The Impact of Fatty Acid Diisopropanolamides on Marine Gas Oil Lubricity
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of Diisopropanolamides
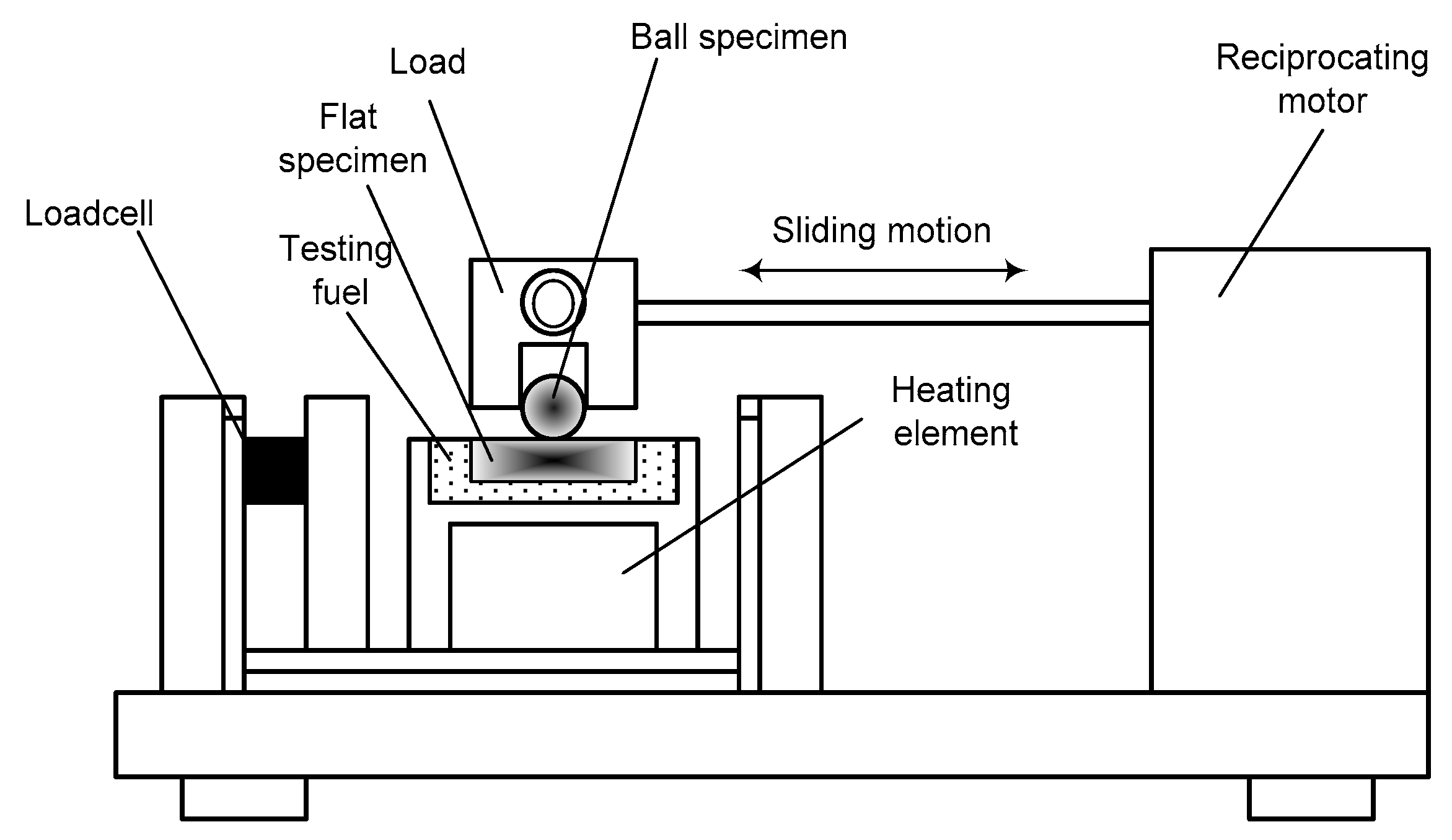
2.3. Lubricity Measurements
