The Preparation of Graphene Oxide and Its Derivatives and Their Application in Bio-Tribological Systems
Abstract
:1. Introduction
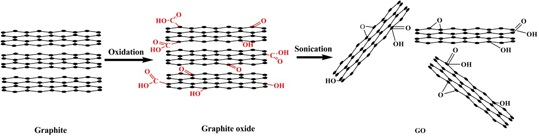
2. Preparation of GO


3. Characterization of GO

| Item | C–C | C–O (Hydroxyl and Epoxy) | C=O | O=C–OH | Reference |
|---|---|---|---|---|---|
| Bonding Energy (eV) | 284.8 | 286.3 | 287.2 | 288.4 | [30] |
| 284.6 | 285.8 | 287.1 | 288.9 | [31] | |
| 285.1 | 286.4 | 287.8 | 288.9 | [32] | |
| 284.8 | 286.2 | 287.8 | 289.0 | [33] |

4. Derivative of GO and Their Characteristics
4.1. RGO


4.2. Functionalized Derivatives of GO
| Reactive Groups | Modifiers | References |
|---|---|---|
| Epoxy | Aliphatic amines | [64,65,66,67,68] |
| Aromatic amines | [69,70] | |
| Dopamine | [71] | |
| Amino acid | [64] | |
| Ionic liquid (amine-terminal) | [72] | |
| Carboxylic acid | ethylenediamine, 1,6-hexanediamine | [73] |
| Amine-functionalized porphyrin Pyrrolidine fullerene | [74] | |
| N-Containing heterocyclic compounds | [75] | |
| Hydroxyl | Polyoxyethylene sorbitol anhydride monolaurate | [76] |
| π–π stacking | Polyaniline | [77] |
4.3. GO-Based Thin Films
| Substrates | Techniques | Applications or Potential Applications | References |
|---|---|---|---|
| Ti | Electrophoretic deposition (EPD) | Corrosion protection | [87,88] |
| Biomedical | |||
| TiO2 | Spin-coating | Photocatalytic | [89] |
| Solvothermal | [90] | ||
| Aqueous deposition | [91] | ||
| SiO2 | Sol-gel approach | Environment | [92] |
| Spin coating | Humidity sensors | [93] | |
| Si | Spin-coating | Transparent conductors | [94] |
| Langmuir-Blodgett/dip-coating | Transparent conducting thin films | [95] | |
| Grafting-onto | Lubricants | [96] | |
| Covalent assembly | Lubricants | [32] | |
| EPD | Lubricants | [97] | |
| PMMA | Covalent immobilization | Microfluidic bioreactors | [98] |
| Cu | EPD | Corrosion resistance | [99,100] |
| Electrochemical deposition | Corrosion resistance | [101] | |
| Cordierite | Hydrogen bonding | Catalysts | [102] |
| Al foil | Spin-coating | Hybrid transparent conductive films | [103] |

4.4. GO-Based Nanocomposites
5. Biological Property of GO-Based Materials

6. Tribological Behavior of GOBMs
7. Application of GOBMs in Bio-Tribological Systems
8. Conclusions and Prospect
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–190. [Google Scholar] [CrossRef]
- Park, S.J.; Ruoff, R.S. Chemical methods for the production of graphene. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Avouris, P. Graphene: Electronic and photonic properties and devices. Nano Lett 2010, 10, 4285–4294. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B: Biointerfaces 2013, 111, 188–202. [Google Scholar]
- Wissler, M. Graphite and carbon powders for electrochemical application. J. Power Sources 2006, 156, 142–150. [Google Scholar] [CrossRef]
- Szabo´, T.; Szeri, A.; De´ka´ny, I. Composite graphitic nanolayers prepared by self-assembly between finely dispersed graphite oxide and a cationic polymer. Carbon 2005, 43, 87–94. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structure model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- lrich, H.; Rudolf, H. The acid nature and methylation of graphitic oxide. Ber. Dtsch. Chem. Ges. 1939, 72, 754–771. [Google Scholar] [CrossRef]
- Ruess, G. Uber das Graphitoxyhydroxyd (Graphitoxyd). Monatsch. Chem. 1946, 76, 381–417. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.J.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Mural, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Ma, C.; Liu, W.; Shi, M.; Lang, X.; Chu, Y.; Chen, Z.; Zhao, D.; Lin, W.; Hardacre, C. Low loading platinum nanoparticles on reduced graphene oxide-supported tungsten carbide crystallites as a highly active electrocatalyst for methanol oxidation. Electrochim. Acta 2013, 114, 133–141. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.; Tasis, D.; Sygellou, L.; Dracopoulos, V.; Galiotis, C.; Lianos, P. Study of the thermal reduction of graphene oxide and of its application as electrocatalyst in quasi-solid state dye-sensitized solar cells in combination with PEDOT. Electrochim. Acta 2013, 111, 698–706. [Google Scholar] [CrossRef]
