [Google Scholar] [CrossRef]
- Roland Berger Strategy Consultants. Fight or flight? Diversification vs. Rx-focus in big pharma’s quest for sustained growth. Available online: http://www.rolandberger.com/media/pdf/Roland_Berger_Fight_or_flight_Shortversion_20101025.pdf (accessed on 1 March 2012).
- Cantrell, R. Outpacing the Competition: Patent-based Business Strategy; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Jaruzelski, B.; Dehoff, K. The Global Innovation 1000: How the Top Innovators Keep Winning. Available online: http://www.booz.com/media/file/sb61_preprint_Global-Innov1000-10408.pdf (accessed on 2 June 2011).
- March, R. Delivering on the promise of personalized healthcare. Pers. Med. 2010, 7, 327–337. [Google Scholar] [CrossRef]
- Personalized Medicine Coalition (PMC). The Case for Personalized Medicine: All about Personalized Medicine. Available online: http://www.personalizedmedicinecoalition.org/about/about-personalized-medicine/the-case-for-personalized-medicine (accessed on 20 August 2012).
- Lenz, C.; Girek, D.; Schmitt, D. Advancing health technology assessment (HTA) and reimbursement processes to promote access to personalized medicine. J. Pharmacol. Ther. 2011, 5, 150–152. [Google Scholar]
- Miller, I.; Pothier, K.; Dunn, M. Advocacy in personalized medicine: A developing strength in a complex space. Pers. Med. 2010, 7, 179–186. [Google Scholar] [CrossRef]
- Moch, H.; Blank, P.R.; Dietel, M.; Elmberger, G.; Kerr, K.M.; Palacios, J.; Penault-Llorca, F.; Rossi, G.; Szucs, T.D. Personalized cancer medicine and the future of pathology. Virchows Arch. 2012, 460, 3–8. [Google Scholar] [CrossRef]
- Korn, D. Interview: A perspective on personalized medicine: Dr. David Korn. Pers. Med. 2012, 9, 259–263. [Google Scholar] [CrossRef]
- Zander, T.; Heukamp, L.C.; Allardt Bos, M.C.; Fassunke, J.; Mattonet, C.; Merkelbach-Bruse, S.; Ko, Y.D.; Schlesinger, A.; Brockmann, M.; Serke, M.; et al. Regional screening network for characterization of the molecular epidemiology of non-small cell lung cancer (NSCLC) and implementation of personalized treatment. J. Clin. Oncol. 2012, 30 Suppl.: Abstr. CRA10529. [Google Scholar]
- The latest oncology headlines. Available online: http://ecancer.org/news/2899 (accessed on 8 August 2012).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Steffen, J.A.; Steffen, J.S. Driving Forces Behind the Past and Future Emergence of Personalized Medicine. J. Pers. Med. 2013, 3, 14-22. https://doi.org/10.3390/jpm3010014
Steffen JA, Steffen JS. Driving Forces Behind the Past and Future Emergence of Personalized Medicine. Journal of Personalized Medicine. 2013; 3(1):14-22. https://doi.org/10.3390/jpm3010014
Chicago/Turabian StyleSteffen, Julius Alexander, and Jan Simon Steffen. 2013. "Driving Forces Behind the Past and Future Emergence of Personalized Medicine" Journal of Personalized Medicine 3, no. 1: 14-22. https://doi.org/10.3390/jpm3010014