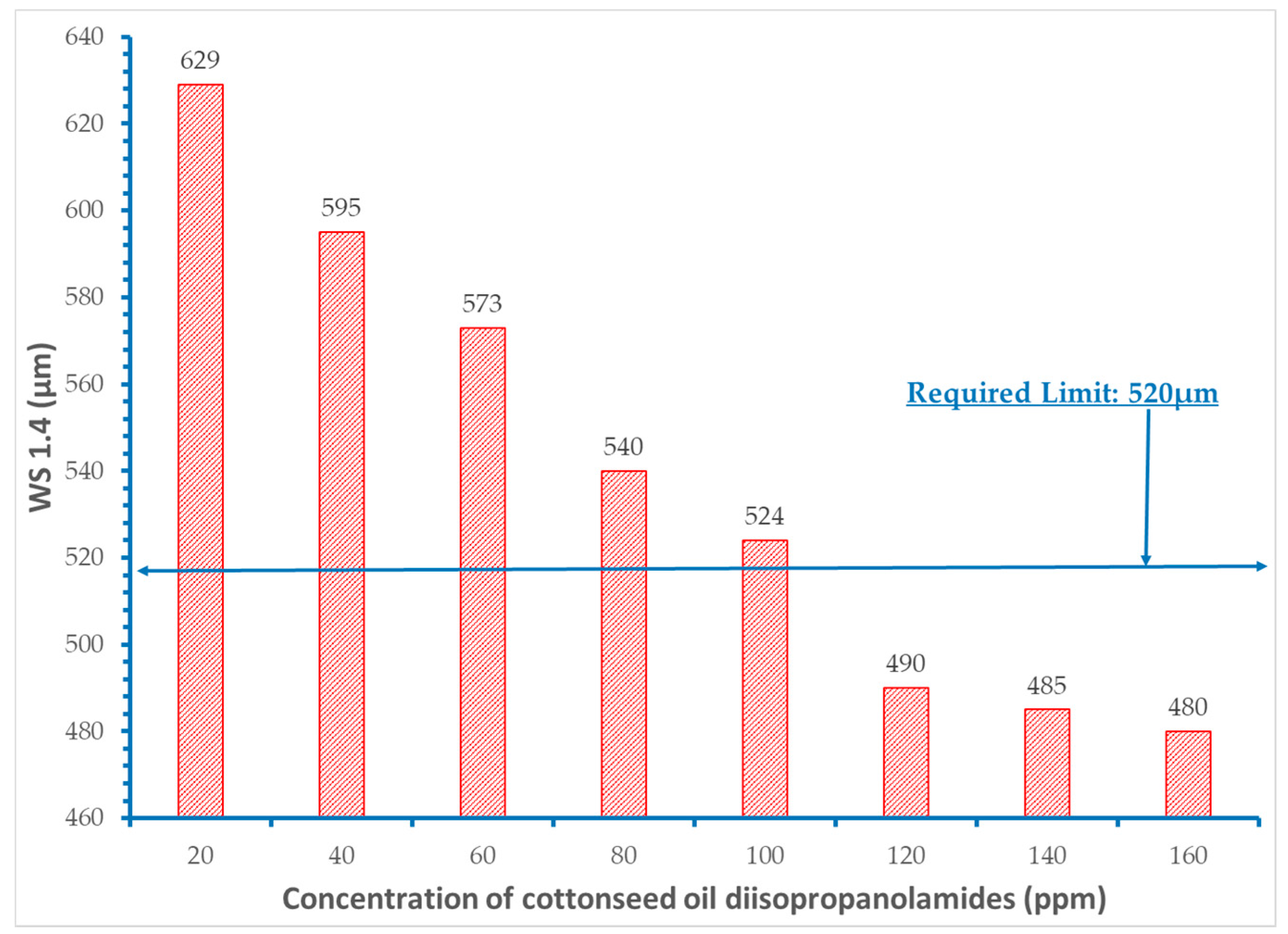
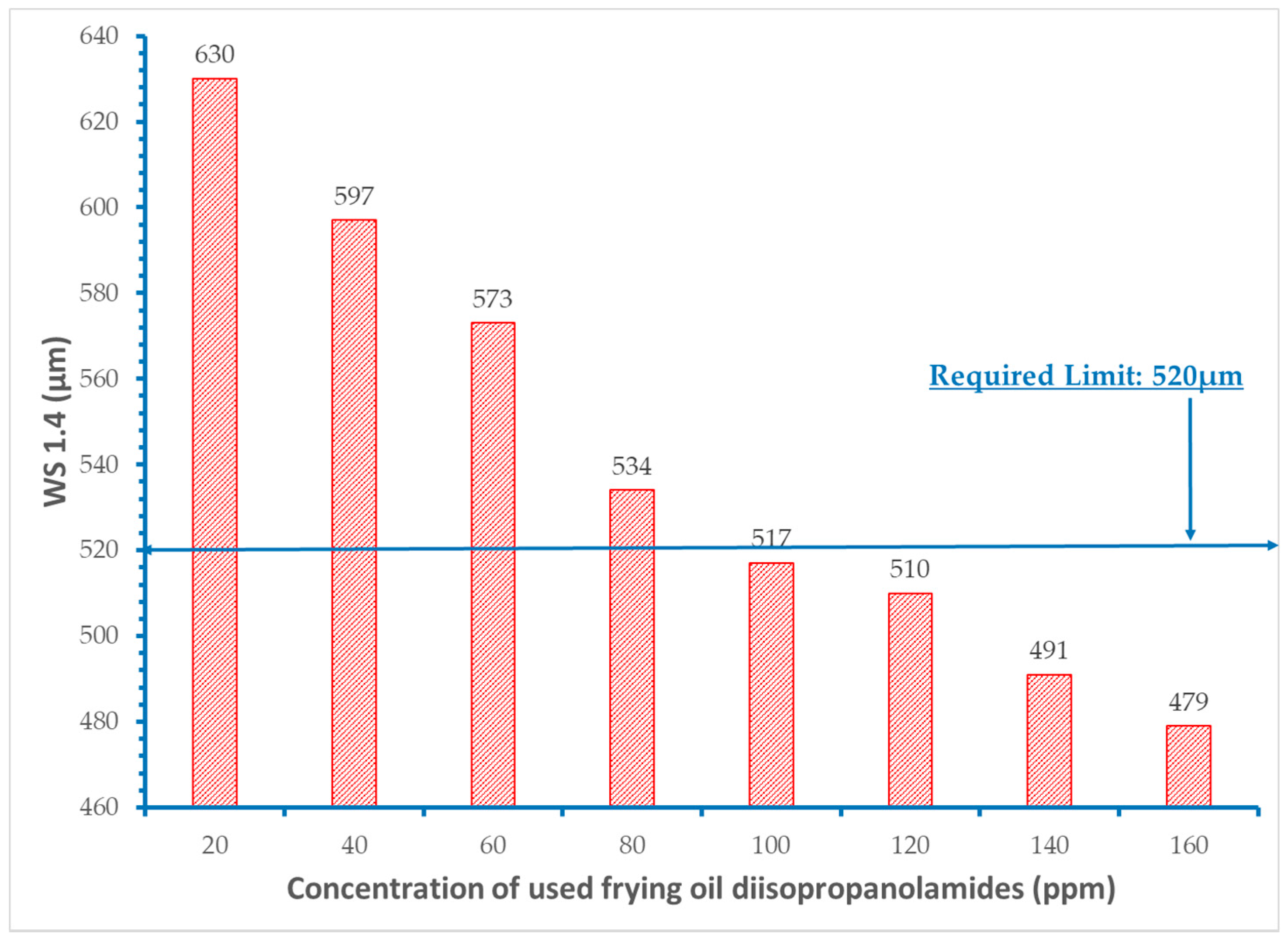
3. Results and Discussion
4. Conclusions
- The low-sulfur marine gas oil does not provide satisfactory lubrication and may lead to damage