- Yu, Y; Kang, B.; Lee, Y.; Lee, S.; Ju, B. Effect of fluorine plasma treatment with chemically reduced graphene oxide thin films as hole transport layer in organic solar cells. Appl. Surf. Sci. 2013, 287, 91–96. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Kosynkin, D.V.; Sinitskii, A.; Sun, Z.; Tour, J.M. Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 2010, 4, 2059–2069. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly (L-lactic acid) as a promising reinforcement for PLLA nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y.; Rousseau, A. FTIR and 13C NMR study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Ramesh, P.; Bhagyalakshmi, S.; Sampath, S. Preparation and physicochemical and electrochemical characterization of exfoliated graphite oxide. J. Colloid Interface Sci. 2004, 274, 95–102. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Alonso, M.H.; Milius, D.L.; Car, R.; Prud’homme, R.K.; Aksay, I.A. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Li, P.; Xu, Y.; Cheng, X. Chemisorption of thermal reduced graphene oxide nano-layer film on TNTZ surface and its tribological behavior. Surf. Coat. Technol. 2013, 232, 331–339. [Google Scholar] [CrossRef]
- Ou, J.; Wang, J.; Liu, S.; Mu, B.; Ren, J.; Wang, H.; Yang, S. Tribology study of reduced graphene oxide sheets on silicon substrate synthesized via covalent assembly. Langmuir 2010, 26, 15830–15836. [Google Scholar] [CrossRef]
- Yang, D.X.; Velamakanni, A.; Bozoklu, G.; Park, S.J.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.J.; Ruoff, R.S. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Kozlowski, C.; Sherwood, P.M.A. X-ray photoelectron spectroscopic studies of carbon-fibresurfaces. J. Chem. Soc. Faraday Trans. I 1984, 80, 2099–2107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef]
- Rourke, J.P.; Pandey, P.A.; Moore, J.J.; Bates, M.; Kinloch, I.A.; Young, R.J.; Wilson, N.R. The real graphene oxide revealed: Stripping the oxidative debris from the graphene-like sheets. Angew. Chem. Int. Ed. 2011, 50, 3173–3177. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Wilson, N.R.; Pandey, P.A.; Beanland, R.; Young, R.J.; Kinloch, I.A.; Gong, L.; Liu, Z.; Suenaga, K.; Rourke, J.P.; York, S.J.; et al. Graphene oxide: Structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 2009, 3, 2547–2556. [Google Scholar] [CrossRef]
- Kang, H.; Kulkarni, A.; Stankovich, S.; Ruoff, R.S.; Baik, S. Restoring electrical conductivity of dielectrophoretically assembled graphite oxide sheets by thermal and chemical reduction techniques. Carbon 2009, 47, 1520–1525. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Pacile´, D.; Meyer, J.C.; Rodrı´guez, A.F.; Papagno, M.; Go´mez-Navarro, C.; Sundaram, R.S.; Burghard, M.; Kern, K.; Carbone, C.; Kaiser, U. Electronic properties and atomic structure of graphene oxide membranes. Carbon 2011, 49, 966–972. [Google Scholar] [CrossRef]
- Li, D.; MÜller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Paci, J.T.; Belytschko, T.; Schatz, G.C. Computational studies of the structure, behavior upon heating, and mechanical properties of graphite oxide. J. Phys. Chem. C 2007, 111, 18099–18111. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical vapor deposition repair of graphene oxide: A route to highly conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar]
- Zhang, H.; Wang, J.; Yan, Q.; Zheng, W.; Chen, C.; Yu, Z. Vacuum-assisted synthesis of graphene from thermal exfoliation and reduction of graphite oxide. J. Mater. Chem. 2011, 21, 5392–5397. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction ofgraphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar]
- Wang, L.; Ye, Y.; Lu, X.; Wu, Y.; Sun, L.; Tan, H.; Xu, F.; Song, Y. Prussian blue nanocubes on nitrobenzene-functionalized reduced graphene oxide and its application for H2O2 biosensing. Electrochim. Acta 2013, 114, 223–232. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating graphene sheets with gold nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef]
- Jang, H.; Yun, J.; Kim, D.Y.; Park, D.W.; Na, S.I.; Kim, S.S. Moderately reduced graphene oxide as transparent counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2012, 81, 301–307. [Google Scholar] [CrossRef]