in the fuel pumps of the marine diesel engines.
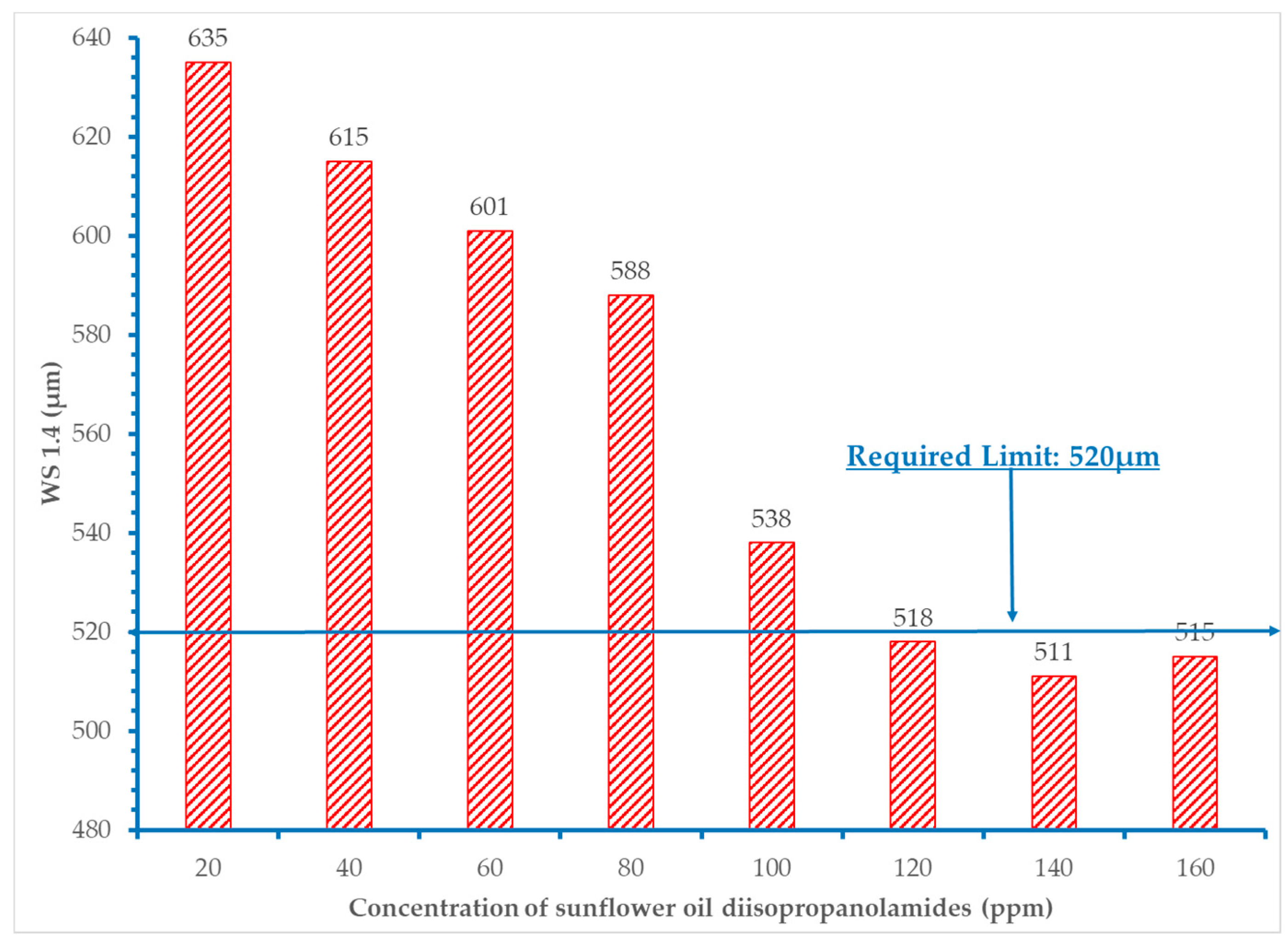
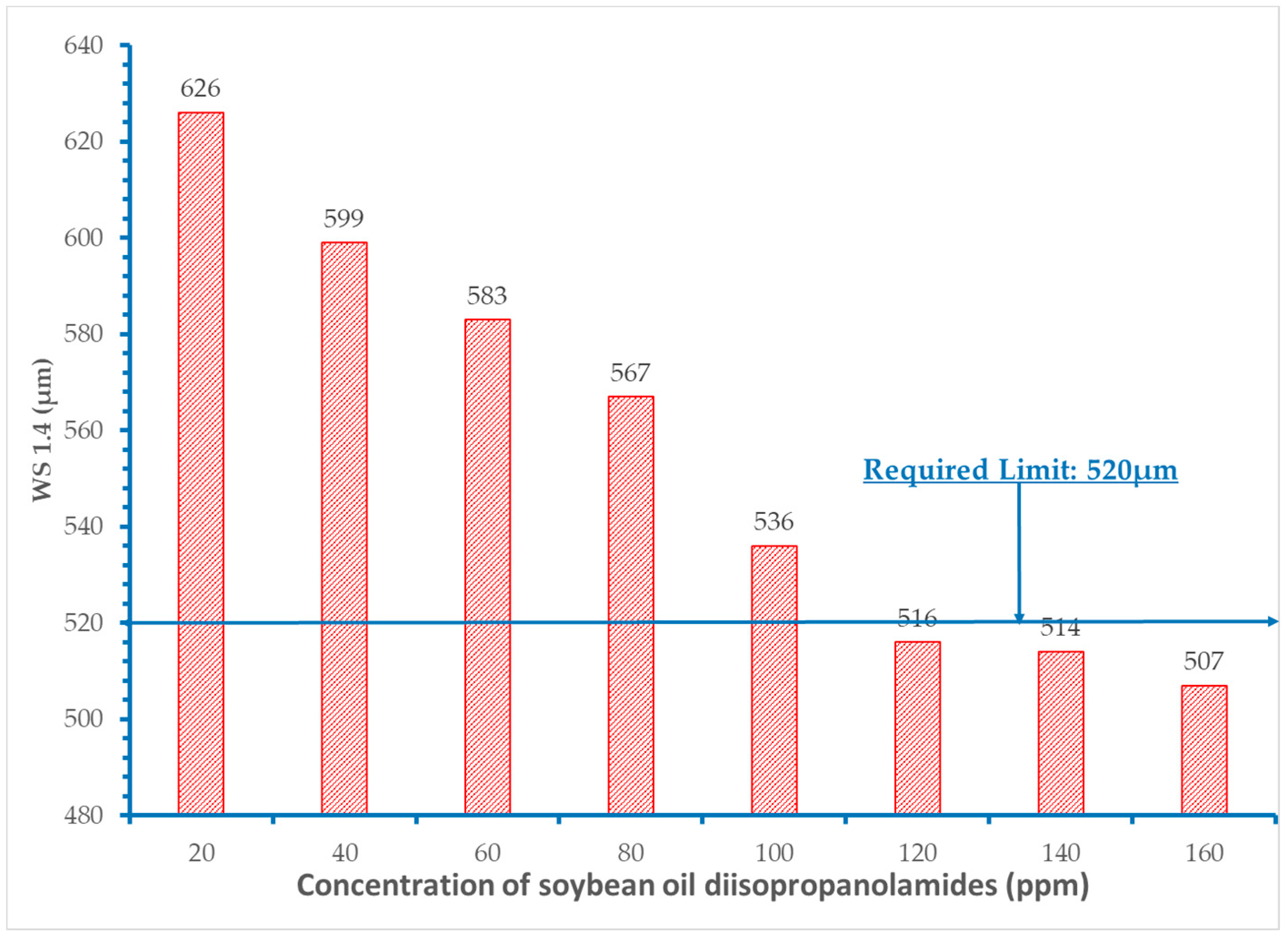
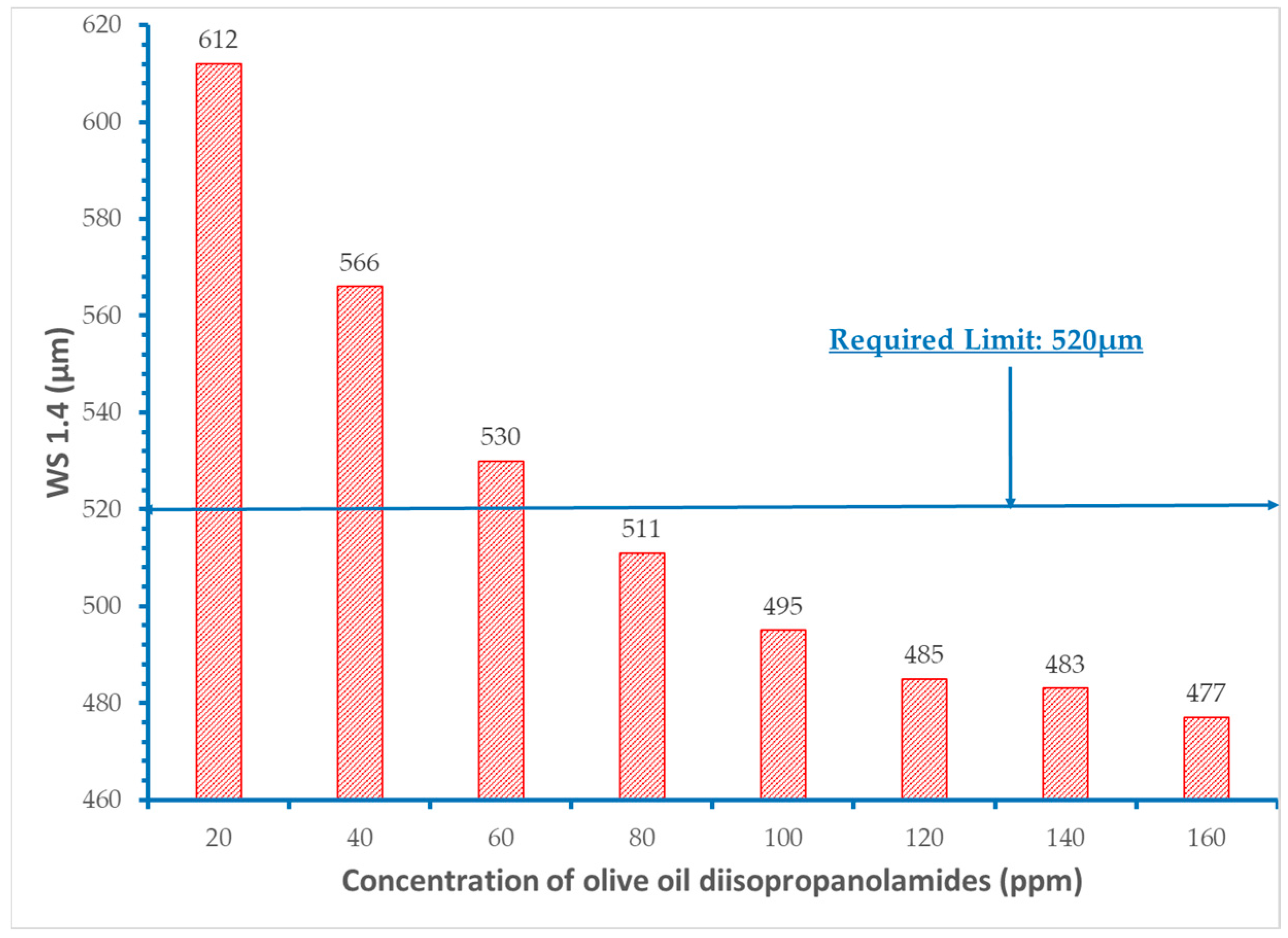
- It was proved that concentration levels of the fatty acid diisopropanolamides from 60 to 120 ppm decrease the initial wear scar diameter value of the base fuel within the acceptable requirement of 520 μm.
- Extra addition of amides did not provide additional improvement in the lubricity of the fuels.
- Among the individual types of fatty acid diisopropanolamides, those derived from non-polyunsaturated oils such as olive oil and coconut oil appear to be better lubricants.
Author Contributions
Conflicts of Interest
References
- Vermeire, M. Development future marine fuels: What has been achieved, what needs to be done. In Proceedings of the European Fuels Conference, Marine Fuels Focus Day, Paris, France, 13 March 2012. [Google Scholar]
- Bengtsson, S.; Fridell, E.; Andersson, K. Environmental assessment of two pathways towards the use of biofuels in shipping. Energy Policy 2012, 36, 451–463. [Google Scholar] [CrossRef]
- European-Commission. White Paper Road Map to a Single European Transport Area Towards a Competitive and Resource Efficient Transport System; European-Commission: Brussels, Belgium, 2011. [Google Scholar]
- Miola, A.; Marra, M.; Ciuffo, B. Designing a climate change policy for the international maritime transport sector: Market-based measures and technological options for global and regional policy actions. Energy Policy 2011, 39, 5490–5498. [Google Scholar] [CrossRef]
- Andersen, K.L.; Haase, F.; Jandles, M.; Paint, J.K. Bio-Fuel Trial on Seagoing Container Vessel; Project Reference: SMIG 09004; The Maersk Group: Copenhagen, Denmark, 2011. [Google Scholar]
- Crutchley, I.; Green, M. Lubricity Characteristics of Marine Distillate Fuels. MTZ Ind. 2012, 2, 58–63. [Google Scholar] [CrossRef]
- Barbour, R.H.; Rickeard, D.J.; Elliott, N.G. Understanding Diesel Lubricity; SAE Technical Paper 2000-01-1918; SAE International: Warrendale, PA, USA; Troy, MI, USA, 2000. [Google Scholar]
- Wei, D.P. The lubricity of fuels I. Wear studies on diesel fuel components. Acta Pet. Sin. 1986, 2, 79–87. [Google Scholar]
- Wei, D.P. The lubricity of fuels II. Wear studies using model compounds. Acta Pet. Sin. 1990, 4, 90–99. [Google Scholar]
- Wei, D.P. The lubricity of fuels III. Wear studies on diesel fuels. Acta Pet. Sin. 1990, 6, 15–19. [Google Scholar]
- Tucker, R.F.; Stradling, R.J.; Wolveridge, P.E.; Rivers, K.J.; Unbbens, A. The Lubricity of Deeply Hydrogenated Diesel Fuels—The Swedish Experience; SAE Technical Paper 942016; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1994. [Google Scholar]
- Caprotti, R.; Bovington, C.; Fowler, W.J.; Taylor, M.G. Additive Technology as a Way to Improve Diesel Fuel Quality; SAE Technical Paper 922183; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1992. [Google Scholar]
- Batt, R.J.; McMillan, J.A.; Bradbury, I.P. Lubricity Additives Performance and No-Harm Effects in Low Sulfur Fuels; SAE Technical Paper 961943; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1996. [Google Scholar]
- Nikanjam, M.; Burk, E. Diesel Fuel Lubricity Additive; SAE Technical Paper 940248; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1994. [Google Scholar]
- Nikanjam, M. Diesel Fuel Lubricity Additive Study; SAE Paper 942014; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1994. [Google Scholar]
- ISO12156–1. Diesel Fuel—Assessment of Lubricity Using the High Frequency Reciprocating Rig; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Bowden, F.P.; Tabor, D. Friction and lubrication of Solids; Clarendo Press: Oxford, UK, 1950. [Google Scholar]
- Karonis, D.; Anastopoulos, G.; Lois, E.; Stournas, S.; Zannikos, F.; Serdari, A. Assessment of the Lubricity of Greek Road Diesel and the Effect of the Addition of Specific Types of Biodiesel; SAE Technical Paper 1999-01-1471; SAE International: Warrendale, PA, USA; Troy, MI, USA, 1999. [Google Scholar]
- Anastopoulos, G.; Lois, E.; Serdari, A.; Zanikos, F.; Stournas, S.; Kalligeros, S. Lubrication Properties of Low-Sulfur Diesel Fuels in the Presence of Specific Types of Fatty Acid Derivatives. Energy Fuel 2001, 15, 106–112. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Lois, E.; Karonis, D.; Kalligeros, S.; Zannikos, F. Impact of Oxygen and Nitrogen Compounds on the Lubrication Properties of Low Sulfur Diesel Fuels. Energy 2005, 30, 415–426. [Google Scholar] [CrossRef]
- Kajdas, C.; Majzner, M. Boundary Lubrication of Low-Sulphur Diesel Fuel in the Presence of Fatty Acids. Lubr. Sci. 2001, 14, 83–108. [Google Scholar] [CrossRef]