- Williams, G.; Serger, B.; Kamat, P.V. TiO2-graphene nanocomposites: UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Zhao, Q.; Lv, T.; Zhu, G.; Chen, T.; Lu, T.; Sun, Z.; Sun, C. UV-assisted photocatalytic synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic activity in reduction of Cr(VI). Chem. Eng. J. 2012, 183, 238–243. [Google Scholar] [CrossRef]
- Choobtashani, M.; Akhavan, O. Visible light-induced photocatalytic reduction of graphene oxide by tungsten oxide thin films. Appl. Surf. Sci. 2013, 276, 628–634. [Google Scholar] [CrossRef]
- Xing, Z.; Chu, Q.; Ren, X.; Tian, J.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. Biomolecule-assisted synthesis of nickel sulfides/reduced graphene oxide nanocomposites as electrode materials for supercapacitors. Electrochem. Commun. 2013, 32, 9–13. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; Cai, L. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photo acoustic imaging and photo thermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells in toneurons on ginseng-reduced graphene oxide sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Kong, C.; Song, W.; Meziani, M.J.; Tackett, K.N.; Cao, L.; Farr, A.J.; Anderson, A.; Sun, Y. Supercritical fluid conversion of graphene oxides. J. Supercrit. Fluids 2012, 61, 206–211. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem. A Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef]
- Sutar, D.S.; Narayanam, P.K.; Singh, G.; Botcha, V.D.; Talwar, S.S.; Srinivasa, R.S.; Major, S.S. Spectroscopic studies of large sheets of graphene oxide and reduced graphene oxide monolayers prepared by Langmuir-Blodgett technique. Thin Solid Film 2012, 520, 5991–5996. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabo, T.; Szeri, A.; Dekany, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Matsuo, Y.; Miyabe, T.; Fukutsuka, T.; Sugie, Y. Preparation and characterization of alkylamine-intercalated graphite oxides. Carbon 2007, 45, 1005–1012. [Google Scholar] [CrossRef]
- Wang, S.; Chia, P.J.; Chua, L.L.; Zhao, L.H.; Png, R.Q.; Sivaramakrishnan, S.; Zhou, M.; Goh, R.G.S.; Friend, R.H.; Wee, A.T.S.; Ho, P.K.H. Band-like transport in surface-functionalized highly solution-processable graphene nanosheets. Adv. Mater. 2008, 20, 3440–3446. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-soluble graphene covalently functionalized by biocompatible poly-L-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterization of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Hu, Y.; Ye, M. Synthesis of hydrophilic and organophilic chemically modified graphene oxide sheets. J. Colloid Interface Sci. 2010, 351, 366–370. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Xu, L.; Yang, W.; Neoh, K.G.; Kang, E.; Fu, G. Dopamine-induced reduction and functionalization of graphene oxide nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Yang, H.; Shan, C.; Li, F.; Han, D.; Zhang, Q.; Niu, L. Covalent functionalization of poly disperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009. [Google Scholar] [CrossRef]
- Yan, J.; Chen, G.; Cao, J.; Yang, W.; Xie, B.; Yang, M. Functionalized graphene oxide with ethylenediamine and 1,6-hexanediamine. New Carbon Mater. 2012, 27, 370–376. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Zhang, X.; Zhang, X.; Chen, Y.; Tian, J. Porphyrin and fullerene covalently functionalized graphene hybrid materials with large nonlinear optical properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nano graphene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y.P. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009. [Google Scholar] [CrossRef]
- Tang, X.; Li, W.; Yu, Z.; Rafiee, M.A.; Rafiee, J.; Yavari, F.; Koratkar, N. Enhanced thermal stability in graphene oxide covalently functionalized with 2-amino-4,6-didodecylamino-1,3,5-triazine. Carbon 2011, 49, 1258–1265. [Google Scholar]
- Lu, W.; Ning, R.; Qin, X.; Zhang, Y.; Chang, G.; Liu, S.; Luo, Y.; Sun, X. Synthesis of Au nanoparticles decorated graphene oxide nanosheets: Noncovalent functionalization by TWEEN 20 in situ reduction of aqueous chloroaurate ions for hydrazine detection and catalytic reduction of 4-nitrophenol. J. Hazard. Mater. 2011, 197, 320–326. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B.; Xiao, Z. Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 2014, 119, 458–466. [Google Scholar] [CrossRef]
- Choi, E.; Han, T.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-soluble poly(N-isopropylacrylamide)-graphene sheets synthesized via click chemistry for drug delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Geng, J.; Jung, H. Porphyrin functionalized graphene sheets in aqueous suspensions: From the preparation of graphene sheets to highly conductive graphene films. J. Phys. Chem. C 2010, 114, 8227–8234. [Google Scholar] [CrossRef]
- Ghosh, A.; Rao, K.V.; George, S.J.; Rao, C.N.R. Noncovalent functionalization, exfoliation, and solubilization of graphene in water by employing a fluorescent coronene carboxylate. Chem. Eur. J. 2010, 16, 2700–2704. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Huang, F.; Li, K. Fabrication of high-concentration and stable aqueous suspensions of graphene nanosheets by noncovalent functionalization with lignin and cellulose derivatives. J. Phys. Chem. C 2010, 114, 3811–3816. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew. Chem. Int. Ed. 2011, 50, 5913–5917. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Electrophoretic deposition and electrochemical behavior of novel graphene oxide-hyaluronic acid-hydroxyapatite nanocomposite coatings. Appl. Surf. Sci. 2013, 284, 804–810. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Khoa, N.T.; Pyun, M.W.; Yoo, D.H.; Kim, S.W.; Leem, J.Y.; Kim, E.J.; Hahn, S.H. Photodecomposition effects of graphene oxide coated on TiO2 thin film prepared by electron-beam evaporation method. Thin Solid Films 2012, 520, 5417–5420. [Google Scholar] [CrossRef]
- Min, Y.; Zhang, K.; Zhao, W.; Zheng, F.; Chen, Y.; Zhang, Y. Enhanced chemical interaction between TiO2 and graphene oxide for photocatalytic decolorization of methylene blue. Chem. Eng. J. 2012, 193–194, 203–210. [Google Scholar]
- Akhavan, O.; Ghaderi, E. Photocatalyticr eduction of graphene oxide nanosheets on TiO2 thin film for photo inactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Li, G. Graphene-supported zinc oxide solid-phase micro extraction coating with enhanced selectivity and sensitivity for the determination of sulfur volatiles in Allium species. J. Chromatogr. A 2012, 1260, 1–8. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene oxide thin film coated quartz crystal microbalance for humidity detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Watcharotone, S.; Dikin, D.A.; Stankovich, S.; Piner, R.; Jung, I.; Dommett, G.H.B.; Evmenenko, G.; Wu, S.; Chen, S.; Liu, C.; et al. Graphene-silica composite thin films as transparent conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir-Blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef]
- Valentini, L.; Bon, S.B.; Monticelli, O.; Kenny, J.M. Deposition of amino-functionalized polyhedral oligomericsilsesquioxanes on graphene oxide sheets immobilized onto an amino-silane modified silicon surface. J. Mater. Chem. 2012, 22, 6213–6217. [Google Scholar] [CrossRef]
- Liang, H.; Bu, Y.; Zhang, J.; Cao, Z.; Liang, A. Graphene oxide film as solid lubricants. ACS Appl. Mater. Interfaces 2013, 5, 6369–6375. [Google Scholar] [CrossRef]
- Fan, H.; Yao, F.; Xu, S.; Chen, G. Microchip bioreactors based on trypsin-immobilized graphene oxide-poly (urea-formaldehyde) composite coating for efficient peptide mapping. Talanta 2013, 117, 119–126. [Google Scholar] [CrossRef]
- Singh, B.P.; Nayak, S.; Nanda, K.K.; Jena, B.K.; Bhattacharjee, S.; Besra, L. The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 2013, 61, 47–56. [Google Scholar] [CrossRef]
- Singh, B.P.; Jena, B.K.; Bhattacharjee, S.; Besra, L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf. Coat. Technol. 2013, 232, 475–481. [Google Scholar] [CrossRef]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A facile electrochemical approach for development of highly corrosion protective coatings using graphene nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, L.; Wang, X.; Zhou, Y.; Ye, H. A novel monolithic Pd catalyst supported on cordierite with graphene coating. Catal. Commun. 2013, 40, 98–102. [Google Scholar] [CrossRef]