- Kajdas, C.; Majzner, M. The Influence of Fatty Acids and Fatty Acids Mixtures on the Lubricity of Low-Sulfur Diesel Fuels; SAE Technical Paper 2001-01-1929; SAE International: Warrendale, PA, USA; Troy, MI, USA, 2001. [Google Scholar]
- Geller, D.P.; Goodrum, J.W. Effect of specific fatty acid methyl ester on diesel fuel lubricity. Fuel 2004, 83, 2351–2356. [Google Scholar] [CrossRef]
- Wain, K.S.; Perez, J.M.; Chapman, E.; Boehman, A.L. Alternative and low sulphur fuel options: Boundary lubrication performance and potential problems. Tribol. Int. 2005, 38, 313–319. [Google Scholar] [CrossRef]
- Schumacher, L.G.; Adams, B.T. Using Biodiesel as a Lubricity Additive for Petroleum Diesel Fuel; ASAE Paper 02-6085; ASAE: Washington, DC, USA, 2002. [Google Scholar]
- Goodrum, J.W.; Geller, D.P. Influence of Fatty Acid Methyl Esters from Hydroxylated Vegetable Oils on Diesel Fuel Lubricity. Bioresour. Technol. 2005, 96, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.E.; Klaus, E.E.; Fein, R.S. Boundary Lubrications—An Appraisal of World Literature; American Society of Mechanical Engineers: New York, NY, USA, 1969; p. 87. [Google Scholar]
- Bhuyan, S.; Sundararajan, S.; Yao, L.; Hammond, E.G.; Wang, T. Boundary lubrication properties of lipid-based compounds evaluated using microtribological methods. Tribol. Lett. 2006, 22, 2–11. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Ara, M.; Salley, S.O.; Ng, K.Y.S. Investigation of Lubricity Characteristics of Biodiesel in Petroleum and Synthetic Fuel. Energy Fuel 2009, 23, 2229–2234. [Google Scholar] [CrossRef]
- Hu, J.; Du, Z.; Li, C.; Min, E. Study on the lubrication properties of biodiesel as fuel lubricity enhancers. Fuel 2005, 84, 1601–1606. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Lubricity of Components of Biodiesel and Petrodiesel. The Origin of Biodiesel Lubricity. Energy Fuel 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- Wei, D.; Spikes, H. The lubricity of diesel fuels. Wear 1986, 111, 217–235. [Google Scholar]
- EN ISO 12185. Crude Petroleum and Petroleum Products—Determination of Density—Oscillating U-Tube Method (ISO 12185:1996); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 1996. [Google Scholar]
- EN ISO 3104. Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 1996. [Google Scholar]
- EN ISO 2719. Determination of Flash Point—Pensky-Martens Closed Cup Method (ISO 2719:2016); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2016. [Google Scholar]
- EN ISO 8754. Petroleum Products—Determination of Sulfur Content—Energy-Dispersive X-Ray Fluorescence Spectrometry (ISO 8754:2003); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2003. [Google Scholar]
- ISO 3015. Petroleum Products—Determination of Cloud Point; International Organization for Standardization, BIBC II, Chemin de Blandonnet 8, CP 401, 1214 Vernier: Geneva, Switzerland, 1992. [Google Scholar]
- ISO 3016. Petroleum Products—Determination of Pour Point; International Organization for Standardization, BIBC II, Chemin de Blandonnet 8, CP 401, 1214 Vernier: Geneva, Switzerland, 1994. [Google Scholar]
- EN ISO 6245. Petroleum Products—Determination of Ash (ISO 6245:2001); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2002. [Google Scholar]
- EN ISO 10370. Petroleum Products—Determination of Carbon Residue—Micro Method (ISO 10370:2014); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2014. [Google Scholar]
- ISO 3733. Petroleum Products and Bituminous Materials—Determination of Water—Distillation Method; International Organization for Standardization, BIBC II, Chemin de Blandonnet 8, CP 401, 1214 Vernier: Geneva, Switzerland, 1999. [Google Scholar]
- EN ISO 4264. Petroleum Products—Calculation of Cetane Index of Middle-Distillate Fuels by the Four-Variable Equation (ISO 4264:2007); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2007. [Google Scholar]