- Domingues, S.H.; Kholmanov, I.N.; Kim, T.Y.; Kim, J.Y.; Tan, C.; Chou, H.; Alieva, Z.A.; Piner, R.; Zarbin, A.J. G.; Ruoff, R.S. Reduction of graphene oxide films on Al foil for hybrid transparent conductive film applications. Carbon 2013, 63, 454–459. [Google Scholar]
- Moniruzzaman, M.; Winey, K. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Tai, Z.; Yan, X.; Zhu, F.; Xue, Q. Preparation, mechanical properties and biocompatibility of graphene oxide/ultrahigh molecular weight polyethylene composites. Eur. Polym. J. 2012, 48, 1026–1033. [Google Scholar] [CrossRef]
- Tai, Z.; Chen, Y.; An, Y.; Yan, X.; Xue, Q. Tribological behavior of UHMWPE reinforced with graphene oxide nanosheets. Tribollett 2012, 46, 55–63. [Google Scholar]
- Suñer, S.; Emami, N. Investigation of graphene oxide as reinforcement for orthopaedic applications. Tribology 2014, 8, 1–6. [Google Scholar]
- Fim, F.C.; Guterres, J.M.; Basso, N.R.S.; Galland, G.B. Polyethylene/graphite nanocomposites obtained by in situ polymerization. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 692–698. [Google Scholar]
- Huang, Y.; Qin, Y.; Zhou, Y.; Niu, H.; Yu, Z.; Dong, J. Polypropylene/graphene oxide nanocomposites prepared by in situ Ziegler–Natta polymerization. Chem. Mater. 2010, 22, 4096–4102. [Google Scholar] [CrossRef]
- Potts, J.R.; Lee, S.H.; Alam, T.M.; An, J.H.; Stoller, M.D.; Piner, R.D.; Ruoff, R.S. Thermomechanical properties of chemically modified graphene/poly(methyl methacrylate) composites made by in situ polymerization. Carbon 2011, 49, 2615–2623. [Google Scholar]
- Wang, J.; Xu, Y.; Zhu, J.; Ren, P. Electrochemical in situ polymerization of reduced graphene oxide/polypyrrole composite with high power density. J. Power Sources 2012, 208, 138–143. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Li, H.D.; Liu, P. Synthesis and electrochemical performance of well-defined flake-shaped sulfonated graphene/polypyrrole composites via facile in situ doping polymerization. Electrochim. Acta 2013, 111, 729–737. [Google Scholar] [CrossRef]
- Lin, Y.; Hsu, F.; Wu, T. Enhanced conductivity and thermal stability of conductive polyaniline/graphene composite synthesized by in situ chemical oxidation polymerization with sodium dodecyl sulfate. Synth. Met. 2013, 184, 29–34. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Yu, B.; Yang, W.; Wang, B.; Hu, Y.; Yuen, R.K.K. One-pot surface functionalization and reduction of graphene oxide with long-chain molecules: Preparation and its enhancement on the thermal and mechanical properties of polyurea. Chem. Eng. J. 2014, 236, 233–241. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Špitalsky´, Z.; Mosnacek, J.; Kwiatkowski, K.; Rosłanie, Z. Structure and properties of nanocomposites based on PTT-block-PTMO copolymer and graphene oxide prepared by in situ polymerization. Eur. Polym. J. 2014, 50, 69–77. [Google Scholar]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.; McNamara, N.D.; Vangsness, M.; Sun, Y.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible graphene oxide-based glucose biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Raob, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2466. [Google Scholar] [CrossRef]
- Dong, H.; Ding, L.; Yan, F.; Ji, H.; Ju, H. The use of poly ethylenimine-grafted graphene nanoribbon for cellular delivery of locked nucleic acid modified molecular beacon for recognition of microRNA. Biomaterials 2011, 32, 3875–3882. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, S.M.; Gama, F.M.; Gonçalves, I.C.; Magalhães, F.D. Biocompatibility of poly (lactic acid) with incorporated graphene-based materials. Colloids Surf. B: Biointerfaces 2013, 104, 229–238. [Google Scholar]
- Ma, H.; Su, W.; Tai, Z.; Sun, D.; Yan, X.; Liu, B.; Xue, Q. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012, 57, 3051–3058. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Yang, J.; Xue, Q.; Miele, P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide-polyaniline and graphene-polyanilinehybrid papers. ACS Appl. Mater. Interfaces 2010, 2, 2521–2529. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Biocompatibility of microbially reduced graphene oxide in primary mouseembryonic fibroblast cells. Colloids Surf. B: Biointerfaces 2013, 105, 58–66. [Google Scholar]
- Chen, H.; Muller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhou, X.; Ye, F.; He, Q.; Chen, G.C.; Soo, J.; Boey, F.; Zhang, H.; Chen, P. Interfacing Live Cells with Nanocarbon Substrates. Langmuir 2010, 26, 2244–2247. [Google Scholar] [CrossRef]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, T.; Jiang, Y.; Liu, X. Synthesis, characterization and cytotoxicity of phosphorylcholine oligomer grafted grapheme oxide. Carbon 2014, 71, 166–175. [Google Scholar] [CrossRef]
- Ou, J.; Wang, Y.; Wang, J.; Liu, S.; Li, Z.; Yang, S. Self-assembly of octadecyltrichlorosilane on graphene oxideand the tribological performances of the resultant film. J. Phys. Chem. C 2011, 115, 10080–10086. [Google Scholar] [CrossRef]
- Li, P.; Zhou, H.; Cheng, X. Nano/micro tribological behaviors of a self-assembled graphene oxide nanolayer on Ti/titanium alloy substrates. Appl. Surface Sci. 2013, 285P, 937–944. [Google Scholar] [CrossRef]
- Shen, X.; Pei, X.; Fu, S.; Friedrich, K. Significantly modified tribological performance of epoxy nanocomposites at very low graphene oxide content. Polymer 2013, 54, 1234–1242. [Google Scholar] [CrossRef]
- Shen, X.; Pei, X.; Liu, Y.; Fu, S. Tribological performance of carbon nanotube-graphene oxide hybrid/epoxy composites. Compos. Part B 2014, 57, 120–125. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Wang, T.; Pan, G. Preparation and tribological properties of graphene oxide/nitrile rubber nanocomposites. J. Mater. Sci. 2012, 47, 730–738. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, M.; Lu, Z.; Huang, F. Preparation and tribological performance of chemically-modified reduced graphene oxide/polyacrylonitrile composites. Compos. Part A 2013, 54, 153–158. [Google Scholar] [CrossRef]
- Thangavel, E.; Ramasundaram, S.; Pitchaimuthu, S.; Hong, S.W.; Lee, S.Y.; Yoo, S.S.; Kim, D.E.; Ito, E.; Kang, Y. Structural and tribological characteristics of poly(vinylidene fluoride)/functionalized graphene oxide nanocomposite thin films. Compos. Sci. Technol. 2014, 90, 187–192. [Google Scholar] [CrossRef]
- Kandanur, S.S.; Rafiee, M.A.; Yavari, F.; Schrameyer, M.; Yu, Z.Z.; lanchet, T.A.; Koratkar, N. Suppression of wear in graphene polymer composites. Carbon 2012, 50, 3178–3183. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; Wu, D. Tribological properties of oleicacid-modified graphene as lubricant oil additives. J. Phys. D: Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Song, H.; Li, N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A: Mater. Sci. Process. 2011, 105, 827–832. [Google Scholar]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Hoseini, M.; Jedenmalm, A.; Boldizar, A. Tribological investigation of coatings for artificial joints. Wear 2008, 264, 958–966. [Google Scholar] [CrossRef]
- Sonntag, R.; Reinders, J.; Kretzer, J.P. What’s next? Alternative materials for articulation in total joint replacement. Acta Biomater. 2012, 8, 2434–2441. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Exp. Opin. Drug Deliv. 2012, 12, 13265–13276. [Google Scholar]
- Kurtz, S.M. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement; Elsevier Academic Press: Boston, UK, 2004. [Google Scholar]
- Fang, L.; Leng, Y.; Gao, P. Processing and mechanical properties of HA/UHMWPE nanocomposites. Biomaterials 2006, 27, 3701–3707. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Li, L. Recent advances in graphene-based nanomaterials for biomedical applications. Nano Life 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.; Zeng, X.; Ren, T.; Van der Heide, E. The Preparation of Graphene Oxide and Its Derivatives and Their Application in Bio-Tribological Systems. Lubricants 2014, 2, 137-161. https://doi.org/10.3390/lubricants2030137
Li J, Zeng X, Ren T, Van der Heide E. The Preparation of Graphene Oxide and Its Derivatives and Their Application in Bio-Tribological Systems. Lubricants. 2014; 2(3):137-161. https://doi.org/10.3390/lubricants2030137
Chicago/Turabian StyleLi, Jianchang, Xiangqiong Zeng, Tianhui Ren, and Emile Van der Heide. 2014. "The Preparation of Graphene Oxide and Its Derivatives and Their Application in Bio-Tribological Systems" Lubricants 2, no. 3: 137-161. https://doi.org/10.3390/lubricants2030137