- EN ISO 12156–1. Diesel Fuel—Assessment of Lubricity Using the High-Frequency Reciprocating Rig (HFRR)—Part 1: Test Method (ISO 12156–1:2016); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2016. [Google Scholar]
- EN ISO 3405. Petroleum Products—Determination of Distillation Characteristics at Atmospheric Pressure (ISO 3405:2011); European Committee for Standardization, CEN-CENELEC Management Centre: Brussels, Belgium, 2011. [Google Scholar]


| Fatty Acid | Chemical Structure | Vegetable Oil Type | ||||||
|---|---|---|---|---|---|---|---|---|
| Sunflower Oil | Soybean Oil | Olive Oil | Cottonseed Oil | Coconut Oil | Used Frying Oil | Tobacco Seed Oil | ||
| Lauric (C12) | CH3(CH2)10COOH | 0.00 | 0.10 ± 0.01 | 0.00 | <0.01 | 49.20 ± 0.02 | 1.98 ± 0.01 | <0.01 |
| Myristic (C14) | CH3(CH2)12COOH | 0.00 | 0.10 ± 0.01 | 0.00 | 0.75 ± 0.01 | 18.50 ± 0.02 | 0.32 ± 0.01 | 0.09 ± 0.01 |
| Palmitic (C16) | CH3(CH2)14COOH | 6.20 ± 0.01 | 11.30 ± 0.01 | 11.60 ± 0.01 | 22.23 ± 0.01 | 9.10 ± 0.01 | 15.65 ± 0.02 | 10.96 ± 0.01 |
| Palmitoleic (C16:1) | CH3(CH2)5CH=CH(CH2)7COOH | 0.10 ± 0.01 | 0.00 | 0.90 ± 0.01 | 0.44 ± 0.01 | 0.10 ± 0.01 | 0.31 ± 0.01 | 0.20 ± 0.02 |
| Stearic (C18) | CH3(CH2)16COOH | 3.70 ± 0.01 | 4.10 ± 0.01 | 3.10 ± 0.01 | 2.17 ± 0.01 | 2.70 ± 0.01 | 3.10 ± 0.01 | 3.34 ± 0.01 |
| Oleic (C18:1) | CH3(CH2)7CH=CH(CH2)7COOH | 25.20 ± 0.01 | 22.70 ± 0.02 | 74.98 ± 0.03 | 17.66 ± 0.02 | 6.50 ± 0.01 | 48.71 ± 0.02 | 15.54 ± 0.01 |
| Linoleic (C18:2) | CH3(CH2)3(CH2CH=CH)2(CH2)7COOH | 63.10 ± 0.01 | 52.60 ± 0.01 | 7.80 ± 0.01 | 55.77 ± 0.01 | 1.70 ± 0.01 | 22.39 ± 0.01 | 69.49 ± 0.02 |
| Linolenic (C18:3) | CH3(CH2CH=CH)3(CH2)7COOH | 0.30 ± 0.01 | 7.40 ± 0.02 | 0.60 ± 0.01 | 0.13 ± 0.01 | 0.00 | 1.04 ± 0.01 | 0.69 ± 0.01 |
| Eicosenoic (C20) | CH3(CH2)8CH=CH(CH2)8COOH | 0.20 ± 0.01 | 0.50 ± 0.01 | 0.01 | 0.20 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.25 ± 0.01 |
| Behenic (C22) | CH3(CH2)20COOH | 0.70 ± 0.01 | 0.50 ± 0.01 | 0.10 ± 0.01 | <0.01 | 0.10 ± 0.01 | 0.24 ± 0.01 | 0.12 ± 0.01 |
| Erucic (C22:1) | CH3(CH2)7CH=CH(CH2)11COOH | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.00 | 0.02 | 0.00 | 0.02 ± 0.01 | <0.01 |
| Lignoceric (C24) | CH3(CH2)22COOH | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.50 ± 0.01 | 0.01 ± (<0.01) | 0.00 | 0.30 ± 0.01 | 0.04 ± (<0.01) |
| Property | Value | Test Method |
|---|---|---|
| Density (kg/m3, 15 °C) | 853.5 ± 0.7 | EN ISO 12185 [33] |
| Kinematic Viscosity (cSt at 40 °C) | 3.27 ± 0.04 | EN ISO 3104 [34] |
| Flash point (°C) | 64 ± 0.5 | EN ISO 2719 [35] |
| Nitrogen content (mg/kg) | 31 ± 1 | - |
| Sulfur content (mg/kg) | 310 ± 8 | EN ISO 8754 [36] |
| Cloud point (°C) | −3 ± 0.1 | ISO 3015 [37] |
| Pour point (°C) | −9 ± 0.2 | ISO 3016 [38] |
| Ash content (% m/m) | <0.01 | EN ISO 6245 [39] |
| Carbon residue (% m/m) | 0.006 ± 0.0005 | EN ISO 10370 [40] |
| Water content (mg/kg) | 29 ± 1 | ISO 3733 [41] |
| Cetane index | 49.7 ± 0.1 | EN ISO 4264 [42] |
| Lubricity | EN ISO 12156-1 [43] | |
| Initial measurement, μm | 645 ± 1 | |
| Repeated measurement, μm | 641 ± 1 | |
| Distillation (°C) | EN ISO 3405 [44] | |
| Initial Boiling Point (IBP) | 177 ± 0.5 | |
| 10% | 231 ± 0.4 | |
| 50% | 295 ± 0.4 | |
| 90% | 352 ± 0.5 | |
| Final Boiling Point (FBP) | 378 ± 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastopoulos, G.; Kaligeros, S.; Schinas, P.; Zannikou, Y.; Karonis, D.; Zannikos, F. The Impact of Fatty Acid Diisopropanolamides on Marine Gas Oil Lubricity. Lubricants 2017, 5, 28. https://doi.org/10.3390/lubricants5030028
Anastopoulos G, Kaligeros S, Schinas P, Zannikou Y, Karonis D, Zannikos F. The Impact of Fatty Acid Diisopropanolamides on Marine Gas Oil Lubricity. Lubricants. 2017; 5(3):28. https://doi.org/10.3390/lubricants5030028
Chicago/Turabian StyleAnastopoulos, George, Stamatios Kaligeros, Petros Schinas, Ypatia Zannikou, Dimitrios Karonis, and Fanourios Zannikos. 2017. "The Impact of Fatty Acid Diisopropanolamides on Marine Gas Oil Lubricity" Lubricants 5, no. 3: 28. https://doi.org/10.3390/lubricants5030028





